Abstract
Introduction:
Prevention of dental caries is one of the main strategies in contemporary pediatric dental practice. Mouth rinses are widely used as an adjunct to maintain oral hygiene. It is important for these products to be effective and safe for regular use in children.
Objective:
The aim of the study was to investigate the efficacy of a newly introduced xylitol, sodium fluoride and triclosan containing mouth rinse in reducing levels of plaque Streptococcus mutans and to compare it with that of a 0.12% chlorhexidine mouth rinse.
Materials and Methods:
Thirty children were randomly divided into two groups of 15 children each. Group I (study group) was given a mouth rinse containing xylitol (5%), sodium fluoride (0.05%) and triclosan (0.03%) and Group II (control group) was given a chlorhexidine (0.12%) mouth rinse. Both mouth rinses were alcohol free. Mouth rinsing was carried out twice daily, half an hour after breakfast and half an hour following dinner, for a period of 21 days under the supervision of the investigator.
Results:
In both groups, there was a significant reduction in the mean S. mutans count at the end of 21 days (P < 0.001). No significant difference was observed between the two mouth rinses.
Conclusion:
The use of a low fluoride–xylitol based mouth rinse appears to be a suitable choice for regular use in children.
Keywords: Alcohol free, chlorhexidine, mouth rinse, sodium fluoride, triclosan, xylitol
Introduction
Prevention of dental caries is one of the main strategies in contemporary pediatric dental practice. The dental plaque is a structure of vital significance as a contributing factor to at least the initiation of the carious lesion.[1] The major strains of streptococci present in plaque are: Streptococcus mutans, Streptococcus sanguis, Streptococcus mitior, Streptococcus milleri and Streptococcus salivarius. Of these, S. mutans is considered to be the chief etiological agent in human dental caries.[1] Although mechanical plaque control by tooth brushing is the most dependable and commonly practiced oral hygiene measure, numerous anti-plaque agents have been tried for improving oral health. These agents are capable of preventing bacterial adhesion, colonization and metabolism, and thus affect the bacterial growth. The efficacy of these antimicrobial agents depends on factors like vehicle used, concentration of active agents, substantivity of the agent and duration of the treatment.[2] An ideal anti-plaque agent for regular use in children should not interfere with biologic processes occurring in the mouth, be harmless to oral mucosa, should have low toxicity if accidentally swallowed, and should be both sugar and alcohol free.
Fluoride is one of the most important and effective components of preventive dental programs in children.[3] Fluorides are abundantly used in oral health products including mouth rinses. Sodium fluoride mouth rinses are effective in reducing caries and inhibit carbohydrate utilization of oral microorganisms by blocking enzymes involved in the bacterial gylcolytic pathway.[4]
Xylitol is a non-sugar sweetener permitted for use in foods.[5] Xylitol is a naturally occurring non-cariogenic sugar substitute that cannot be metabolized by oral bacteria.[6] This polyol possesses various properties favorable for caries prevention. Xylitol reduces the amount of adherent extracellular polysaccharides, lipoteichoic acids, resulting in the formation of loosely attached bio-films to the tooth surfaces.[7] Most in vivo studies have focused on the application of xylitol chewing gums, inextricably combining physicochemical with mechanical effects.[8] Triclosan is a non-ionic phenolic anti-plaque agent. Although it is incorporated in certain toothpastes, data available on mouth rinses containing triclosan are limited.
Thus, the aim of this study was to investigate the efficacy of a newly introduced xylitol, sodium fluoride and triclosan containing mouth rinse in reducing the levels of plaque S. mutans and to compare it with that of a 0.12% chlorhexidine mouth rinse.
Materials and Methods
Eighty-five normal children aged between 7 and 13 years and residing in an orphanage in Bangalore city were screened as part of a routine dental examination. These children had the same dietary pattern and followed similar oral hygiene practices. Prior to the study, written consent was obtained from the authorities of the orphanage. Ethical clearance was obtained from the ethical committee of the institution. To be included in the study, each child had to have a dental caries score (deft/DMFT score) equal to or greater than 3.
