Abstract
Aim:
Gingival crevicular fluid (GCF) is regarded as a promising medium for detection of markers of periodontal disease activity. Very few investigators have examined concentration of electrolytes in GCF, but most results are not in agreement to one another. This study was undertaken with an objective of quantitative estimation of sodium, potassium and calcium concentrations of GCF in gingivitis and periodontitis, to find the reliability of these ions as diagnostic markers and to analyze the relation of these ions to one another. This will indicate stage of disease activity which helps in early diagnosis, prevention and treatment of periodontal diseases.
Materials and Methods:
The patients selected for the study included both sexes, aging from 18 to 55 years, divided into two groups: gingivitis (group I) and periodontitis (group II). Using volumetric microcapillary pipette, 5 μl GCF was collected for quantitative analysis of sodium, potassium and calcium using flame photometry.
Results:
The concentrations of sodium, potassium and calcium in GCF and their significant correlation with gingival index and pocket depth measurements reflect the clinical status of gingival and periodontal tissues.
Conclusions:
Estimation of these electrolytes may be used as potential diagnostic markers of active disease status in periodontal tissues and to predict the effective methods of prevention and treatment.
Keywords: Biochemical markers, disease activity, electrolytes, gingival crevicular fluid, periodontitis
Introduction
The gingival tissue is constantly subjected to mechanical and bacterial aggregation. Resistance to these actions is provided by saliva, sulcular fluid, epithelial surface keratinization and initial stages of inflammation. Information on the structure and function of the marginal periodontium in health and disease are more precise now. The origin, composition and clinical significance of gingival crevicular fluid (GCF) have significantly helped us in understanding the pathogenesis of periodontal disease. Analysis of very small amount of fluid may reveal important clinical changes taking place within the gingiva. These changes may be valuable in diagnosis, prevention and treatment of periodontal disease.[1,2]
Studies on GCF extend over a period of about 50 years. The pioneer research of Waerhaug in the early 1950s was focused on the anatomy of the sulcus and its transformation into a gingival pocket during the course of periodontitis. In the late 1950s and early 1960s, a series of groundbreaking studies by Brill et al. laid the foundation for understanding the physiology of GCF formation and its composition. The studies of Loe et al. contributed to this understanding and started to explore the use of GCF as an indicator of periodontal diseases. Egelberg continued to analyze GCF and focused his studies on the dentogingival blood vessels and their permeability as they relate to GCF flow. The GCF studies boomed in the 1970s. The rationale for understanding dentogingival structure and physiology was created by the outstanding electron microscopic studies of Schroeder and Listgartan.[3–6]
New morphological, biochemical, immunological and bacteriological research has been performed in periodontology, allowing a better understanding of the significance of crevicular fluid production. A few attempts have been made to measure the concentration of common electrolytes in GCF. In the last few years, it is evident that inflammation of the marginal gingiva, elicited by any kind of stimuli, was the primary and probably the only reason for the presence of fluid around a tooth.[7]
GCF is regarded as a promising medium for the detection of markers of periodontal disease activity. The collection protocols are straightforward, non-invasive and can be performed at specificities of interest in the periodontium. The first quantitative study on the absolute contents of sodium and potassium in GCF was performed by Matsue.[2,8,9] Pioneer work was performed in periodontology by Waerhaug, Brill and Krasse, Egelberg and others, allowing a better understanding of the significance of crevicular fluid.[1,6]
Nevertheless, the potential of using the crevicular fluid as a diagnostic and prognostic marker was realized and there was an intense interest in the qualitative assessment of GCF. As periodontal diseases are characterized by destruction of tooth supporting tissues, quantitation of tissue breakdown products in GCF has been pursued as a means of identifying sites undergoing active disease.[1,9,10] The qualitative and quantitative measurements of its components may act as a gradient for the evaluation of the extent of gingival and periodontal inflammation. So far, more than 40 compounds have been analyzed, but their origin is not known with certainty.[2,6,11]
More is known about the clinical significance of crevicular fluid, for instance, as a possible carrier of antibiotics from the general circulation into the oral cavity. A diagnostic test seeks to establish the presence or absence of a disease. Crevicular fluid based diagnostic tests for various periodontal diseases are currently attracting much interest in clinical, academic and industrial circles. This is because the existing clinical diagnostic tests have many shortcomings. By the use of these techniques it is hoped that treatment will become more effective and that over treatment will be avoided thus resulting in a more cost-effective outcome.[9,12]
In the search for a useful biochemical marker to assess disease activity, very few investigators have examined the concentration of electrolytes in GCF in health and disease, but most of the results are not in agreement to one another and need confirmation.[8]
Therefore, this short-term clinical study of quantitative estimation of sodium, potassium and calcium concentrations in GCF in gingivitis and periodontitis was carried out. This will help to find the reliability of these ions as a diagnostic marker in gingivitis and periodontitis and to analyze the relation of these ions to one another, which would help in prevention and treatment of periodontal disease and to indicate disease activity in gingivitis and periodontitis.
