Abstract
Use of immunohistochemical technique is increasing in diagnosing various diseases. In many situations it may not be possible to differentiate entities with overlapping clinical and histopathological features. Immunostaining of cellular antigens is immensely helpful in such cases. Immunohistochemistry (IHC) has also been in use for targeted cancer therapy. In this article, the discussion will be restricted to use of IHC in dermatological disorders and use of classical antigens with brief updating of some important newly discovered antigens.
Keywords: Immunohistochemistry, dermatopathology, immunostaining
Introduction
Use of immunohistochemical technique is increasing in diagnosing various diseases. Dermatopathology plays a pivotal role in the diagnosis of many skin disorders. However, in many a situation it may not be possible to differentiate entities with overlapping clinical and histopathological features. Immunostaining of cellular antigens is immensely helpful in such cases. Immunohistochemical methods enhance the diagnostic value of dermatopathology as well as help in assessing the prognostic markers of some disorders. Immunohistochemistry (IHC) has also been in use for targeted cancer therapy.
The principles of immunohistochemical analysis were first conceptualized by Coons et al.[1] Since then it has been in use to detect abnormal cells, as in various malignant tumors, especially lymphoma and melanoma. Currently, there has been a wide expansion in this field, with usage of IHC in various medical streams. With the access to arrays of commercially available antibodies, the scope of immunohistochemical diagnostic methods is fast evolving, and the lists of newly detected cellular markers and antibodies are exhaustive. In the following section, the discussion will be restricted to use of IHC in dermatological disorders and use of classical antigens with brief updating of some important newly discovered antigens.
Basic Principles of Immunohistochemical Analysis
IHC is the method of localization of antigens in tissue sections using labeled antibodies, visualized by markers (chromogen).[2,3] Theoretically, any antigenic cellular component, which can be retained (even if partially) in tissue section, should be demonstrable by IHC.[1] The antibody is aimed to locate a specific antigen expression site (epitope) which remains masked normally.[3] The marker used may be a fluorescent dye, enzyme-system, radioactive element or colloidal gold.
Procedure
Immunohistochemical analysis is usually performed on formalin-fixed, paraffin-embedded tissue sections, which are mounted on specially coated or charged glass slides for better adherence.[2] Immediate tissue fixation is usually recommended as it ensures adequate preservation of cellular structure. However, too long duration of fixation reduces the chances of antigen-antibody reaction. Some antigens can not be preserved in chemically fixed tissue and require rapid freezing in liquid nitrogen, followed by cryostat sectioning and thereafter fixed in cold acetone or alcohol.
There are several techniques to localize the antigens, the ultimate selection of which should be guided by the type of specimen to be studied and the sensitivity deserved.
During formalin fixation, the antigenic sites may become masked and requires retrieval (antigen/epitope retrieval system) by physical methods like heating in microwave oven / pressure cooker /steamer / autoclave / water bath (heat induced epitope retrieval;HIER) or enzyme-induced proteolysis (proteolytic-induced epitope retrieval; PIER).[1] PIER is the most commonly used method. A combination of both the methods may be used.
Other techniques of antigen retrieval include freeze-thaw method and chemical treatment with 1% sodium borohydrate in phosphate buffer.[1]
The most widely used chromogen is diaminobenzidine (DAB), which imparts a brown color to the reaction site.[4] Others are ‘Fast Red’ and aminoethyl carbazole (AEC) which produce a red color.[4]
The antibodies used may be polyclonal or monoclonal; the polyclonal antibodies are heterogenous and recognize several epitopes whereas the monoclonal antibodies are more specific.[3] A panel of antibodies is frequently used to stain tissue sections to overcome the cross-reactivity of antibodies with other antigens.
The prepared tissue specimen is observed under light microscope or sometimes by electron microscope. Immunohistochemical analysis may also be carried out on smears, imprint preparations and cytocentrifuge preparations.[3]
Normal Antigen Expression in Skin and Some Other Tissues
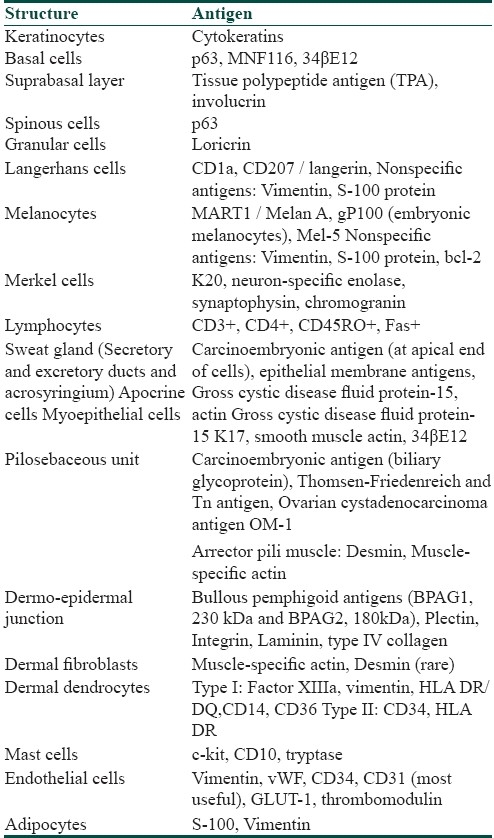
Human skin is an important area of antigen expression, many of which may contribute to various skin disorders. Some crucial antigens expressed in skin have been listed in [Table 1].[5,6] The antigens expressed in dermoepidermal junction (DEJ) contribute to the pathogenesis of immunobullous and hereditary or acquired mechanobullous disorders. Methods of detection of these antigens are beyond the scope of this article and will not be discussed further.
Table 1.
