Abstract
Background:
Preservation of pulp vitality is of utmost importance to the normal physiological functioning of tooth in situ and physiological process of exfoliation in a tooth affected by disease or trauma. Pulpotomy serves such a purpose using various medicaments applied directly on vital pulp.
Aim:
The aim of this study was to evaluate clinically and radiographically the effects of mineral trioxide aggregate (MTA) and formocresol (FC) as a pulp dressing after coronal pulp amputation (pulpotomy) in primary molars prospectively over a period of 1 year.
Materials and Methods:
Thirty-three healthy children, aged between 5 and 8 years, requiring pulp therapy were selected after clinical and radiographic assessment. A total of 50 maxillary and mandibular primary molars were treated by the conventional pulpotomy technique. The teeth were divided into two equal groups. In Group A, FC was used as the pulp dressing agent, and in Group B, MTA was used before restoration with stainless steel crowns. The research employed was a prospective study. The teeth treated were assessed postoperatively after 1, 3, 6 and 12 months. The observations were tabulated and statistically analyzed.
Results:
Clinically, both the groups showed 100% success at 1, 3, 6 and 12 months. At 3 months, the radiographic success rates of FC and MTA were 92% and 96%, respectively, and at 6 and 12 months, the radiographic success rates of FC and MTA were 88% and 96%, respectively.
Conclusion:
MTA showed a higher success rate than FC and may be a favorable material for pulpotomy in primary molars whose pulps have been compromised by a carious or mechanical pulp exposure
Keywords: Formocresol, mineral trioxide aggregate, primary molars, pulpotomy
Introduction
A major goal in pediatric dentistry is to maintain primary dentition in an intact state until permanent successors erupt.[1] Primary dentition is essential for maintenance of arch length, mastication, speech and esthetics and prevention of abnormal oral habits. Pulp diseases and trauma to pulp can lead to loss of vitality. This does not mean that tooth needs removal; it can be retained in oral cavity in a functional state with appropriate treatment. This is accomplished through various procedures which include indirect pulp capping, direct pulp capping, pulpotomy, pulpectomy and apexification. Pulpotomy is a procedure performed in a tooth with deep carious lesion adjacent to pulp, where coronal pulp is amputated to preserve the vitality of radicular pulp.[2] A wide range of materials such as formocresol (FC), glutaraldehyde, ferric sulfate, zinc oxide eugenol and calcium hydroxide have been used over the years for pulpotomy. FC has been a popular pulpotomy medicament in primary dentition for the past 60 years.[3] Concerns have been raised about the toxicity and potential carcinogenicity of FC in humans.[4–6] Studies have demonstrated systemic uptake of FC from pulpotomized teeth and have also shown FC to produce defects in succedaneous teeth.[7] Alternative technique and pulp therapy agents have been proposed to maintain partial pulp vitality. These include glutaraldehyde, electrosurgery, laser, freeze-dried bone, bone morphogenic protein, osteogenic protein and mineral trioxide aggregate (MTA).[4,8]
In recent times, with the introduction of new materials, which are not only biocompatible but also bioinductive, the emphasis has shifted from mere preservation to regeneration.[9] MTA attracted attention in the field of endodontics with its excellent sealing ability, biocompatibility, and ability to form dentinal bridge, and cementum and periodontal ligament regeneration.[8] MTA has the ability to stimulate cytokine release from bone cells, indicating that it actively promotes hard tissue formation.[10] It has also been proved that MTA has antimicrobial properties similar to Zinc Oxide Eugenol (ZOE)[11] and has no cytotoxic effect. MTA has been proposed as a potential medicament for pulpotomy procedure, capping of pulps with reversible pulpitis, apexification, repair of root perforation and repair of resorptive defects.[12,13] Hence, the present study was conducted with an aim to assess clinically and radiographically the effects of MTA as a pulp dressing after coronal pulp amputation in human primary molars and compare them to those of FC.