Materials and Methods
Source of data
The patients for this study were selected from the Department of Periodontics, P. M. N. M. Dental College and Hospital, Bagalkot. This study included 30 patients, both males and females in the age group ranging from 18 to 55 years. The selected patients were divided into two groups: group I (gingivitis group) consisted of 15 patients with gingivitis and group II (periodontitis group) consisted of 15 patients with pocket depth of ≥5 to ≤7 mm. The selection of patients was done on the same day before the collection of sample. Gingival index (Loe and Silness 1963) and probing pocket depth were recorded.
Inclusion criteria
Patients who had not received any periodontal treatment during the past 6 months, those who had not taken any antibiotic therapy during the past 6 months and those who were not suffering from any known systemic diseases or conditions that influence the tissues were included in the study. No oral hygiene instructions were given priory which might change home care habits.
Procedure for gingival crevicular fluid collection
The selected patients were seated in an upright position on the dental chair with proper lighting condition. The selected test site was dried and isolated with cotton rolls. GCF samples were obtained by placing calibrated, volumetric microcapillary pipette of internal diameter of 1.1 mm with a capacity of 5 μl extracrevicularly over test sites. From each test site, a standardized volume of 5 μl was collected. Sites which did not express appropriate volume of fluid and micropipettes which were contaminated with blood and saliva were not included in the study.[1,8,13]
Biochemical assay
The collected GCF samples were transferred to a volumetric flask containing 2 ml of double-distilled water and then centrifuged. The samples were analyzed for GCF sodium, potassium and calcium concentrations using flame photometry at Department of Pharmachemistry, H. S. K. College of Pharmacy, Bagalkot.[14–16]
Statistical analysis
Descriptive data were presented as mean and standard deviations. Students's t test was used. A Pearson's correlation coefficient P-value of less than 0.05 was considered for statistical significance.
Results
Comparison of sodium, potassium and calcium levels in GCF and serum of the gingivitis group showed significantly higher levels in GCF than serum. But the sodium:potassium ratio in serum was higher than GCF values [Table 1]. In periodontitis group, the sodium, potassium and calcium levels in GCF were significantly higher than the corresponding serum values of the same patients. The sodium:potassium ratio in GCF was lower than the serum values of the same patients [Table 2].
Table 1.
Comparison of sodium, potassium, calcium levels and sodium:potassium ratio (Na+:K+) in GCF and serum with gingivitis (group I)
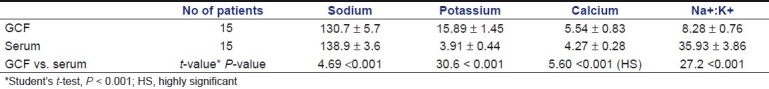
Table 2.
Comparison of sodium, potassium, calcium levels and sodium:potassium ratio (Na+:K+) in GCF and serum with periodontitis (group II)

The comparison of GCF sodium, potassium, calcium levels and sodium:potassium ratio between gingivitis and periodontitis groups showed higher levels of sodium and potassium in periodontitis group than gingivitis group. The calcium levels in periodontitis patients were slightly higher than gingivitis patients; however, the difference was statistically not significant. The sodium:potassium ratio was highly significant in group II than group I [Table 3].
Table 3.
Comparison of sodium, potassium, calcium levels and sodium:potassium ratio (Na+:K+) in GCF between gingivitis group I and periodontitis group II

Correlation between gingival index scores and GCF sodium, potassium, calcium levels and sodium:potassium ratio in the gingivitis group was not significant. But the correlation values between pocket depth and sodium, potassium, calcium levels and sodium:potassium ratio in GCF with periodontitis were significant, which indicates increase in these ion levels as the pocket depth increases. Correlation of calcium and sodium:potassium ratio with pocket depth showed no significance.