Practical application of IHC in dermatology
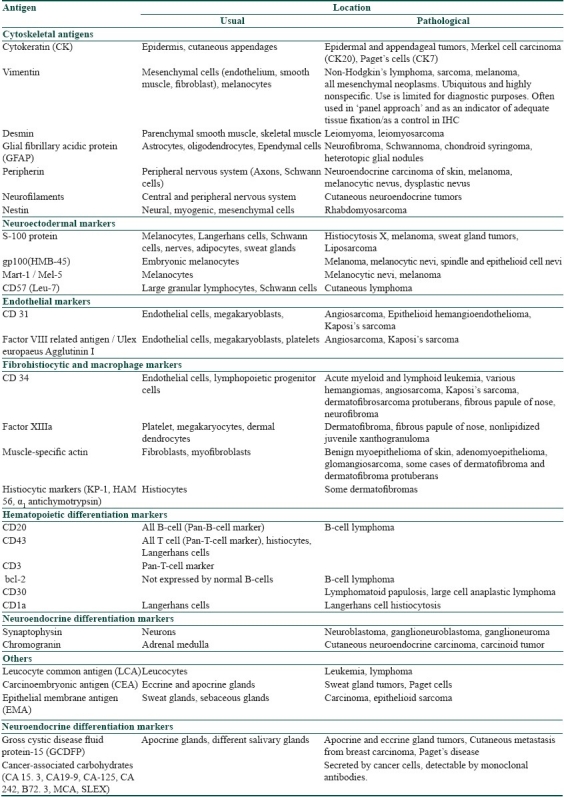
Table 2 lists the common antigens, which are helpful in diagnosing skin lesions by immunohistochemical analysis.[1,2,7]
Table 2.
Application of IHC in diagnosing skin tumors
Melanoma
Immunohistochemical analysis is of value in the diagnosis of various clinical subtypes of primary cutaneous melanoma and metastatic melanoma from unknown primary. Moreover, it can be utilized to assess the prognosis of individual tumors. Important markers of melanoma cells are as follows:[4,7,8]
S100 protein: It is the most sensitive (not as specific) marker of cutaneous melanoma, showing positivity in >95% cases of primary cutaneous melanoma.[4]
gp100 (antibody HMB45): This is a relatively specific and most useful marker for melanoma cells.[4] However, this may be detectable in many other benign melanocytic nevi. Some variants (e.g., desmoplastic melanoma, some cases of nevoid melanoma and cytokeratin positive metastatic melanoma) may be negative for HMB45. It is a helpful marker in frozen sections for delineating surgical margins for residual or recurrent melanoma during Mohs surgery.[7]
MART-1 (antibody Melan-A/A-103): Currently it is considered as one of the most important melanoma markers, producing strong and diffuse expression. It may also be present in some benign melanocytic lesions. Desmoplastic melanoma is the only type, consistently negative for this marker.[4,8]
NK1/C3.
Mel-5: An antibody detecting a 75-kDa glycoprotein expressed by normal and neoplastic melanocytes.[7] It is a helpful marker in differentiating malignant melanocytes from nonmelanocytic pigmented lesions, e.g., lentigo maligna from pigmented actinic keratosis in sun-exposed areas.[7]
Ki67 (detectable by MIB1antibody): It is a proliferation marker for melanoma cells. It is helpful in doubtful cases and >10% Ki67 positivity in melanocytic population of cells is suggestive of melanoma (helpful in differentiating spitz nevus and melanoma).[8]
Microphthalmia transcription factor (MiTF): This is a melanocytic nuclear protein crucial for growth, development and viability of melanocytes in embryonic and postnatal life. It also plays a role in synthesis of melanin. It is not a specific marker and the specificity is much lower in spindle cell variant of melanoma.
Multiple myeloma-1/interferon regulatory factor 4(MUM1/IRF4):[9] Positive in 92% primary cutaneous and metastatic melanoma. It is more sensitive than HMB45 and Melan-A. However, its specificity is less as it stains melanocytic nevi equally strongly and hence its utility is limited. It is not effective to detect desmoplastic melanoma.
Non specific markers: Vimentin, epithelial membrane antigen, cytokeratin, muscle-specific actin, neuron-specific enolase; cytokeratins may be positive in some metastatic melanoma.
Distinction between melanoma and melanocytic nevi
The clinical and histopathological features of melanoma may often be overlapping with melanocytic nevi. IHC may help in differentiating these difficult to diagnose cases. Following are some of the recently recognized immunohistochemical markers which show differential expressivity in melanocytic neoplasms; e.g., melanocytic nevus, spitz nevus and melanoma.
The gp100 expression in primary melanoma cells is in a patchy pattern involving isolated / clusters of cells in contrast to melanocytic nevi, where it is diffuse.[4]
CD99 expression is focally positive for some cases of spitz nevi whereas spitzoid melanoma shows predominantly diffuse staining pattern.[6]
Nucleolin protein is a major nucleolar argyrophilic protein involved in carcinogenesis. Dysplastic and malignant melanocytes show high positivity (multiple, irregular positive dots/coarse, irregular positivity) of this antigen, whereas benign lesions never express this pattern.[10]
Minichromosome maintainance proteins (MCM3 and MCM4),[11] Microtubule-associated protein-2(MAP-2),[12] Versican;[13] phospho-Histone H3 (pHH3):[14] expressed differentially in benign and malignant melanocytic tumors (enhanced expression in dysplastic nevi and superficial spreading melanoma).
Heat shock protein 105 (HSP105):[15] Expression in melanocytic nevi is equivalent to normal skin. There is overexpression in melanoma cells, especially in metastatic melanoma.
Glucose transporters (Glut-1, Glut-3):[16] play role in glucose metabolism of melanocytes. Glut-1 is expressed in benign melanocytic nevi, which is downregulated in majority of the melanoma cells.