Materials and Methods
The procedure, possible discomfort and benefits were explained fully to parents of the children involved and their written consent was obtained prior to the treatment. Ethical clearance to conduct the study was obtained from institutional review board. Normal, healthy and cooperative children for the study were selected from the patients attending the Department of Pedodontics and Preventive Dentistry, I. T. S. – Centre for Dental Studies and Research, Ghaziabad, Uttar Pradesh, India. The study was started in October 2007 and completed in July 2009. A total of 50 maxillary and mandibular primary molar teeth were selected from 33 children requiring pulp therapy and aged between 5 and 8 years, who were selected after clinical and radiographic assessment. The criteria for selection of teeth to be included in the study are given in Table 1. Primary molars were treated by conventional pulpotomy technique. The teeth were divided into two equal groups: in Group A, FC (Pharmadent Remedies Pvt. Ltd., Gundlav, India) was used as the pulp dressing agent and gray MTA (Proroot MTA, Dentsply, Tulsa Dental, Tulsa OK, USA) was used in Group B. Cases which came under the exclusion criteria were eliminated and they are not represented in the study. In case a child had two molars needing pulpotomy, the second tooth was assigned to alternative group.[8,14] The teeth treated were assessed postoperatively after 1, 3, 6 and 12 months.
Table 1.

The conventional pulpotomy procedure was carried out step-by-step in one visit using local anesthesia and isolating the teeth with rubber dam. After caries removal with round bur, coronal access was obtained using a #330 high-speed bur with water spray to expose the pulp chamber. De-roofing of pulp chamber was done by connecting the pulp horns by a non-end cutting bur. The coronal pulp was excised until root canal orifices could be seen, with no tags remaining on the pulpal floor, by using Hu-friedy sharp spoon excavator. After the pulp was amputated, the pulp chamber was irrigated with saline to wash away dentin debris. Following irrigation, sterile, saline-wetted cotton pellets were applied for 5 minutes on the amputated pulp stumps to achieve hemostasis.
After the standardized technique was completed, the teeth assigned for Group A were treated with sterile cotton pellet moistened with FC squeezed twice, which was placed over the radicular pulp for 5 minutes and then removed. A thick mix of zinc oxide eugenol paste was placed over the FC fixed tissue. Intermediate restorative material (IRM) was placed over the zinc oxide eugenol layer at the same appointment. Within 1 week, the tooth was restored with a preformed stainless steel crown.
In Group B, pulp stumps were covered with MTA paste, obtained by mixing MTA powder with distilled water provided by the manufacturer in 3:1 (powder:liquid) ratio, which was placed over the exposure site with a plastic instrument. Then, the mixture was compressed against the exposure site with a moist cotton pellet. Wet cotton pellet was placed in pulp chamber and the cavity was covered with IRM. In the second session (after 1 day), the patient was recalled, cotton pellet was removed and the cavity was restored with IRM. Within 1 week, the tooth was restored with a preformed stainless steel crown. In both the groups, intraoral periapical radiographs were taken immediately after placement of IRM.
The children were recalled for clinical and radiographic examination postoperatively after 1, 3, 6 and 12 months. Intraoral periapical radiograph was taken for all the treated molars except at 1 month. The children were examined at follow-up clinically for the signs and symptoms like pain, swelling and sinus/fistula and radiographically for periapical changes, furcation radiolucency and internal resorption.
The treatment was regarded as a failure when one or more of the above-mentioned signs and symptoms were present, but pulp canal obliteration (PCO) was not regarded as a failure.
All data were entered in MS Excel sheet and analyzed by Epi-Info 2002 software to assess the success rate of the treatment with MTA and FC after 12 months. The difference between the effects of two materials was statistically analyzed by Fisher's exact test and P <0.05 was considered statistically significant.
Results
All 50 teeth in 33 children were available for analysis of success/failure rate. The age of the subjects ranged from 5 to 8 years, with a mean (±SD) age of 6.04 (+0.84) years in FC group and 6.44 (±1.12) years in MTA group. The distribution of teeth by type of tooth and material used is shown in Table 2.
Table 2.