Discussion
The composition of GCF seems promising as a potential medium for the detection of early changes which could indicate the onset of disease. The origin, composition and the clinical significance of the gingival fluid are now known with more precision and have significantly helped our understanding of pathogenesis of periodontal disease. On the other hand, some of the legitimate that were inspired when GCF was first discovered have been dashedUp to now, for instance, the multiple components analyzed in the fluid have improved clinical judgment of the rate of progress of gingivitis and periodontitis or the rate of repair of these conditions.[1]
The requirement for a reliable biochemical marker in GCF for disease activity and susceptibility in periodontology was the relative frustration experienced by research workers in this quest for last three decades.[9] The flow and composition of GCF serve as a gauge or barometer of the intensity of the inflammation. This fluid contains all the plasma proteins as well as cellular elements such as Polymorphonuclear (PMN)s in mild inflammation, and the composition of GCF is characterized by the appearance of bacterial products, degradation products of the host immune system, mediator of inflammation and by-products of the host immune system and the by-products of connective tissue breakdown in severe inflammation. Additionally, according to “Alfano's theory,” this increase in concentration may be attributed to the modulation by the extent of plasma protein exudation.[1,11,17]
Clinically, the monitoring of the GCF flow and the quality of its contents may be useful diagnostically to assess the severity of gingival and periodontal inflammation, the effectiveness of oral hygiene, the response of tissues to periodontal therapy and the effectiveness of antibiotics as adjuncts of periodontal therapy. Many research efforts have attempted to use GCF components to detect or diagnose active disease or to predict patients at risk of periodontal disease. So far, various compounds found in GCF have been analyzed, but their origin is not known with certainty.[3,2,6,11]
The collection protocols for GCF are straightforward and non-invasive and can be performed at specific sites of interest in periodontium. Because the fluid accumulates at the gingival margin, it will contain potential markers derived not only from the host tissues and serum but also from the subgingival microbial plaque, and thus an extremely broad range of candidate molecules may be investigated. However, the ability to successfully describe indicators of current disease activity and predictors of future disease is dependent not only upon the choice of the biochemical marker but also on the accurate description of the health status of the sample sites, using currently available clinical and radiographic methods.[1,18]
To a certain extent, the method chosen reflects the type of analysis to be performed on the sample subsequently; for example, when cell types and numbers are to be examined, the gingival washing procedure[19] is the most suitable, and when large volumes of GCF are required, the capillary tubing procedure may be the most useful. However, the insertion of a filter paper strip into the sulcus has been shown to cause trauma to the tissues, accompanied by increased permeability of the vessels beneath the epithelium.[20,21] Curtis[22] clearly stated the sampling method of GCF collection must be very carefully standardized in order to minimize compositional differences. Because of lack of uniformity in the methods used for collection and quantitation, comparisons between different studies in the literature are not often feasible.
Quantitative research on GCF was greatly improved after appropriate standardized techniques of collecting a known volume of fluids were devised. Krasse and Egelberg[1] proposed the use of micropipettes. However, the detailed procedure of GCF collection by using glass capillary tubes of known internal diameter and length was described by Kaslick and Mann.[13,23] In the present study, the micropipettes with an internal diameter of 1.1 mm were used for the collection of GCF samples, where the fluid collection takes place through capillary action. These micropipettes were placed at the entrance of gingival crevice to avoid air entrapment during collection of fluid. As done in the present study, the area should be completely isolated and dried before the collection of GCF to avoid salivary contamination.
Qualitative and quantitative investigation of sodium, potassium and calcium in gingival fluid has greatly contributed to a better understanding of its nature and significance. In the present study, sodium concentration in GCF significantly exceeded that in serum, which was similar to that reported in other studies done by Kaslick.[2,13,24] The tendency for sodium to increase as inflammation increased was found to be statistically significant as the sodium levels in periodontitis patients (163.8 ± 3.6) were higher than in gingivitis patients (130.7 ± 5.7). This can be attributed to the presence of a large quantity of sodium in bone, from where only a small part (10–15%) of sodium enters into immediate exchange with that in the remainder of extracellular spaces. It is conceivable that with alveolar bone destruction, increased quantities of sodium may be made available to the extracellular compartment and to the gingival fluid.[24]
It is possible that under normal conditions with slow fluid flow, sodium may be reabsorbed actively from fluid in the crevice by crevicular epithelium. So, high sodium levels in fluid from group II may be due to a failure of active sodium reabsorption in the pocket epithelium in severe inflammation.[24] Many studies of Cimasoni and Kaslick[1,2,13,24] report that the concentration of sodium tends to increase in cases of more severe inflammation. But the present finding did not coincide with that of Bang[8] who reported lower sodium levels in periodontitis than gingivitis cases.