Prognostic markers for melanoma
Ki67 is associated with vertical growth phase of the tumor and hence is a prognostic marker.[8]
Nucleolin protein is the second powerful prognostic indicator after melanoma thickness.[10]
MAP-2 expression is a predictor of disease progression and significantly correlates with Breslow vertical tumor thickness, Clark level and stage of disease.[12]
Estrogen receptors α and β (ER-α and ER-β);[17] comparison of expression of these receptors in melanoma skin and surrounding skin helps in detecting spread of melanoma cells. ER-β is an important prognostic indicator.
Fatty acid-binding protein 7 (FABP7) expression may be associated with tumor progression and invasion; it is associated with Ki67 score.[18]
Expressions of cycloxygenase-2 (Cox-2) and peroxisome proliferator-activated receptor γ (PPAR-γ) on melanoma cells are indicative of progression to metastasis.[19]
Expression of HSP105 on melanoma cells is associated with widespread metastasis and poor prognosis.[15]
Phospho-histone H3 (pHH3) rapidly assesses the mitotic activity of malignant cells and is considered as a mitotic marker.[6,14]
Dysregulation of cell adhesion molecules is associated with progression of melanoma. Carcinoembryonic antigen cell adhesion molecule-1 (CEACAM-1) may have a role in progression of melanoma and serves as a potential prognostic marker in melanoma.[20]
RUNX3 is an important tumor suppressor playing role in cell proliferation, apoptosis and tumor metastasis. Positive RUNX3 expression in human melanoma is an important prognostic factor in patient outcome.[21]
Basal cell carcinoma
Neuronal differentiation is characteristic of the cells of basal cell carcinoma (BCC), as evidenced by enhanced expression of neuronal differentiation markers ARC ULK1, β-tubulin III, GAP-43 and neurofilaments. Expression of these markers shows a strong correlation with the BCC subtype; a lower expression is seen in morphoeic and more aggressive form of BCC, as compared to nodular, micronodular and superficial variants.[22] Immunostaining for CD10 helps in differentiating BCC and trichoepithelioma;[6] in BCC, CD10 expression is seen in the cells of basaloid nests, but absent in stroma whereas in trichoepithelioma it is in the stroma and tumor papillae.[6]
BCC and squamous cell carcinoma (SCC) can be differentiated by higher expression of Ki67 and negativity for BerEP4 antigen in the latter; the basal cells in BCC shows immunostaining with BerEP4.[8]
Cutaneous SCC
Immunostaining for p63 is positive in the cells of SCC, and helps in diagnosis of the cases with atypical presentation.[6] Immunohistochemical analysis of the epidermal cells in SCC for PGP 9.5 and Cyclin D1 expression reveals that co-occurrence of these two markers indicate tumor aggressiveness.[23] These can be utilized as a prognostic indicator in postsurgical patients.
Benign and malignant fibrous and fibrohistiocytic tumors of skin
The different tumors in this group are as follows:[24]
Deep dermatofibroma (extension to subcutaneous tissue)
Dermatofibroma with atypical cells
Pleomorphic fibroma of the skin
Epithelioid cell histiocytoma
Dermatomyofibroma
Sclerotic fibroma of the skin
Atypical fibroxanthoma
Dermatofibrosarcoma protuberans
Fibrosarcoma
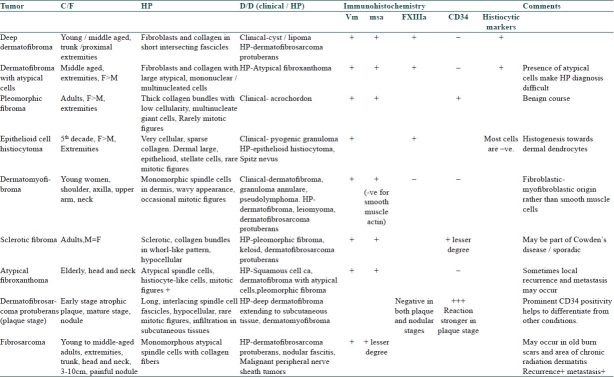
These tumors are mainly composed of a variable proportion of the components like fibroblasts and myofibroblasts, histiocytes, dermal dendrocytes, collagen, elastin and mucin. Immunohistochemical analysis for the above components includes detection of vimentin (vm), CD34, muscle-specific actin (msa), factor XIIIa, and histiocytic markers.[24]
Usually these are the tumors of adults and elderly, located mostly on extremities. These occur as solitary, well-circumscribed papule or nodule (1-2.5 cm), skin-colored/waxy/brown, smooth, firm and sessile or pedunculated. Some lesions like fibrosarcoma have a deeper location. Clinically, it is important to differentiate these lesions from other entities like cysts, lipoma and cutaneous fibroma or acrochordon. Epithelioid cell histiocytoma has a vascular component and frequently mistaken as pyogenic granuloma. Some of these lesions have the potential of local recurrence following excision and tendency to deeper invasion. Some others present with atypical cells and mitotic figures histopathologically.[24] Moreover, it may be difficult to differentiate the tumors with deeper invasion (deep dermatofibroma with extension to subcutaneous tissue) from dermatofibrosarcoma protuberans.
The classical histopathological features of dermatofibrosarcoma protuberans are evident at the mature nodular stage. The initial plaque stage may bear histopathological resemblance to many other fibrohistiocytic tumors and may cause diagnostic difficulty.
Help of IHC is occasionally required in differentiating these conditions if diagnostic confusion arises. In addition, the predominant immunohistochemical staining pattern of the constituent cells helps in understanding the histogenesis of such tumors (e.g., increased factor XIIIa staining in dermatofibroma with atypical cells indicates predominance of dermal dendritic cells).[23] The distinguishing features of these tumors have been presented in Table 3.