Distribution of assessed teeth according to type of material
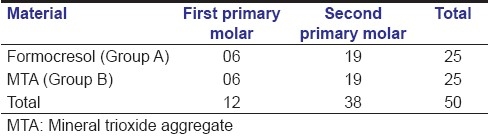
A total of 12 first primary molars and 38 second primary molars were treated by conventional pulpotomy technique. Children from both the groups were evaluated for 12 months postoperatively. The follow-up evaluation revealed 100% success with regard to the clinical signs and symptoms for both the groups. None of the teeth in either group showed any clinical pathology at the end of 12 months when observed by the same observer. Fisher's exact test was not necessary for the clinical signs as none of the samples showed signs of clinical evidence of failure, giving 100% success.
The radiographic evaluation at 3, 6 and 12 months follow-up period for both the groups is shown in Table 3. The cases which showed furcation radiolucency and internal resorption with FC pulpotomy and regarded as failure are shown in Figures 1 and 2. The case which showed internal resorption with MTA pulpotomy is shown in Figure 3. However, both the groups did not show any periapical changes at the above intervals. Pulp canal obliteration (calcific metamorphosis) was found in the three cases in MTA treated teeth (Group B) and was not considered as failure. Out of 25 teeth treated in each group, 22 in Group A and 24 in Group B did not show any radiographic changes, giving 88% and 96% success, respectively.
Table 3.
Assessment of radiographic signs at 3, 6 and 12 months
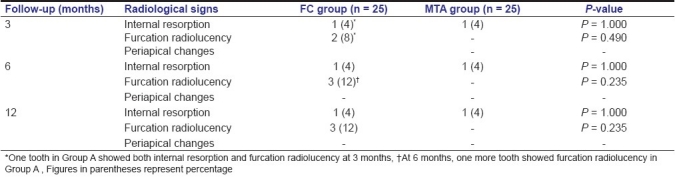
Figure 1.
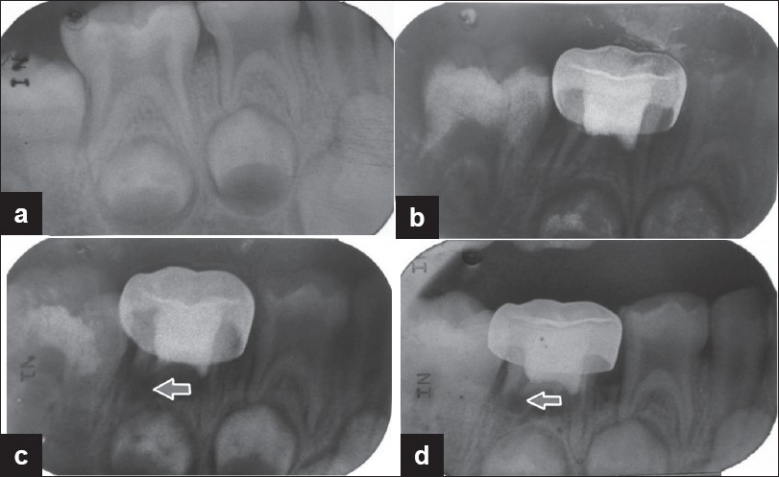
Preoperative and postoperative radiographs of mandibular second primary molar treated with formocresol pulpotomy revealed internal resorption and furcation radiolucency. (a) Preoperative radiograph; (b) radiograph taken 3 months postoperatively showing features of failure in pulpotomy; (c) radiograph taken 6 months postoperatively showing features of failure in pulpotomy; (d) radiograph taken 12 months postoperatively showing features of failure in pulpotomy
Figure 2.