The potassium content of crevicular exudate was much higher than that of serum in both the groups, which coincides with the findings of other studies carried out by Menkin and Kaslick[2,24] who also stated higher levels of potassium in GCF. A clear tendency for potassium to increase along with inflammation from gingivitis (15.89 ± 1.45) to periodontitis (16.95 ± 0.78) proved to be statistically significant and was evident when potassium concentration and pocket depth were correlated. The increased potassium is probably derived from intracellular sources in the inflamed tissues and from degenerating epithelial, connective tissue and blood cells in the pocket area.[2,24] The present results mimic the findings on potassium levels in other investigations done by Menkin and Kaslick.[2,24,25] But lower values were found in the study of Bang, and in Matsue's study the values were found to be very high.[2,8]
Sodium:potassium ratio was lower in GCF than serum in both groups I and II, which is concordant with the reports of Krasse and Egelberg[1] and Bang.[8] It was suggested by these investigators that the fluid passes through damaged tissues, and decreased sodium:potassium ratio is found because of accumulation of intracellular potassium from disrupted cells. Significantly higher sodium:potassium ratio was found in group II (9.68 ± 0.37) than group I (8.28 ± 0.76). In the investigations of Kaslick,[2,24] highly significant sodium:potassium ratio was found, which states that as the inflammation increases, the sodium:potassium ratio increases. A negative but statistically not significant correlation could be shown between the sodium:potassium ratio and gingival index scores and mean pocket depth. This finding is the logical corollary of results concerning sodium and potassium concentrations. Similar reports were obtained by Bang.[8]
The mean calcium concentration found in this study was much lower as compared to those reported from other studies of Kaslick.[2,24] But the present values were similar to the findings of Bang et al.[8] Mean calcium concentration of GCF was found to be significantly higher than the serum concentration in both groups I and II. The mean calcium concentration in group II (6.13 ± 0.77) was slightly higher than in group I (5.54 ± 0.83). However, this was statistically not significant. These results are similar to the reports given by Bang et al.,[4] but Kaslick[2,24] reported higher values of calcium concentration.
But calcium concentration did not show any correlation with the various parameters like gingival index scores and pocket depth. Jenkins has formulated an interesting hypothesis that the presence of calcium in gingival fluid plays a role in the genesis of plaque. As shown by Dawes, adding calcium ions to saliva will favor the precipitation of proteins. It is possible that calcium from gingival fluid, where it is present in higher concentration than in saliva, could help triggering the precipitation of mucoproteins along the enamel surface. This seems even more plausible when thinking that the very first acquired deposits along the enamel surface seem to take place toward the sulcular area.[8]
Hence, it can be suggested that the concentration of sodium, potassium and calcium ions in GCF reflects the clinical status of the periodontal tissues, so that the estimation of these may be used as a potential diagnostic marker of an active disease status in periodontal tissues.
Even though the above-mentioned conclusions could bedrawn in this present study, further investigations need to be done as the high way of science is broad straight throughfare, but it cannot be traveled in a straight line because the way is glittered with wreckage of discarded scientific theories that were formerly delivered to be facts. Diagnostic techniques have advanced since then, and the goal to instil appropriate oral health early in adulthood so that when an individual advances to retirement years, he/she will be able to sustain and retain natural dentition in health, will not be accomplished overnight but will be realized during the coming decades of this century.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Cimasoni G. The crevicular fluid updated.Monographs in oral science. In: S Karger AG, editor. Chapter 2, The gingival sulcus. 2nd ed. Basel (Switzerland): 1983. pp. 1–14. [PubMed] [Google Scholar]
- 2.Kaslick RS, Chasens AI, Mandel ID, Weinstein D, Waldman R, Pluhar T, et al. Quantitative analysis of sodium, potassium and calcium in gingival fluid from gingiva in varying degrees of inflammation. J Periodontol. 1970;41:93–7. doi: 10.1902/jop.1970.41.2.93. [DOI] [PubMed] [Google Scholar]
- 3.Bang JS, Cimasoni G. Total protein in human crevicular fluid. J Dent Res. 1971;50:1683. doi: 10.1177/00220345710500065701. [DOI] [PubMed] [Google Scholar]