Table 3.
Distinguishing features of different benign and malignant fibrous tumors of the skin[24]
Adnexal and epidermal tumors
Though most of these tumors present with classical clinical and histopathological pictures, overlapping features may pose diagnostic difficulty at times.
Desmoplastic trichoepithelioma (DT) may show morphological and histopathological similarities with morpheiform BCC.[8] Immunohistochemical analysis helps in differentiating these two conditions; bcl2 positivity is diffuse in BCC, whereas basal in DT; stromal cells are CD34-negative in BCC, whereas cellular islands are surrounded by CD34-positive cells in DT; fibroblasts in BCC are stromelysin-3 positive, negative in DT; Ki67 expression is strong and diffuse in BCC, which is less so in DT.[8]
Syringoma and DT can be differentiated by CEA positivity and involucrin negativity in the former and vice versa.[8]
SCC is negative for both CEA and S100, whereas sweat gland carcinoma is positive for CEA and shows occasional positivity for S100.[8]
Epithelial membrane antigen (EMA) and GCDFP-15 immunostainings are also seen in sweat gland tumors.[7]
Most primary cutaneous adnexal carcinomas express p63, CK5/6 and D2-40, whereas cutaneous metastasis from visceral adenocarcinoma show negativity (rarely weakly positive) for these markers.[6]
Paget's disease
Cytokeratin 7 (CK7), EMA and CEA immunostainings are positive for Paget's cells in extramammary Paget's disease, of which EMA positivity is a very useful marker in diagnosis.[7] CK7 also constitutes a sensitive marker for Paget's cells, producing intense diffuse staining. GCDFP-15 staining is positive for both mammary and extramammary Paget's disease.[7]
Merkel cell carcinoma/Neuroendocrine carcinoma of the skin
The tumor cells show positive immunostaining with CK20, EMA, synaptophysin, neuron-specific enolase (NSE) and neurofilaments.[7] Positive CK20 expression in association with negative thyroid transcription factor is very useful in differentiating this tumor from metastatic small cell carcinoma of lungs.[7] The tumor cells also show positivity for peripherin (occasional) and neurofilaments. This is the most common skin lesion showing neurofilament immunostaining.[7] Coexpression of cytokeratins and neurofilaments in tumor cells is a strong differentiating point from non-neuroendocrine carcinomas.
Minichromosome maintenance nuclear protein expression is a helpful marker in detecting cell proliferation in Merkel cell carcinoma.[25]
Cutaneous metastasis of unknown origin
Metastatic cutaneous nodules may be the first indicator of an internal malignancy.[7] In such circumstances, it may be difficult to locate the primary organ of tumor origin. Clinical symptoms related to the primary organ, if present, may be helpful. Some metastatic tumors may retain the characteristics of parent organ cells (thyroid acini, renal tubular cells) and help in diagnosis. If such clinical and histopathological indicators are absent, immunohistochemical staining methods may be of diagnostic help.[7]
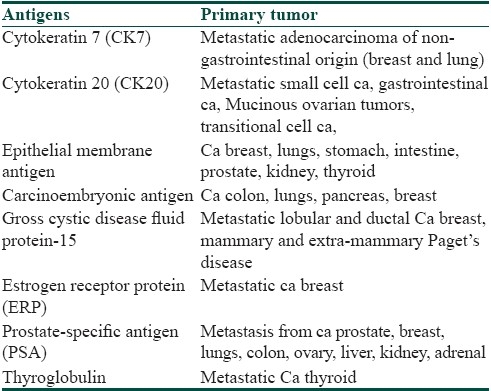
Table 4 lists some of the markers helpful in identifying the source organ for unknown cutaneous metastasis.
Table 4.
Some immunohistochemical markers for unknown cutaneous metastasis[7]
Application of IHC in diagnosing hair disorders
The infundibular area of hair follicle is rich with CD1a+ Langerhans cells, involved in antigen processing and immunosurveillance.[26] Many of the hair disorders are immune mediated and IHC may play an important role in distinguishing these disorders. CD10 antigen (a 100-kDa zinc metalloendopeptidase), is expressed in dermal sheath cells of hair follicles, which are involved in regeneration of dermal papillae and thus key factor in hair growth.[6]
Alopecia areata
Immunological alteration play major role in the pathogenesis of alopecia areata. In active lesions of the disease, there is a diffuse infiltration of CD1a+ Langerhans cells in and around the hair bulb. There may also be a perivascular and peribulbar dense infiltrate in the affected area composed of large number of lymphocytes (CD4+), dendritic cells (CD1a+) and monocytes (CD36+).[27]
Some adhesion molecule receptors play important role in the pathogenesis of alopecia areata by enhancing adhesion of leukocytes to endothelial cells and thereafter their trafficking to dermis. These include ICAM-1, endothelial leukocyte adhesion molecule-1 and lymphocyte function associated molecule-1. In the progressive stage of alopecia areata, these are over expressed in both affected and unaffected skin, whereas these are only weakly positive in the stable phase of the disease.
Scarring alopecia
In scarring alopecia, there is inflammation involving the permanent portion of the hair follicle, resulting in irreversible hair loss. Immunohistochemical staining may help to establish the underlying etiology in a case of scarring alopecia;
In lichen planopilaris, a cellular infiltrate rich in CD8+ T-lymphocytes is observed.
In discoid lupus erythematosus causing scarring alopecia, there is a T-lymphocytic infiltrate involving the dermoepidermal junction, extending along the hair follicle and its appendages. There are apoptotic basal keratinocytes, which show abnormal expression of certain antigens like Ki67, p53 and bcl-2.
