Abstract
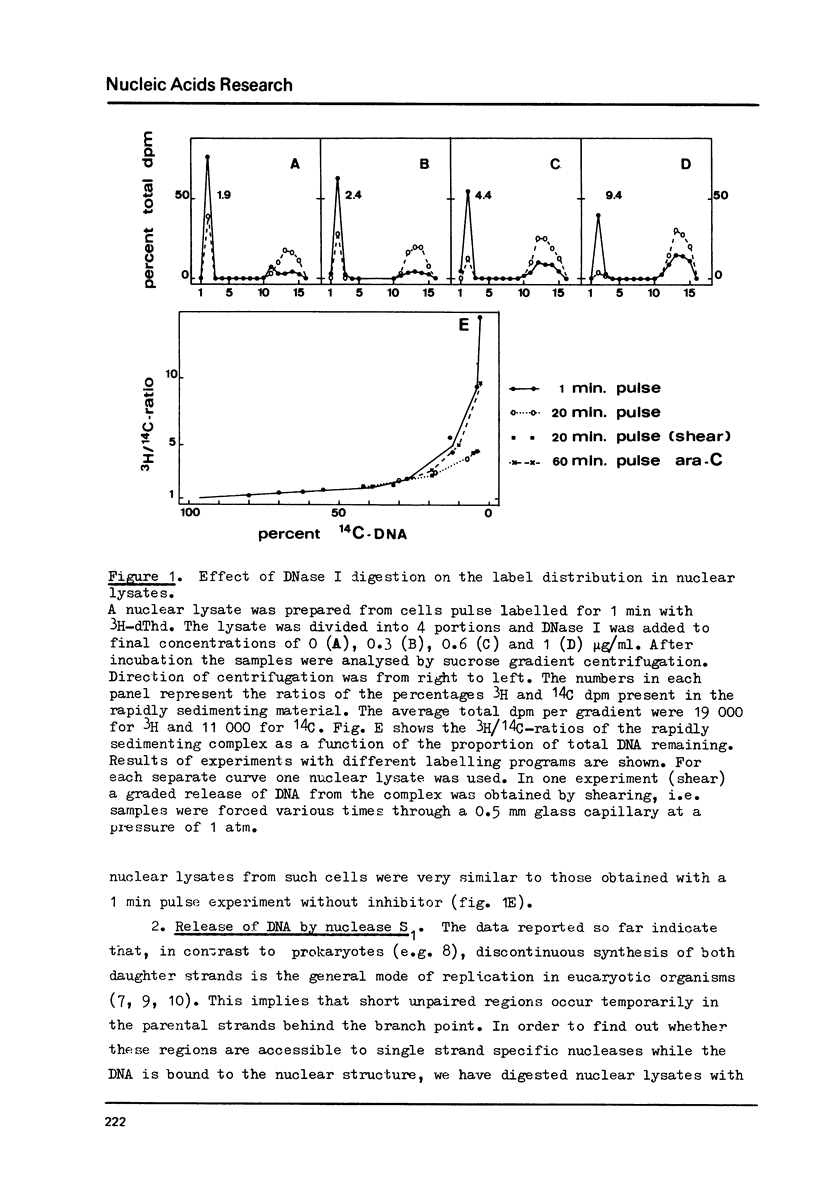
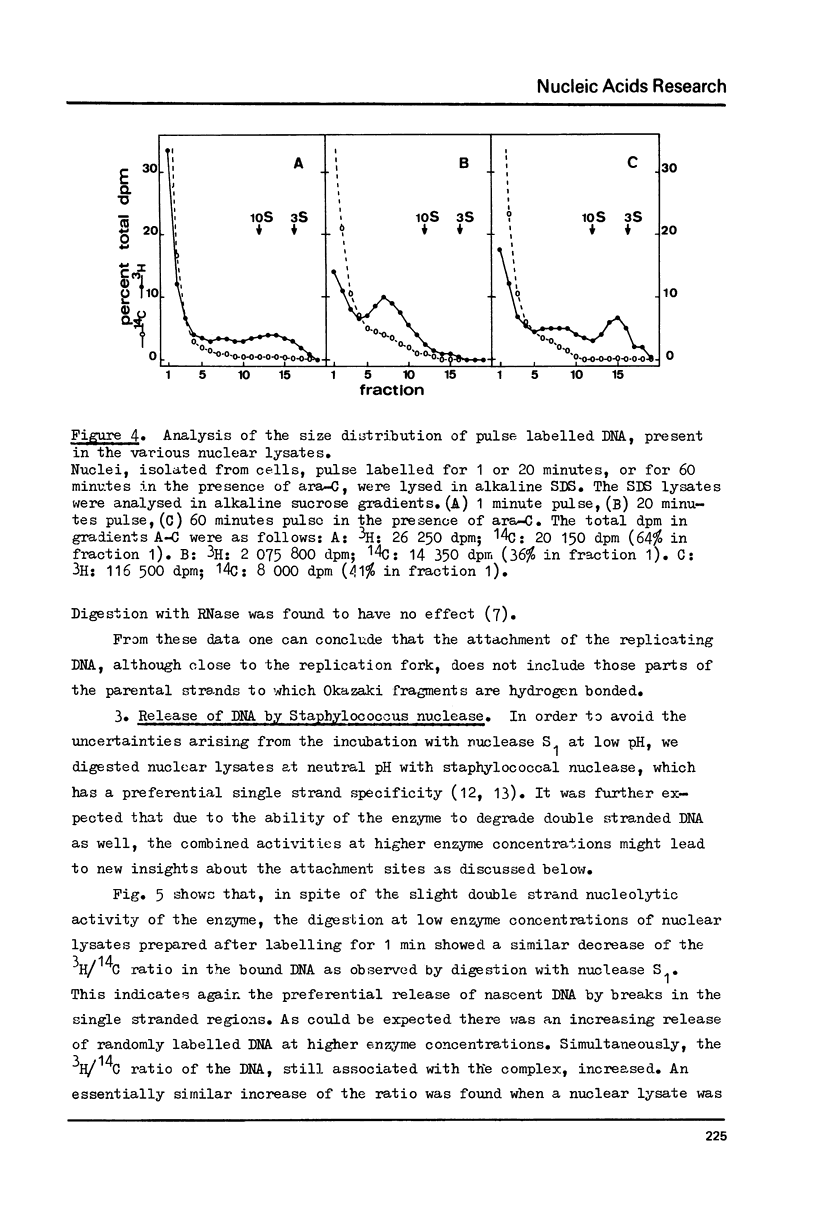
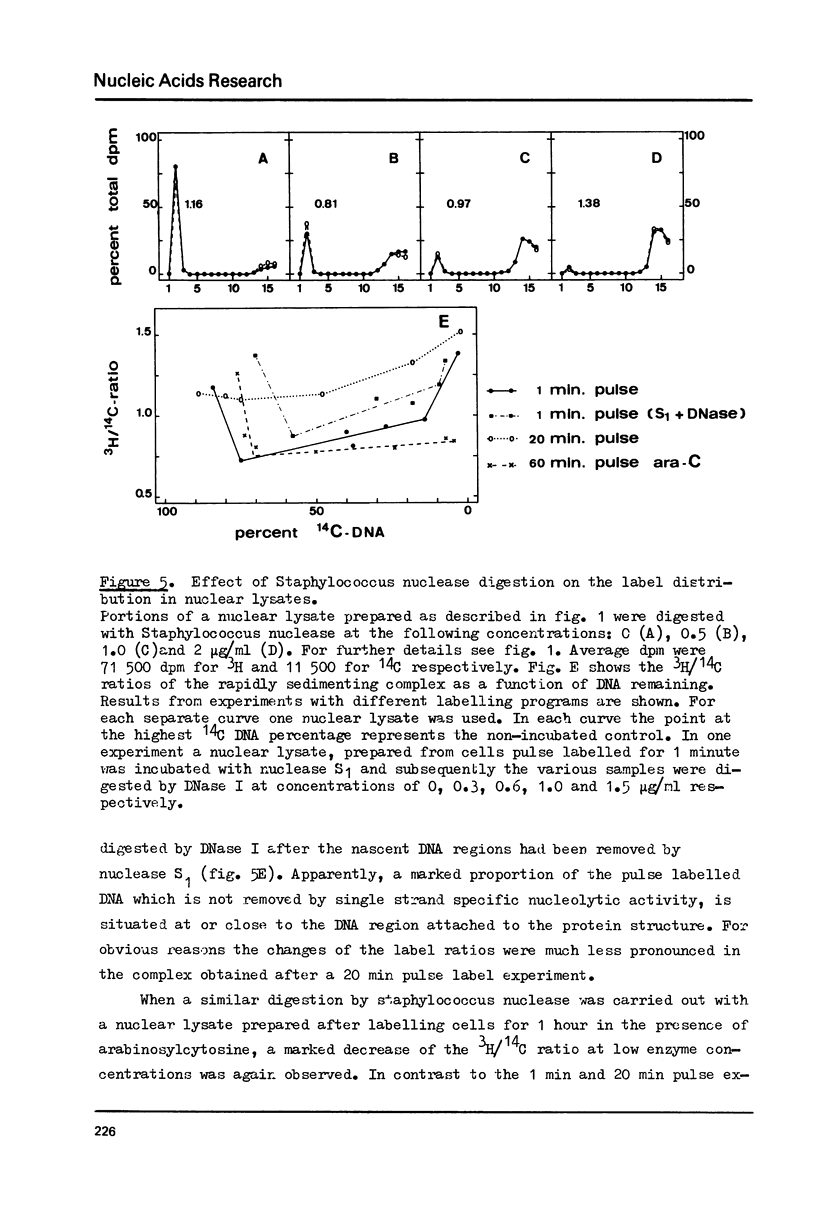
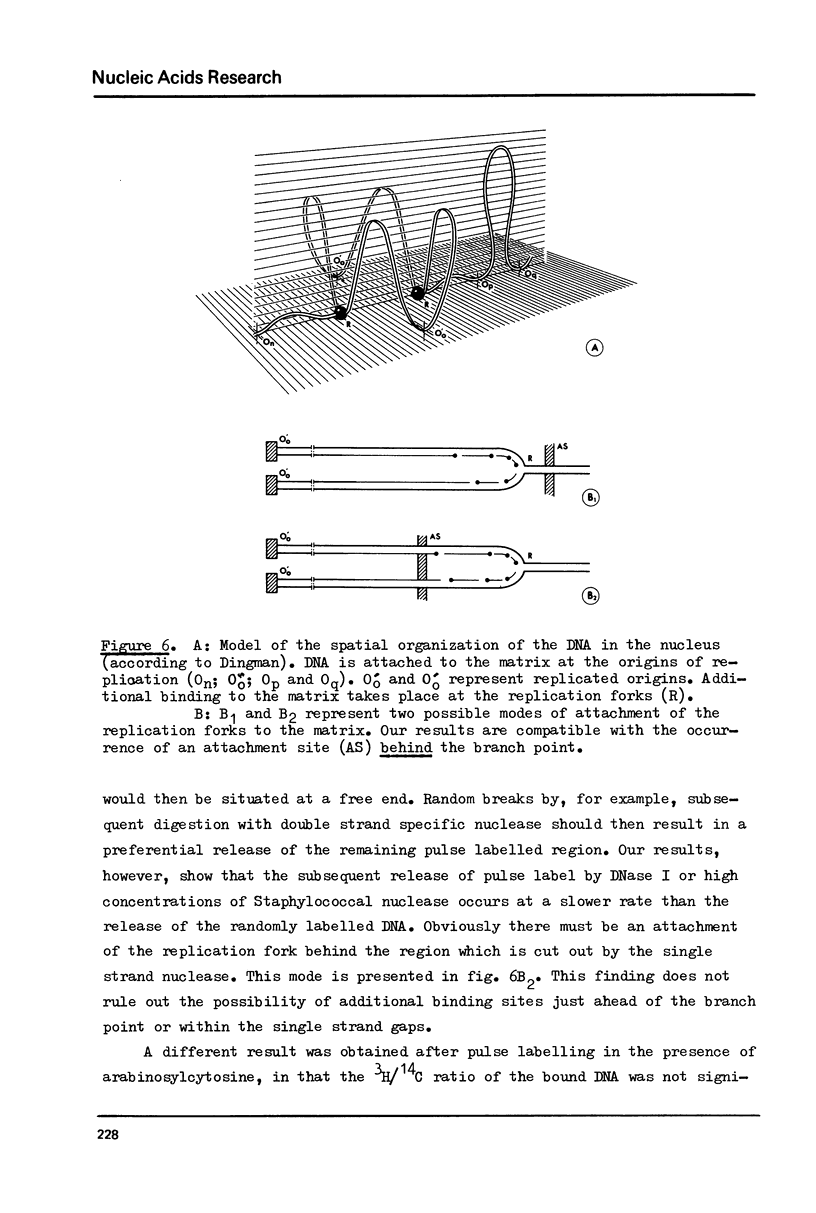
The attachment of replicating DNA to a rapidly sedimenting nuclear structure was investigated by digestion with various nucleases. When DNA was gradually removed by DNase I, pulse label incorporated during either 1 min or during 1 hour in the presence of arabinosylcytosine, remained preferentially attached to the nuclear structure. Single strand specific digestion by nuclease S1 or staphylococcal nuclease at low concentrations caused a release of about 30% of the pulse label, without significantly affecting the attachment of randomly labelled DNA. The released material had a low sedimentation coefficient and contained most of the Okasaki fragments. The remaining pulse label was less accessible to further digestion by double strand specific nuclease activity than the bulk DNA. The results suggest that an attachment of the replication fork to the nuclear structure occurs at sites behind but close to the branch point.