Exclusion criteria
Medically compromised children
Children with a history of taking antibiotics 3 months prior to and during the study period
Children undergoing orthodontic treatment or with an intraoral prosthesis
Children who could not brush their teeth or rinse on their own
Presence of any intraoral soft tissue pathology
Thirty children formed the study group. The authorities of the orphanage were instructed not to take these children for any dental treatment during the study period. At the onset of the study, each child was given a new soft toothbrush and non-fluoridated toothpaste. Demonstration of tooth brushing using scrub technique was given to all children by the investigator. They were instructed to brush before breakfast in the morning and after meals in the night.
Prior to commencement of the oral rinse regime, autoclaved wooden toothpicks were used to take plaque samples from the buccal surface of a noncarious permanent mandibular first molar or a primary mandibular second molar. The plaque sample was taken before breakfast. It was transferred to an autoclaved Eppendorf tube containing 1 ml of saline. The samples were taken to the laboratory within an hour, serial dilutions prepared and vortexed. One milliliter of the dilution was inoculated onto Mitis Salivarius agar and incubated at 37°C for 48 hours. Colony forming units (CFUs) of S. mutans were counted at baseline with the help of a digital colony counter.
In this single-blind trial, the children were randomly divided into two groups of 15 children each. Group I (study group) was given a mouth rinse containing xylitol (5%), sodium fluoride (0.05%) and triclosan (0.03%) (Kidodent® mouthwash, Indoco Remedies Ltd, Mumbai, India), and Group II (control group) was given a chlorhexidine (0.12%) mouth rinse (Nitra hex®, Micro Labs Limited, Bangalore, India). Both mouth rinses were alcohol free.
The children were asked to gather in a large room, where each child was given 10 ml of the mouth rinse and asked to rinse for 1 minute. Mouth rinsing was carried out twice daily,[2,9] half an hour after breakfast and half an hour following dinner, for a period of 21 days under the supervision of the investigator. On the 22nd day, prior to breakfast, plaque samples were collected, processed and S. mutans counts were assessed and compared with baseline values.
Data obtained were subjected to statistical analysis using Student's t-test. In order to ascertain preference of mouth rinse, all the children were asked to taste both mouth rinses at the end of the study. Their preference was recorded based on taste acceptability.
Results
In both groups, there was a significant reduction in the mean S. mutans count at the end of 21 days (P < 0.001). No significant difference was observed between the two mouth rinses [Table 1]. Of the 30 children, 83.33% preferred the taste of xylitol, sodium fluoride and triclosan containing mouth rinse, while 16.67% preferred the taste of 0.12% chlorhexidine mouth rinse [Figure 1].
Table 1.
Streptococcus mutans count in plaque samples
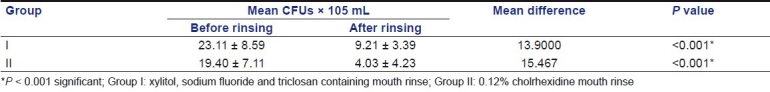
Figure 1.
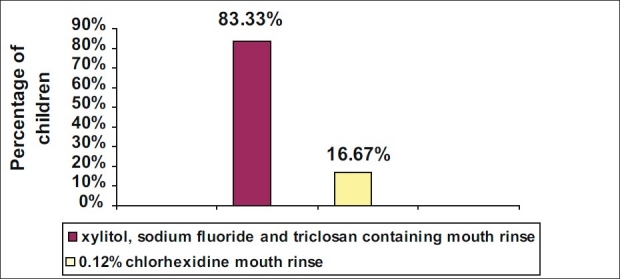
Preferences of mouth rinse
Discussion
Mouth rinses are widely used as an adjunct to maintain oral hygiene. It is important for these products to be effective and safe for regular use in children. Mouth rinses are recommended only for those who have the ability to swish and expectorate without swallowing. Most mouth rinses contain alcohol as one of their ingredients and they are also available “over the counter.” Alcohol-free preparations should be recommended over those containing alcohol.[3] The major side effects of alcohol containing mouth rinses include the presence of oral pain, burning sensation, difficulty of use in patients with oral sensitivity and the risk of accidental alcohol ingestion in children.[10–12]
The extrahepatic metabolism of alcohol has been demonstrated in oral tissue.[13] In the human mouth, aldehyde dehydrogenase (ALDH), an enzyme that converts acetaldehyde into a nontoxic acetate compound, occurs less frequently than alcohol dehydrogenase (ADH). This imbalance allows for the accumulation in oral tissues of a toxic, reactive and irritating acetaldehyde.[14] The continuous use of mouth rinses containing alcohol should be avoided. Due to a point mutation, aldehyde dehydrogenase 2 (ALDH2) isoenzyme is deficient in 30–50% of Asians. These individuals have a genetic inability to remove acetaldehyde and consequently have very high salivary acetaldehyde levels after moderate dose of alcohol.[15] Hence, in this study, it was primarily necessary to select two mouth rinses that were alcohol free.