In graft vs. host disease, the perifollicular lymphocytic infiltrate is predominantly CD4+.
Folliculotropic cutaneous T-cell lymphoma
In this malignant disorder of the hair follicle, patient presents with non-specific features; clinical pictures simulating follicular mucinosis or dissecting cellulitis have been reported. Other presenting skin lesions include alopecia, papules, cysts or comedones. The infiltrating T-cells in this condition have a CD3+, CD4+ and CD8 negative phenotype. CD30+ blast cells may also be seen. Because of the deep perifollicular location of the neoplastic cell infiltrate, the disorder is less accessible to skin-targeted therapy.[28]
Application of IHC in diagnosing cutaneous lymphomas
Primary cutaneous lymphomas are a group of disorders characterized by localization of neoplastic lymphocytes (T cells and B-cells) to the skin. Skin is the second most common extranodal site of lymphoma (first being gastrointestinal tract).[29] There are two broad categories; ‘primary cutaneous T cell lymphoma (CTCL)” and ‘primary cutaneous B-cell lymphoma (CBCL)’, which present with sole cutaneous disease at diagnosis.[28] Of all primary cutaneous lymphomas, 65% are CTCL.[30] Mycosis fungoides is the commonest type of CTCL (50% of all cutaneous lymphomas) followed by primary cutaneous CD30+ lymphoproliferative disorders (30% of all CTCL).[30] Approximately 20% of the primary cutaneous lymphomas are of B-cell type.[31]
The different types of cutaneous lymphomas vary widely in clinical features, histopathological characteristics, immunophenotypic features and therapeutic outcome. IHC play an important role in diagnosis of primary cutaneous lymphomas. Each type expresses multiple antigen markers on the surface of cells and there are several subclassifications based upon these. With the advent of IHC, many of the controversial CTCL types are now well-defined. Table 5 lists the different types of primary CTCLs and their immunophenotypic expressions.[27,28]
Table 5.
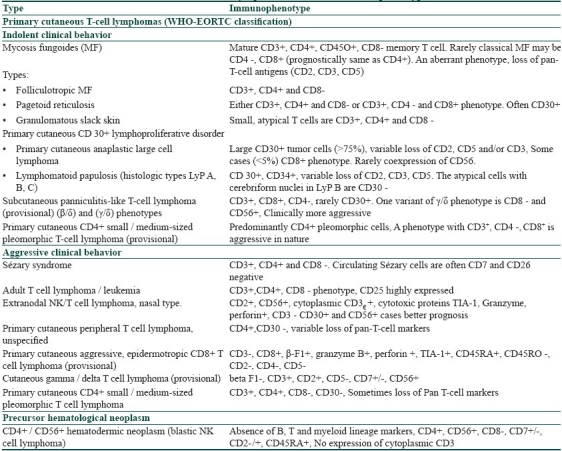
In Sézary syndrome, the malignant cells are CD3+, CD4+ and CD8 negative. With a similar clinical picture, if the predominant T-cell population in skin and peripheral blood show CD3+, CD4 negative and CD8+ phenotype, diagnosis of actinic reticuloid should be considered.[28] The current criteria proposed (International Society for Cutaneous Lymphoma, ISCL) for diagnosis of Sézary syndrome (under validation) include one or more of the following:[28]
Absolute Sézary cell count of at least 1000 cells/mm3
Immunophenotypical abnormalities; e.g., increased CD4+ T-cell population, resulting in CD4+ / CD8+ T-cell ratio >10, loss of any of the T-cell antigens (or total loss of T-cell antigens), CD2, CD3, CD4, CD5 or both.
Demonstration of T-cell clone in peripheral blood by molecular/cytogenetic methods.
Currently, the minimal criteria suggested to differentiate between Sézary syndrome and benign cutaneous inflammatory infiltrate include demonstration of similar T-cell clone in skin and peripheral blood in combination with one of the ICSL-proposed cytomorphological or immunophenotypic criteria.[28]
CD4+ / CD56+ hematodermic neoplasm presents as solitary or multiple skin nodules and should be differentiated from leukemia cutis resulting from myelomonocytic leukemia. To differentiate from lymphoblastic or myeloblastic neoplasms, CD3 and myeloperoxidase immunostaining should be performed, negativity of which exclude these cases.
Lymphoproliferative malignancies of regulatory T-cells (Treg cells) with aggressive course, necessitating polychemotherapy have been reported.[32] These malignant clone of Treg cells showed positive staining for CD4, CD25, and there was strong expression of FOXP3.[32] Primary cutaneous peripheral T-cell lymphoma with expression of usual T-cell markers as well as aberrant coexpression of cytotoxic T-cell marker CD8 and B-cell marker CD20, with an aggressive course has been reported.[33]
CD99, a useful marker of Ewing's sarcoma, has been shown to be expressed frequently in nodal, extranodal and primary cutaneous cases (64%) of anaplastic large cell lymphoma, and this should be included under the differential diagnosis of CD99+ neoplasms.[34]
The WHO-EORTC classification of primary cutaneous B-cell lymphoma is as follows:[28]
Primary cutaneous follicle center lymphoma (PCFCL); this tumor has excellent prognosis with 5-year survival rate >95%. The neoplastic B-cells show positive immunostaining for CD20 and CD79a. There is consistent expression of bcl-6. CD5 and CD43 expression are not seen.
Primary cutaneous marginal zone B-cell lymphoma (PCMZL); the prognosis of this tumor is excellent with 5-year survival rate of nearly 100%. Generally CD20-, CD5+, bcl-2+ and bcl-6- cells help to distinguish this tumor from PCFCL.