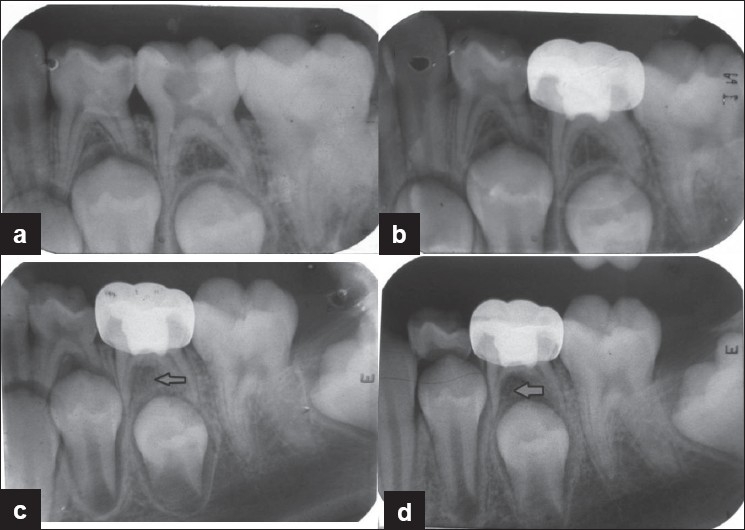
Preoperative and postoperative radiographs of mandibular second primary molar treated with formocresol pulpotomy revealed furcation radiolucency. (a) Preoperative radiograph; (b) postoperative radiograph taken immediately after formocresol pulpotomy and restored with stainless steel crown; (c) radiograph taken 6 months postoperatively showing feature of failure in pulpotomy; (d) radiograph taken 12 months postoperatively showing feature of failure in pulpotomy
Figure 3.
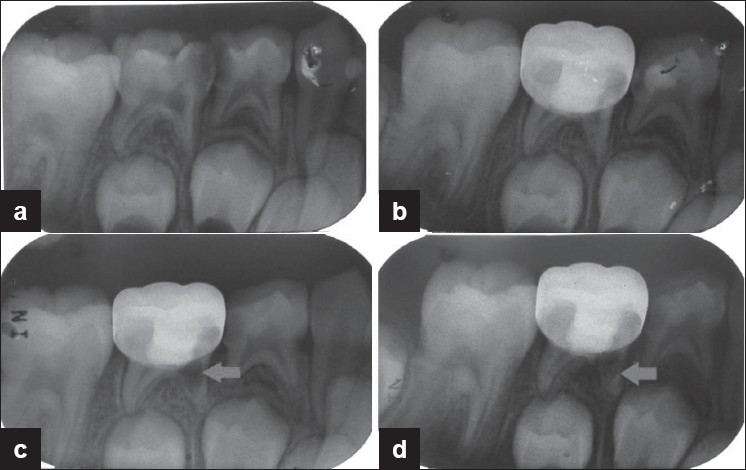
Preoperative and postoperative radiographs of mandibular second primary molar treated with MTA pulpotomy revealed internal resorption. (a) Preoperative radiograph; (b) postoperative radiograph taken immediately after MTA pulpotomy and restored with stainless steel crown; (c) radiograph taken 3 months postoperatively showing feature of failure in pulpotomy; (d) radiograph taken 6 months postoperatively showing feature of failure in pulpotomy
The statistical analysis was carried out by Fisher's exact test pertaining to radiographic findings in both the groups. There was no significant difference found between the two groups after 12 months of radiographic evaluation (P = 0.61).
Discussion
The objective of pulp therapy in a child patient is the successful treatment of a pulpally involved tooth and to retain the tooth in a healthy condition so that it may fulfil its role as a useful component of a primary and young permanent dentition.[15]
This report intended to examine the clinical and radiographic success rates of pulpotomies with MTA, a material with evidence-based success in many endodontic procedures. Several in vitro and in vivo studies have shown that MTA prevents microleakage, is biocompatible, and promotes regeneration of the original tissues when it is placed in contact with the pulp or periradicular tissues. FC was selected as it is still considered “gold standard” in pediatric dentistry, may be mainly because of its ease in use and excellent clinical success.[5,16] The estimated dose associated with one pulpotomy procedure, assuming a 1:5 dilution of FC placed on a no. 4 cotton pellet that has been squeezed dry, is approximately 0.02–0.10 mg.[17].