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Blobel G. Isolation of nuclear pore complexes in association with a lamina. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1007–1011. doi: 10.1073/pnas.72.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Dijkwel P. A., Wanka F. Enhanced release of nascent single strands from DNA synthesized in the presence of arabinosylcytosine. Biochim Biophys Acta. 1978 Oct 24;520(3):461–471. doi: 10.1016/0005-2787(78)90131-4. [DOI] [PubMed] [Google Scholar]
- Dingman C. W. Bidirectional chromosome replication: some topological considerations. J Theor Biol. 1974 Jan;43(1):187–195. doi: 10.1016/s0022-5193(74)80052-4. [DOI] [PubMed] [Google Scholar]
- Fridland A. Effect of cytosine arabinoside on replicon initiation in human lymphoblasts. Biochem Biophys Res Commun. 1977 Jan 10;74(1):72–78. doi: 10.1016/0006-291x(77)91376-6. [DOI] [PubMed] [Google Scholar]
- Housman D., Huberman J. A. Changes in the rate of DNA replication fork movement during S phase in mammalian cells. J Mol Biol. 1975 May 15;94(2):173–181. doi: 10.1016/0022-2836(75)90076-5. [DOI] [PubMed] [Google Scholar]
- Olivera B. M. DNA intermediates at the Escherichia coli replication fork: effect of dUTP. Proc Natl Acad Sci U S A. 1978 Jan;75(1):238–242. doi: 10.1073/pnas.75.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson J. R., Laemmli U. K. The structure of histone-depleted metaphase chromosomes. Cell. 1977 Nov;12(3):817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Chien J., Lehman I. R., Duncan B. K., Warner H. R. Uracil incorporation: a source of pulse-labeled DNA fragments in the replication of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1978 Jan;75(1):233–237. doi: 10.1073/pnas.75.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye B. K., Nyman P. O., Lehman I. R., Hochhauser S., Weiss B. Transient accumulation of Okazaki fragments as a result of uracil incorporation into nascent DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):154–157. doi: 10.1073/pnas.74.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wanka F., Brouns R. M., Aelen J. M., Eygensteyn A., Eygensteyn J. The origin of nascent single-stranded DNA extracted from mammalian cells. Nucleic Acids Res. 1977 Jun;4(6):2083–2097. doi: 10.1093/nar/4.6.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanka F. Decreased DNA synthesis in mammalian cells after exposure to deoxyadenosine. Exp Cell Res. 1974 Apr;85(2):409–414. doi: 10.1016/0014-4827(74)90143-8. [DOI] [PubMed] [Google Scholar]
- Wanka F., Mullenders L. H., Bekers A. G., Pennings L. J., Aelen J. M., Eygensteyn J. Association of nuclear DNA with a rapidly sedimenting structure. Biochem Biophys Res Commun. 1977 Jan 24;74(2):739–747. doi: 10.1016/0006-291x(77)90364-3. [DOI] [PubMed] [Google Scholar]
- Wingert L., Von Hippel P. H. The conformation dependent hydrolysis of DNA by micrococcal nuclease. Biochim Biophys Acta. 1968 Mar 18;157(1):114–126. doi: 10.1016/0005-2787(68)90270-0. [DOI] [PubMed] [Google Scholar]