Primary cutaneous diffuse large B-cell lymphoma, leg type (PCLBCL); this is a tumor common in elderly females with unfavorable prognosis and tendency to disseminate. The cells are positive for CD20 and CD79a and there is strong expression of bcl-2. Expression of bcl-6 is seen in most cases and CD10 is usually negative. There is expression of MUM-1/IRF4 protein, which is negative in PCFCL.
Primary cutaneous diffuse large B-cell lymphoma, other; variant, intravascular large B-cell lymphoma; these are usually cutaneous manifestations of systemic lymphoma, sometimes presenting with only skin lesions. The prognosis is good except in the intravascular variant.
The distinction between different CBCLs is important to differentiate between indolent or aggressive nature of individual tumor. IHC is an important tool in this regard and help in selecting accurate therapeutic option, radiotherapy or systemic chemotherapy. Diagnostic and prognostic value of some of the markers like bcl-2 and MUM-1/IRF4 protein is under evaluation.[28]
Application of IHC in diagnosing other disorders
Becker's nevus
There is an increased expression of androgen receptors in dermal fibroblasts of Becker's nevus, compared to normal skin, in both males and females.[35] It may be indicative of role of androgens in the pathogenesis of Becker's nevus.
Leprosy
Increased expression of Langerhans cell and dermal dendrocyte markers like CD1a and factor XIIIa in skin lesions of tuberculoid leprosy indicates a role of dendritic cells in eliciting host response toward Mycobacterium leprae.[36] These cells may be the determining factor in evolution of the disease.
Acute graft vs. host disease (aGVHD)
Upregulation of FOXP3+ Treg cells in the skin of patients with aGVHD is associated with less severity of the disease as well as good response to treatment.[37]
Lymphatic metastasis
M2A antigen (D2-40 monoclonal antibody) is expressed in lymphatic endothelial cells; it is a marker to identify intralymphatic malignant cells and sentinel lymph node positivity.[6] Hence it may be helpful in selecting candidates suitable for sentinel lymph node biopsy. Normal lymphatics, Kaposi's sarcoma, lymphangioma and some angiosarcomas also express this antigen.
Vascular tumors
GLUT1 antigen is expressed in all types of congenital hemangiomas, pyogenic granuloma and granulation tissues.[6] It is highly specific for juvenile hemangioma.[6]
Schamberg's disease
Immunohistochemical analysis of skin specimens from patients with Schamberg's disease reveals that there is expression of adhesion receptors (leukocyte function adhesion 1 [LFA-1],[38] endothelial leukocyte adhesion molecule 1 [ELAM-1],[38] intercellular adhesion molecule 1 [ICAM-1])[38] on the endothelial cells in the early stage of the disease, highlighting its pathogenesis.
In detection of microorganisms
Immunohistochemical methods to detect antigens of various microorganisms are now available. These are as follows:[1]
Bacterial; Treponema pallidum and other spirochetes, Mycobacteria sp., Helicobacter pylori
Viral; Cytomegalovirus, herpes simplex, human papilloma virus, hepatitis B virus, Epstein-Barr virus
Fungal
Protozoal; Pneumocystis carinii
Parasitic; Leishmania sp.[39]
These antigen detections by immunohistochemical methods help in diagnosing infectious diseases (e.g., T. pallidum in tissue section, P. carinii in sputum).
Psoriasis
Deficiency of FOXP3+ Treg cells in lesional skin of psoriasis may be responsible for acute flare up of the disease and thus may predict severity and clinical course.[40] High expression of hypoxia-inducible factor-1α (HIF-1 α) has been observed in psoriatic lesions and this may be operative in the development of psoriasis.[41]
Allergic contact dermatitis
Serotonin is involved in the pathogenesis of allergic contact dermatitis (ACD) and immunohistochemical detection of serotonin receptors 5-HT1AR and 5-HT2AR in the early lesions may help in targeted therapy by blocking these receptors.[42]
Therapeutic application of IHC
In the pathogenesis of malignant transformation of cells, a variety of cellular metabolic pathways are altered, which may be targeted for cancer therapy. IHC can be utilized to detect the elevated levels of cellular markers involved in such alterations and thus the ability of these tumors to respond to therapy can be assessed.[6]
Similarly, hormone receptors can be studied in tumor tissue specimens to assess its responsiveness to antihormone therapy.[43]
The basis of invention of targeted therapy with monoclonal antibodies is immunohistochemical detection of cell-surface antigens (e.g., proteins of epidermal growth factor family) which may be aimed as targets for action of such antibodies.[44]
Advantages of IHC
Good sensitivity and specificity.
The technique is performed on paraffin-embedded tissue sections, which allows preservation of cellular details in a better way compared to frozen sections.[2] Such a preparation has the advantage of being preserved and stored effectively.[2]
IHC may show positivity in decalcified or totally necrotic material
It can be used upon specimens stored for a long duration and previously stained tissue sections.[1]
IHC has an edge over routine enzymatic tissue staining by its ability to identify wider arrays of tissue antigens.
Immunohistochemical preparations can be utilized for electron microscopy (immuno-electron microscopy).
Immunohistochemical methods have equivalent sensitivity as direct immunofluorescence technique and it has replaced many uses of electron microscopy.
Disadvantages of IHC
False positive and false negative results.
Non-specific staining as well as aberrant immunoreactivity are the technical snags associated with IHC. Hence, diagnosis by IHC should always be supplemented by some confirmatory method.
The chromogen DAB imparts a brown color to the tissue section creating confusion with melanin pigment.[4] Hence, for evaluation of melanin containing lesions, other chromogens are to be used.
IHC is a rapidly expanding field with invention and application of newer techniques each day. The principle of IHC can be utilized in diagnosis and as prognostic markers as well. Application of IHC has opened new avenues in the therapy of some of the malignant tumors. However, the practical applications of these modalities are not widespread because of nonavailability of the facilities, especially in the developing world.