In recent times, various polyalcohols, particularly xylitol, have been incorporated in a number of different products for children, including mouth rinses. Xylitol is a non-nutritive sweetener that has demonstrated effectiveness for preventing caries. Xylitol is not fermented by plaque. The nonspecific effect of xylitol is due its non-fermentability, and thus does not encourage bacterial growth.[16] The selective effect on mutans streptococci results in the development of mutant resistant strains which may be less virulent in the oral environment.[17] Some streptococcal strains take up xylitol and participate in a “futile” metabolic cycle in which xylitol is taken into the cell, phosphorylated to xylitol-5-phosphate, split into sugar phosphate phosphatases and the resulting xylitol is expelled from the cell.[18]
The ability of plaque to produce acids by the metabolism of sugars is reduced by xylitol. This is explained by selective decrease in mutans streptococci in plaque exposed to xylitol. Xylitol appears to have a unique effect in reducing adhesion and it is expected that other polyols might not show this clinical effect. The concentrations of ammonia and basic amino acids increase when plaque is exposed to xylitol, resulting in neutralization of plaque acids.[19]
The presence of fluoride in this mouth rinse could have also contributed to the reduction of S. mutans in plaque. The effects of fluoride on streptococcal cells are partly ascribed to the inhibition of enolase, one of the series of gylcolytic enzymes.[20] This inhibition decreases the intracellular level of phosphoenolpyruvate (PEP), and thus decreases bacterial sugar uptake via PEP-dependent phosphotransferase system (PEP-PTS).[20] In addition, fluoride can directly inhibit bacterial proton-translocating ATPase that is considered to partly contribute to the proton excertion out of the cells, leading to acidification of intracellular pH. The dissociation of unionized hydrofluoric acid into H+ and F– in the cells also promotes intracellular acidification.[20] This can further reduce the bacterial metabolic activity. Analyses of intracellular gylcolytic intermediates revealed that xylitol inhibited the upper part of the gylcolytic pathway, while fluoride inhibited the lower part. This suggests that fluoride and xylitol together have synergistic inhibitory effects on the acid production of mutans streptococci and that xylitol has the potential to enhance inhibitory effects of low concentrations of fluoride.[21] However, fluoride mouth rinses should be reserved for use with children judged to be at moderate or high risk for dental caries.[3] It is also important to select a fluoridated mouth rinse with a fluoride concentration of 0.05% for regular use.[3]
The other ingredient present is triclosan, a broad-spectrum antimicrobial, having an anti-plaque potential. Triclosan (2,4,4’-trichloro 2’-hydroxydiphenyl ether) is used to increase the ability of mouthwashes to bind to the oral mucosa, and thus be available for longer periods of time. Jenkins et al. compared the magnitude and duration of salivary bacterial count reductions produced by a single rinse of 0.2% triclosan, 1% sodium lauryl sulfate (SLS) and 0.2% chlorhexidine mouthwashes. They found considerable reductions in bacterial counts which remained significant for 3 hours with triclosan and for 7 hours with SLS and chlorhexidine.[22] The use of 0.3% triclosan mouth rinse showed significant reduction in salivary mutans streptococci count.[2]
Hence, the significant reduction in plaque S. mutans observed in our study could be attributed to the synergistic effect of all three constituents, viz., xylitol, sodium fluoride and triclosan.