- 4.Loe H, Holm Pederson P. Absence and presence of fluid from normal and inflamed gingiva. Periodontics. 1965;3:171. [PubMed] [Google Scholar]
- 5.Sueda T, Bang J, Cimasoni G. Collection of gingival fluid for quantitative analysis. J Dent Res. 1969;48:159. doi: 10.1177/00220345690480011501. [DOI] [PubMed] [Google Scholar]
- 6.Uitto VJ. Gingival crevice fluid: An introduction. Periodontal 2000. 2003;31:9–11. doi: 10.1034/j.1600-0757.2003.03101.x. [DOI] [PubMed] [Google Scholar]
- 7.Curtis MA, Gillett IR, Griffiths GS, Maiden MF, Sterne JA, Wilson DT, et al. Detection of high risk groups and individuals for periodontal diseases: Laboratory markers from analysis of gingival crevicular fluid. J Clin Periodontal. 1989;16:1–11. doi: 10.1111/j.1600-051x.1989.tb01604.x. [DOI] [PubMed] [Google Scholar]
- 8.Bang J, Cimasoni G, Rosenbusch C, Duckert A. Sodium, potassium and calcium contents of crevicular exudate: Their relations to Gingivitis and Periodontitis. J Periodontal. 1973;44:770–4. doi: 10.1902/jop.1973.44.12.770. [DOI] [PubMed] [Google Scholar]
- 9.Manson JD, Eley BM. Chapter 13, Diagnostic tests of disease activity. 3rd ed. Oxford: Reed educational and Publishing Ltd; 1995. Outline of Periodontics; pp. 144–57. [Google Scholar]
- 10.Adonogianaski E, Mooney J, Kinane DE. Detection of stable and active periodontitis sites by clinical assessment and gingival crevicular acute phase protein levels. J Periodont Res. 1996;31:135–43. doi: 10.1111/j.1600-0765.1996.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 11.Page RC. Host response test for diagnosing periodontal diseases. J Periodontol. 1992;63:356–66. doi: 10.1902/jop.1992.63.4s.356. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee S. The significance of crevicular fluid. Compendium Continuing Educ. 1985;8:611–6. [PubMed] [Google Scholar]
- 13.Kaslick RS, Chasens AI, Weinstein D, Waldman R. Ultra micro method for the collection of gingival fluid and quantitative analyses of sodium content. J Dent Res. 1968;47:1192. doi: 10.1177/00220345680470063801. [DOI] [PubMed] [Google Scholar]
- 14.Spectrophotometry. Appendix 5.1-5.4. Ghaziabad, India: 2007. The Indian pharmacopoeia commission. Government of India ministry of health and family welfare. [Google Scholar]
- 15.Lowery OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 16.Schacterele GR, Pollack LR. A simplified method for the quantitative assay of small amount of protein in biologic material. Anal Biochem. 1973;51:654–5. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- 17.Harvey AS, Genco RJ. Gingival fluid and serum in periodontal disease: I, quantitative study of immunoglobulins, compliment components and other plasma proteins. J Periodontol. 1977;48:772–7. doi: 10.1902/jop.1977.48.12.772. [DOI] [PubMed] [Google Scholar]
- 18.Lamster IB. The host response in gingival fluid: potential application in periodontitis clinical trial. J Periodontol. 1992;63:1117–23. doi: 10.1902/jop.1992.63.12s.1117. [DOI] [PubMed] [Google Scholar]
- 19.Skapski H, Lehner T. A crevicular washing method for investigating immune components or crevicular fluid in man. J Periodontol Res. 1976;11:19–24. doi: 10.1111/j.1600-0765.1976.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 20.Egelberg J. Permeability of the dento-gingival blood vessels: II, Clinically healthy gingiva. J Periodont Res. 1966;1:276–86. doi: 10.1111/j.1600-0765.1966.tb01872.x. [DOI] [PubMed] [Google Scholar]
- 21.Theilade J, Egelberg J, Attström R. Vascular permeability to colloidal carbon in chronically inflamed gingiva. J Periodontal Res. 1971;6:100–9. doi: 10.1111/j.1600-0765.1971.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 22.Curtis MA, Griffiths GS, Price SJ, Coulthurst SK, Johnson NW. The total protein concentration of gingival crevicular fluid, variation with sampling time and gingival inflammation. J Clin Periodontal. 1988;15:628–32. doi: 10.1111/j.1600-051x.1988.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 23.Mann WV. The correlation of gingivitis and pocket depth and exudate from gingival crevice. J Periodontol. 1963;34:379. [Google Scholar]
- 24.Kaslick RS, Chasens AI, Mandel ID, Weinstein D, Waldman R, Pluhar T, et al. Relationship of sodium, potassium and calcium to one another, to circadian rhythms, gingival bleeding, purulence and to conservative periodontal therapy. J Periodontol. 1970;41:442–8. doi: 10.1902/jop.1970.41.8.442. [DOI] [PubMed] [Google Scholar]
- 25.Kaslick RS, Chasens AI, Bressman E, Lazzara R, Egitto J. Investigation of periodontosis with periodontitis: Ultra microanalysis of gingival fluid, gross examination of the periodontal ligament and approach to treatment. J Periodontol. 1971;42:428–34. doi: 10.1902/jop.1971.42.7.428. [DOI] [PubMed] [Google Scholar]