Children from 5 to 8 years of age were selected as per the inclusion and exclusion criteria for the study, irrespective of their sex. The age group was selected taking into consideration the lack of cooperation of children less than 5 years of age and physiologic root resorption (>3/4 of root) above 8 years of age. The first and second primary molars of both arches were included in the present study. Clinically, the success rate was 100% with both the groups at all observation periods. The results are in agreement with the results of various previous studies.[4,15,18–20]
The clinical success of FC pulpotomy in this study could be attributed to proper case selection, high aseptic standards, proper technique protocol and appropriate use of medicament. In addition to this, the germicidal and bactericidal action of FC, and also its fixative qualities, might have contributed to the success. The clinical success of MTA in this study could also be considered to be due to the above-mentioned factors and also its excellent sealing ability, biocompatibility, alkalinity and ability to regenerate the hard tissues.[12] Effects of MTA on amputated pulpal tissue seem to suggest that the material preserves the pulp tissue and promotes the regeneration of hard tissues.[19]
Radiographically, in Group A, at 3 months, internal resorption and furcal changes were seen in one tooth and furcal changes alone in another tooth. At 6 months follow-up, furcal changes were seen in one more tooth, similar to the results of a previous study.[15,18]
Internal resorption is the result of odontoclastic activity and suggests that the tooth is retaining some degree of vitality and function over time.[1] Although the cause of internal resorption is unknown, there is a speculation that whatever the precipitating factor, it produces a vascular change in the pulp that involves an inflammation and formation of granulation tissue.[1] The reason for internal resorption following pulpotomy is believed to be the irritating effects of the medicaments present in the paste.[21] Previous investigation of ZOE as a pulpotomy agent or as a base for pulpotomies suggests that ZOE can cause pulp inflammation, with a risk for subsequent internal resorption.[8] Smith et al.[1] claimed that internal resorption was associated with eugenol. ZOE, when used as a base in pulpotomies, comes in contact with highly perfused environment of pulp and undergoes hydrolysis of the zinc eugenolate to yield free eugenol and zinc hydroxide.[22,23] This eugenol comes in direct contact with the vital tissue and causes moderate to severe inflammatory response, resulting in chronic inflammation and necrosis.[1,22,23] This, however, cannot explain the internal resorption in the present study because ZOE paste was placed as a sub-base on FC fixed tissue. Cotes et al.[24] claimed that if the pulp tissue is fixed by FC it will not be affected by eugenol, and Smith et al.[1] also claimed that application of FC on pulp serves as a barrier to eugenol.
In the present study, it is more likely that internal resorption was the result of undiagnosed chronic inflammation existing in radicular pulp prior to pulpotomy rather than due to exposure of radicular pulp to eugenol. Due to this, the recovery of pulpal tissue to the normality might have been hampered, causing a delay in the completion of technique. Increasing time of procedure leading to a certain amount of inflammation in the pulpal tissue might have led to exaggerated response in the form of internal resorption. However, since the progress of such activity was limited, such response cannot be taken as failure of the procedure at microlevel.
Another probable reason for internal resorption could be the reversible fixative effect of FC and its irritative pH.[18]
Furcation changes might be seen due to the presence of micro pulp remnants even after the amputation of coronal pulp tissue. The occurrence of such changes might be dependent on the medicament concentration and application time ofFC.[9]
In the MTA group, one tooth showed internal resorption at 3 months, which did not progress any further over a period of the next 9 months. This could be more likely the result of undiagnosed chronic inflammation existing in the radicular pulp prior to pulpotomy.[25] A similar finding was seen at a duration of 25–38 months in two cases in a study by Holen et al.[8] and at a duration of 12 months in a study by Jabbarifar et al.[18] Since MTA contains highly alkaline substances, the possibility of exaggerated response of pulp cannot be ruled out. It is known from various studies[20,26] that calcium hydroxide placed on primary pulp reacts by producing internal resorption. Internal resorption may result from overstimulation of primary pulp by the highly alkaline calcium hydroxide. Overstimulation induced by high alkalinity could cause metaplasia within the pulp tissue, leading to the formation of odontoclasts. Though MTA does not contain calcium hydroxide, it has calcium oxide that forms calcium hydroxide when mixed with water. According to Holland et al.,[27] the reaction of calcium from calcium hydroxide with the carbon dioxide from pulpal tissue produces calcite crystals. These calcite crystals attract fibronectin, which is responsible for cellular adhesion and differentiation. Therefore, we believe that the mechanism of action of MTA is similar to that of calcium hydroxide.[28]
In the present study, in Group B, internal resorption was noticed at a duration of 3 months and did not progress to root perforation and osseous changes. Therefore, such internal resorption may be ignored as being a reason for the amount of inflammation at the time of pulpotomy.