Majority of the immunohistochemical markers are nonspecific and training in this field as well as experience is required to interpret the results in each individual case. Although IHC forwards us steps ahead in the diagnosis of difficult tumors it should be kept in mind that use of this technique must always be supplemented with adequate history, proper clinical examination and histopathological study of the tissue specimen, failure of which may lead to grossly misleading interpretation.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Rosai J. Gross Techniques in surgical pathology. In: Rosai J, editor. Rosai and Ackerman's surgical pathology. 9th ed. Vol. 1. Mosby: St. Louis, Missouri; 2004. pp. 25–91. [Google Scholar]
- 2.Elenitsas R, Nousari CH, Seykora JT. Laboratory methods. In: Elder DE, editor. Lever's histopathology of the skin, th ed. 9th ed. Lippincott Williams and Wilkins: Philadelphia; 2005. pp. 59–73. [Google Scholar]
- 3.Shariff S, editor. Laboratory techniques in surgical pathology. Prism Books Pvt Ltd: Bangalore; 1999. pp. 186–97. [Google Scholar]
- 4.Prieto VG, Shea CR. Use of immunohistochemistry in melanocytic lesions. J Cutan Pathol. 2008;35(Suppl 2):1–10. doi: 10.1111/j.1600-0560.2008.01130.x. [DOI] [PubMed] [Google Scholar]
- 5.Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. 2002;12:390–401. [PubMed] [Google Scholar]
- 6.Justin W, Maddox J, Racz M, Petronic-Rosic V. Update on immunohistochemical methods relevant to dermatopathology. Arch Pathol Lab Med. 2009;133:1053–61. doi: 10.5858/133.7.1053. [DOI] [PubMed] [Google Scholar]
- 7.Hudson AR, Smoller BR. Immunohistochemistry in diagnostic dermatopathology. Dermatol Clin. 1999;17:667–89. doi: 10.1016/s0733-8635(05)70115-7. [DOI] [PubMed] [Google Scholar]
- 8.Roy S. The role of immunohistochemistry in dermatopathology. [Accessed on 2010 December 10]. Available from; http://www.histopathology-india.net/dermpath.htm .
- 9.Sundram U, Harvell JD, Rouse RV, Natkunam Y. Expression of B-cell proliferation marker MUM1 by melanocytic lesions and comparison with S100, gP100 (HMB45), and Melan A. Mod Pathol. 2003;168:802–10. doi: 10.1097/01.MP.0000081726.49886.CF. [DOI] [PubMed] [Google Scholar]
- 10.Mourmouras V, Cevenini G, Cosci E, Epistolato MC, Biagioli M, Barbagli L, et al. Nucleolin protein expression in cutaneous melanocytic lesions. J Cutan Pathol. 2009;36:637–46. doi: 10.1111/j.1600-0560.2008.01126.x. [DOI] [PubMed] [Google Scholar]
- 11.Gambichler T, Shtern M, Rotterdam S, Bechara FG, Stuckee M, Altmever P, et al. Minichromosome maintainance proteins are useful adjuncts to differentiate between benign and malignant melanocytic skin lesions. J Am Acad Dermatol. 2009;60:808–13. doi: 10.1016/j.jaad.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Gambichler T, Rotterdam S, Radkowski K, Altmever P, Kreuter A. Differential expression of microtubule-associated protein-2 in melanocytic skin lesions. Am J Clin Pathol. 2009;131:710–4. doi: 10.1309/AJCPR84ULYVMNJHG. [DOI] [PubMed] [Google Scholar]
- 13.Gambichler T, Kreuter A, Grothe S, Altmever P, Brockmever NH, Rotterdam S. Versican overexpression in cutaneous malignant melanoma. Eur J Med Res. 2008;13:500–4. [PubMed] [Google Scholar]
- 14.Nasr MR, El-Zammar O. Comparison of pHH3, Ki67 and surviving immunoreactivity in benign and malignant melanocytic lesions. Am J Dermatopathol. 2008;30:117–22. doi: 10.1097/DAD.0b013e3181624054. [DOI] [PubMed] [Google Scholar]
- 15.Muchemwa FC, Nakatsura T, Fukushima S, Nishimura Y, Kageshita T, Ihn H. Differential expression of heat shock protein 105 in melanoma and melanocytic naevi. Melanoma Res. 2008;18:166–71. doi: 10.1097/CMR.0b013e3282fe9a16. [DOI] [PubMed] [Google Scholar]
- 16.Parente P, Coli A, Massi G, Mangoni A, Fabrizzi MM, Bigotti G. Immunohistochemical expression of the glucose transporters Glut-1 and Glut-3 in human malignant melanomas and benign melanocytic lesions. J Exp Clin Cancer Res. 2008;27:34. doi: 10.1186/1756-9966-27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.deGiorgi V, Mavilla C, Massi D, Gozzini A, Aragona P, Tanini A, et al. Estrogen receptor expression in cutaneous melanoma: A real-time reverse transcriptase- polymerase chain reaction and immunohistochemical study. Arch Dermatol. 2009;145:30–6. doi: 10.1001/archdermatol.2008.537. [DOI] [PubMed] [Google Scholar]