An intensely researched preventive agent in dentistry has been chlorhexidine. It is an effective anti-plaque agent and is widely available as a mouth rinse. Chlorhexidine is a bis-biguanide which is effective against Gram-positive bacteria, Gram-negative bacteria and yeast. At relatively high concentration, chlorhexidine is bactericidal; but at low concentration, it is bacteriostatic.[23] The positively charged chlorhexidine binds readily to the negatively charged microbial cell surface. This is followed by disorganization of cytoplasmic membrane. Low concentrations of chlorhexidine allow cytoplasmic constituents to leak out, while a high concentration coagulates them. It inhibits the membrane ATPase and anaerobic process.[24]
In a study on 12–14-year-old children with high caries risk, 0.12% chlorhexidine mouth rinse was more efficient in reducing mutans streptococci count in saliva, as compared to other mouth rinses. However, it gave more negative findings when taste sensation and side effects were compared.[2]
A recent review concluded that due to the current lack of long-term clinical evidence for caries prevention and reported side effects, chlorhexidine rinses should not be recommended for caries prevention.[25]
Since we found no significant difference between both the mouth rinses, with regard to their efficacy in reducing S. mutans, the use of a low fluoride–xylitol based mouth rinse appears to be a suitable choice for regular use in children. Also, children preferred the taste of the bubble gum flavored, xylitol-based mouth rinse.
Since this study was a brief clinical trial on a newly introduced mouth rinse for children, studies of longer duration are needed. Further research on individual components and their effect on saliva, teeth and remineralization potential would enable clinicians to select an appropriate mouth rinse for use in children.
Conclusion
Significant reduction inplaque levels of S. mutans was observed with the use of xylitol, sodium fluoride and triclosan containing mouth rinse.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Shafer WG, Maynard KH, Barnet ML. 4th ed. Philadelphia: WB Saunders; 1993. A textbook of oral pathology; pp. 412–414. [Google Scholar]
- 2.Kulkarni VV, Damle SG. Comparative evaluation of efficacy of sodium fluoride, chlorhexidine and triclosan mouth rinses in reducing the mutans streptococci count in saliva: An in vivo study. J Indian Soc Pedod Prev. 2003;21:98–104. [PubMed] [Google Scholar]
- 3.Adair SM. Evidence-based use of flouride in contemporary pediatric dental practice. Pediatr Dent. 2006;28:133–41. [PubMed] [Google Scholar]
- 4.Grant, Daniel A, Stern, Irring B, Everett, Frank G. 5th ed. 1979. Periodontics in the tradition of Orban and Gottleib; pp. 130–51. [Google Scholar]
- 5.The sweetners in foods regulations. London: HMSO; 1983. Department of Health. SI 1983, 1211 as amended by SI 1988, 2122. [Google Scholar]
- 6.Scheinin A, Makinen KK. Turku sugar studies I-XI. Acta Odontol Scand. 1975;33(suppl 70):1–351. [Google Scholar]
- 7.Makinen KK, Saag M, Isotupa KP, Olak J, Nõmmela R, Söderling E, et al. Similarity of the effects of erythritol and xylitol on some risk factors of dental caries. Caries Res. 2005;39:207–15. doi: 10.1159/000084800. [DOI] [PubMed] [Google Scholar]
- 8.Decker EM, Maier G, Axmann D, Brecx M, von Ohle C. Efect of xylitol/chlorhexidine versus xylitol or chlorhexidine as single rinses on initial biofilm formation of cariogenic streptococci. Quintessence Int. 2008;39:17–22. [PubMed] [Google Scholar]
- 9.Sharma U, Jain RL, Pathak A. A clinical assessment of the effectiveness of mouthwashes in comparison to toothbrushing in children. J Indian Soc Pedo Prev Dent. 2004;22:38–44. [PubMed] [Google Scholar]