Pulp canal obliteration (calcific metamorphosis) was found in the three cases in MTA treated teeth (Group B) and was not considered as failure, but was a result of the odontoblastic activity, and this might suggest that the tooth is retaining some degree of vitality and function overtime.[1]
In the current study, radiographic success rates of FC and MTA were 88% and 96%, respectively, at 12 months follow-up. However, this difference in success rates was not statistically significant (P = 0.061) as reported in previous studies.[14,15,18,19]
The mummification of the pulp induced by FC only treats the symptoms, but does not have any healing capacity. The objectives of FC pulpotomy are solely clinical, that is, maintaining the tooth in an asymptomatic condition until normal exfoliation. Enough evidence is present to suggest that the objective should no longer be complete “mummification.” Stated in modern terms, the rationale of the FC pulpotomy is to fix the radicular tissue while allowing recovery of cells and possible replacement of fixed tissue. FC treated teeth have shown earlier exfoliation of primary teeth.[29] There is also a reported case of dentigerous cyst[30] and necrosis of crestal bone[31] associated with the use of FC in pulpotomy.
FC has recently come to critical review and three concerns about the material should be under immediate inspection: firstly local toxicity, secondly the effects of the material systemically and lastly its effects of mutagenicity and carcinogenicity.[32,33]
MTA appears to meet the requirements for pulp capping materials. MTA's advantages might be related to its sealing ability to prevent bacterial penetration and to its high level of biocompatibility. It stimulates dentin bridge formation and prevents microleakage. MTA is a technique-sensitive material and takes about 4 hours to set when in contact with moisture. The material sets slowly, but this slow setting time prevents setting shrinkage.
Histological evaluation of pulpal tissue in animals and humans demonstrated that MTA produces thicker dentin bridge formation, with less inflammation, hyperemia, and pulpal necrosis compared to calcium hydroxide. MTA also appears to induce dentin bridge at a faster rate than calcium hydroxide at amputation sites.[34] MTA demonstrates a pulp architecture nearest to normal pulp by preserving odontoblastic layer.[19]
An important clinical advantage of MTA over FC is the fact that less time is required for the procedure. While FC requires 3–5 minutes application before the cotton pellet is removed, with MTA the pulp chamber is filled with IRM immediately after application of the dressing material. Moreover, during the removal of FC-soaked cotton pellet, there is a possibility of the cotton fibers adhering to clot, resulting in reoccurrence of bleeding. This does not occur with MTA as it is applied directly without cotton pellet.[8]
Presently one of the factors limiting the routine use of MTA is the high cost of the material. Although high success rates have been reported with MTA, re-entry into canal in cases further indicated for pulp therapy may not be possible in teeth with pulp canal obliteration.
Conclusions
MTA showed clinical and radiographic success as a dressing material following pulpotomy in primary teeth after a short-term evaluation period and has a promising potential to become a replacement for FC in primary teeth.
Though FC is in the center of much controversy for the past 20 years, it is still used extensively in pulpotomy procedure. However, MTA seems to have more promising potential to become a replacement for FC in primary teeth except for the cost factor and technique sensitivity. Further histological studies using larger sample size and longer observational period should be carried out in future.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Smith NL, Seale NS, Nunn ME. Ferric sulfate pulpotomy in primary molars : A retrospective study. Pediatr Dent. 2000;22:192–9. [PubMed] [Google Scholar]
- 2.American Academy of Pediatric Dentistry. Guidelines on pulp therapy for primary and young permanent teeth. Pediatr Dent. 2006-2007;28:144–8. [Google Scholar]
- 3.Primosch RE, Glom TA, Jerrell RG. Primary tooth pulp therapy as taught in predoctoral pediatric dental programs in the United States. Pediatr Dent. 1997;19:118–22. [PubMed] [Google Scholar]
- 4.Eidelman E, Odont, Holan G, Fuks AB. Mineral trioxide aggregate vs formocresol in pulpotomized primary molars: A preliminary report. Pediatr Dent. 2001;23:15–8. [PubMed] [Google Scholar]
- 5.Myers DR, Shoaf HK, Dirksen TR, Pashley DH, Whitford GM, Reynolds KE. Distribution of 14C-formaldehyde after pulpotomy with formocresol. J Am Dent Assoc. 1978;96:805–13. doi: 10.14219/jada.archive.1978.0187. [DOI] [PubMed] [Google Scholar]
- 6.Ranly DM. Pulpotomy therapy in primary teeth: New modalities for old rationales. Pediatr Dent. 1994;16:403–9. [PubMed] [Google Scholar]
- 7.Pruhs RJ, Olen GA, Sharma PS. Relationship between formocresol pulpotomies on primary teeth and enamel defects on their permanent successors. J Am Dent Assoc. 1977;94:698–700. doi: 10.14219/jada.archive.1977.0353. [DOI] [PubMed] [Google Scholar]
- 8.Holah G, Eidelman E, Fuks AB. Long-term evaluation of pulpotomy in primary molars using mineral trioxide aggregate or formocresol. Pediatr Dent. 2005;27:129–36. [PubMed] [Google Scholar]
- 9.Salako N, Joseph B, Ritwik P, Salonen J, John P, Junaid TA. Comparison of bioactive glass, mineral trioxide aggregate, ferric sulfate and formocresol as pulpotomy agents in rat molar. Dent Traumatol. 2003;19:314–20. doi: 10.1046/j.1600-9657.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 10.Koh ET, Pittford TR, Torabinejad M, McDonald F. Mineral trioxide aggregate stimulates cytokine production in human osteoblasts. J Bone Min Res. 1995;10S:S406. [Google Scholar]
- 11.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root end filling materials. J Endod. 1995;21:349–53. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 12.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 13.Castellucci A. The use of mineral trioxide aggregate in clinical and surgical endodontics. Dent Today. 2003;22:74–80. [PubMed] [Google Scholar]
- 14.Farsi N, Alamoudi N, Balto K, Mushayt A. Success of mineral trioxide aggregate in pulpotomized primary molars. J Clin Pediatr Dent. 2005;29:307–11. doi: 10.17796/jcpd.29.4.n80t77w625118k73. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniam P, Konde S, Mathew S, Sugnani S. Mineral trioxide aggregate as pulp capping agent for primary: 2 year follow-up study. J Clin Pediatr Dent. 2009;33:311–4. doi: 10.17796/jcpd.33.4.r83r38423x58h38w. [DOI] [PubMed] [Google Scholar]
- 16.Myer DR, Pashley DH, Whitford GM, McKinney RV. Tissue changes induced by the absorption of formocresol from pulpotomy sites in dogs. Pediatr Dent. 1983;5:6–8. [PubMed] [Google Scholar]
- 17.Milnes AR. Is formocresol obsolete.A fresh look at the evidence concerning safety issues? Pediatr Dent. 2008;30:237–46. [PubMed] [Google Scholar]