- 10.Bolanowski SJ, Gescheider GA, Sutton SV. Relationship between oral pain and ethanol concentration in mouth rinses. J Periodont Res. 1995;30:192–7. doi: 10.1111/j.1600-0765.1995.tb01273.x. [DOI] [PubMed] [Google Scholar]
- 11.Ciancio SG. Agents for the management of plaque and gingivitis. J Dent Res. 1992;71:1450–4. doi: 10.1177/00220345920710071701. [DOI] [PubMed] [Google Scholar]
- 12.Shulman JD, Wells LM. Acute ethanol toxicity from ingesting mouthwash in children younger than 6 years of age. Pediatr Dent. 1997;19:404–8. [PubMed] [Google Scholar]
- 13.Lieber CS. Metabolic effects of acetaldehyde. Biochem Soc Trans. 1988;16:241–8. doi: 10.1042/bst0160241. [DOI] [PubMed] [Google Scholar]
- 14.Dong YJ, Peng TK, Yin SJ. Expression and activities of class IV alcohol dehydrogenase and class III aldehyde dehydrogenase in human mouth. Alcohol. 1996;13:257–62. doi: 10.1016/0741-8329(95)02052-7. [DOI] [PubMed] [Google Scholar]
- 15.Vaikevainen S, Tillonen J, Agarwal DP, Srivastava N, Salaspuro M. High salivary acetaldehyde after a moderate dose of alcohol in ALDH- 2 deficient subjects: Strong evidence for the local carcinogenic action of acetaldehyde. Alcohol Clin Exp Res. 2000;24:872–7. [PubMed] [Google Scholar]
- 16.Gehring F, Makinen KK, Larmas M, Scheinin A. Turku sugar studies X.Occurrence of polysaccharide-forming streptococci and ability of the mixed plaque microbiota to ferment various carbohydrates. Acta Odontol Scand. 1976;34:329–43. doi: 10.3109/00016357609004645. [DOI] [PubMed] [Google Scholar]
- 17.Trahan L, Soderling E, Drean MF, Cheverier MC, Isokangas P. Effect of xylitol consumption on the plaque-saliva distribution of mutans streptococci and the occurrence and long term survival of xylitol-resistant strains. J Dent Res. 1992;71:1785–91. doi: 10.1177/00220345920710110401. (published erratum appears in J Dent Res 1993;72:87-8. [DOI] [PubMed] [Google Scholar]
- 18.Philanto-Leppala A, Soderling E, Makinen KK. Expulsion mechanism of xylitol-5-phosphate in Streptococcus mutans. Scand J Dent Res. 1990;98:112–9. doi: 10.1111/j.1600-0722.1990.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 19.Soderling E, Talonpoika J, Makinen KK. Effect of xylitol-containing carbohydrate mixtures on acid and ammonia production in suspensions of salivary sediment. Scand J Dent Res. 1987;95:405–10. doi: 10.1111/j.1600-0722.1987.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins GN. Review of fluoride research since 1959. Arch Oral Biol. 1999;44:985–92. doi: 10.1016/s0003-9969(99)00110-7. [DOI] [PubMed] [Google Scholar]
- 21.Maehara H, Iwami Y, Mayanagi H, Takahashi N. Synergistic inhibition by combination of fluoride and xylitol on glycolysis by mutans streptococci and its biochemical mechanism. Caries Res. 2005;39:521–8. doi: 10.1159/000088190. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins S, Addy M, Newcombe R. Triclosan and sodium lauryl sulphate mouthwashes (I): Effects on salivary bacterial counts. J Clin Periodontol. 1991;18:140–4. doi: 10.1111/j.1600-051x.1991.tb01703.x. [DOI] [PubMed] [Google Scholar]
- 23.Hennessey TD. Some antibacterial properties of chlorhexidine. J Periodont Res Suppl. 1973;12:61–7. doi: 10.1111/j.1600-0765.1973.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 24.Rolla G, Melsen V. On the mechanism of plaque inhibition of chlorhexidine. J Periodont Res. 1975;54:B57–62. doi: 10.1177/00220345750540022601. [DOI] [PubMed] [Google Scholar]
- 25.Autio-Gold J. The role of chlorhexidine in caries prevention. Oper Dent. 2008;33:710–6. doi: 10.2341/08-3. [DOI] [PubMed] [Google Scholar]