- 18.Jabbarifar SE, Khademi D, Ghasemi D. Success rate of formocresol in pulpotomy versus mineral trioxide aggregate in human primary molar tooth. J Res Med Sci. 2004;6:304–7. [Google Scholar]
- 19.Agamy HA, Bakry NS, Mounir MM, Avery DR. Comparison of mineral trioxide aggreagate and formocresol as pulp capping agents in pulpotomized primary teeth. Pediatr Dent. 2004;26:302–9. [PubMed] [Google Scholar]
- 20.Moretti AB, Sakai VT, Oliveira , Fornetti AP, Santos CF, Machado MA, Abdo RC. The effectiveness of mineral trioxide aggregate, calcium hydroxide and formocresol for pulpotomies in primary teeth. Int Endod J. 2008;41:547–55. doi: 10.1111/j.1365-2591.2008.01377.x. [DOI] [PubMed] [Google Scholar]
- 21.Hicks JM, Barr ES, Flaitz CM. Formocresol pulpotomies in primary molars: A radiographic study in a pediatric dentistry practice. J Pedod. 1986;10:331–9. [PubMed] [Google Scholar]
- 22.Strange DM, Seale NS, Nunn ME, Strange M. Outcome of formocresol / ZOE sub-base pulpotomies utilizing alternative radiographic success criteria. Pediatr Dent. 2001;23:331–6. [PubMed] [Google Scholar]
- 23.Huma WR. The pharmacological and toxicological properties of zinc oxide eugenol. J Am Dent Assoc. 1986;113:789–91. doi: 10.14219/jada.archive.1986.0256. [DOI] [PubMed] [Google Scholar]
- 24.Cotes O, Boj JR, Canalda C, Carreras M. Pulpal tissue reaction to formocresol vs ferric sulphate in pulpotomized rat teeth. J Clin Pediatr Dent. 1997;21:247–53. [PubMed] [Google Scholar]
- 25.Sonmez D, Sari S, Cetinbas T. A comparison of four pulpotomy techniques in primary molars: A long term follow-up. J Endod. 2008;34:950–5. doi: 10.1016/j.joen.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Magnusson BO. Pulpotomy in primary molars: Long-term clinical and histological evaluation. Int Dent J. 1980;13:143–55. [Google Scholar]
- 27.Holland R, de Souza V, Murata SS, Nery MJ, Bernabé PF, Otoboni Filho JA, et al. Healing process of dog dental pulp after pulpotomy and pulp covering with mineral trioxide aggregate or portland cement. Braz Dent J. 2001;12:109–13. [PubMed] [Google Scholar]
- 28.Faraco IM, Jr, Holland R. Response of pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001;17:163–6. doi: 10.1034/j.1600-9657.2001.170405.x. [DOI] [PubMed] [Google Scholar]
- 29.Hunter ML. Premature exfoliation of primary molars related to the use of formocresol in a multivisit pulpotomy technique. Int J Pediatr Dent. 2003;13:362–4. doi: 10.1046/j.1365-263x.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- 30.Asian-Gonzalez E, Pereira-Maestre M, Conde-Fernandez D, Vilchez I, Segura-Egea JJ, Gutierrez-Perez JL. Dentigerous cyst associated with a formocresol pulpotamized decidous molar. J Endod. 2007;33:488–92. doi: 10.1016/j.joen.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Kawakami J, Muto T, Shigeo K, Takeda S, Kanazawa M, Hokkaido Tooth exfoliation and necrosis of the crestal bone caused by the use of formocresol. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:736–8. doi: 10.1067/moe.2003.100. [DOI] [PubMed] [Google Scholar]
- 32.Ranly DM, Horn D. Assessment of the systemic distribution and toxicity of formaldehyde following pulpotomy treatment: Part two. J Dent Child. 1987;54:40–4. [PubMed] [Google Scholar]
- 33.Lewis BB, Chestner SB. Formaldehyde in dentistry: A review of mutagenic and carcinogenic potential. J Am Dent Assoc. 1981;103:429–34. doi: 10.14219/jada.archive.1981.0341. [DOI] [PubMed] [Google Scholar]
- 34.Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate (MTA) and calcium hydroxide as pulp capping agents in human teeth: a preliminary report. Int Endod J. 2003;36:225–31. doi: 10.1046/j.1365-2591.2003.00652.x. [DOI] [PubMed] [Google Scholar]