- 18.Slipicevic A, Jorgensen K, Skrede M, Rosnes AK, Troen G, Davidson B, et al. The fatty acid binding protein 7 (FABP7) is involved in proliferation and invasion of melanoma cells. BMC Cancer. 2008;8:276. doi: 10.1186/1471-2407-8-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C, Ramirez JA, Guitart JA, Diaz LK. Expression of cycloxygenase-2 and peroxisome proliferator-activated receptor gamma during malignant melanoma progression. J Cutan Pathol. 2008;35:989–94. doi: 10.1111/j.1600-0560.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- 20.Gambichler T, Grothe S, Rotterdam S, Altmever P, Kreuter A. Protein expression of carcinoembryonic antigen cell adhesion molecules in benign and malignant melanocytic skin lesions. Am J Clin Pathol. 2009;131:782–7. doi: 10.1309/AJCP24KXJVBZXENS. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Chen G, Cheng Y, Martinka M, Li G. Prognostic significance of RUNX3 expression in human melanoma. Cancer. 2011;117:2719–27. doi: 10.1002/cncr.25838. [DOI] [PubMed] [Google Scholar]
- 22.Gore SM, Casper M, Williams T, Reql G, Aberqer F, Cerio R, et al. Neuronal differentiation in basal cell carcinoma: Possible relationship to Hedghog pathway activation? J Pathol. 2009;219:61–8. doi: 10.1002/path.2568. [DOI] [PubMed] [Google Scholar]
- 23.Mastoraki A, Ioannidis E, Apostolaki A, Patsouris E, Aroni K. Pgp9.5 and Cyclin D1 co-expression in cutaneous squamous cell carcinoma. Int J Surg Pathol. 2009;17:413–20. doi: 10.1177/1066896909336018. [DOI] [PubMed] [Google Scholar]
- 24.Kamino H, Salcedo E. Histopathologic and Immunohistochemical diagnosis of benign and malignant fibrous and fibrohistiocytic tumors of the skin. Dermatol Clin. 1999;17:487–505. doi: 10.1016/s0733-8635(05)70103-0. [DOI] [PubMed] [Google Scholar]
- 25.Gambichler T, Breininger A, Rotterdam S, Altmever P, Stucker M, Kreuter A. Expression of minichromosome maintainance proteins in Merkel cell carcinoma. J Eur Acad Dermatol Venereol. 2009;23:1184–8. doi: 10.1111/j.1468-3083.2009.03285.x. [DOI] [PubMed] [Google Scholar]
- 26.Jaworsky C, Gilliam A. Immunopathology of the human hair follicle. Dermatol Clin. 1999;17:561–8. doi: 10.1016/s0733-8635(05)70107-8. [DOI] [PubMed] [Google Scholar]
- 27.Ghersetich I, Campanile G, Lotti T. Alopecia areata: Immunohistochemistry and ultrastructure of infiltrate and identification of adhesion molecule receptors. Int J Dermatol. 1996;35:28–33. doi: 10.1111/j.1365-4362.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 28.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO- EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–85. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 29.Pinter-Brown LC. Lymphoma, Cutaneous T-cell. [Accessed on 2009 August 12]. Available from; http://www.emedicine.medscape.com/article/209091 -
- 30.Cutaneous T cell lymphoma. [Accessed on 2010 December 12]. Available from; http://www.dermnetnz.org/dermal- infiltrative / cutaneous T cell lymphoma.html .
- 31.Cutaneous B-cell lymphoma. [Accessed on 2010 December 12]. Available from; http://www.dermnetnz.org/dermal- infiltrative/ cutaneous B-cell lymphoma.html .
- 32.Marzano AV, Vezzoli P, Fanoni D, Venegoni L, Berti E. Primary cutaneous T-cell lymphoma expressing FOXP3: A case report supporting the existence of malignancies of regulatory T cells. J Am Acad Dermatol. 2009;61:348–55. doi: 10.1016/j.jaad.2008.11.894. [DOI] [PubMed] [Google Scholar]
- 33.Balmer NN, Hughey L, Busam KJ, Reddy V, Andea AA. Immunochemotherapy for Bcl-2 and MUM-negative aggressive primary cutaneous B-cell non-Hodgkin's lymphoma. Am J Dermatopathol. 2009;31:187–92. doi: 10.1097/DAD.0b013e31818cc039. [DOI] [PubMed] [Google Scholar]
- 34.Buxton D, Bacchi CE, Gualco G, Weiss LM, Zuppan CW, Rowsell EH, et al. Frequent expression of CD99 in anaplastic large cell lymphoma: A clinicopathologic and immunohistochemical study of 160 cases. Am J Clin Pathol. 2009;131:574–9. doi: 10.1309/AJCPE68HZXCGWTKK. [DOI] [PubMed] [Google Scholar]
- 35.Grande Sarpa H, Harris R, Hansen CD, Callis Duffin KP, Florell SR, Hadley ML. Androgen receptor expression patterns in Becker's nevi: An immunohistochemical study. J Am Acad Dermatol. 2008;59:834–8. doi: 10.1016/j.jaad.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Simões Quaresma JA, de Oliveira MF, Ribeiro Guimarães AC, de Brito EB, de Brito RB, Pagliari C, et al. CD1a and factor XIIIa immunohistochemistry in leprosy: A possible role of dendritic cells in the pathogenesis of Mycobacterium leprae infection. Am J Dermatopathol. 2009;31:527–31. doi: 10.1097/DAD.0b013e31819f1997. [DOI] [PubMed] [Google Scholar]
- 37.Fondi C, Nozzoli C, Benemei S, Baroni G, Saccardi R, Guidi S, et al. Increase in FOXP3+ regulatory T cells in GVHD skin biopsies is associated with lower disease severity and treatment response. Biol Blood Marrow Transplant. 2009;15:938–47. doi: 10.1016/j.bbmt.2009.04.009. (Abstract) [DOI] [PubMed] [Google Scholar]
- 38.Ghersetich I, Lotti T, Bacci S, Comacchi C, Campanile G, Romagnoli P. Cell infiltrate in progressive pigmented purpura (Schamberg's disease): Immunophenotype, adhesion receptors, and intercellular relationships. Int J Dermatol. 1995;34:846–50. doi: 10.1111/j.1365-4362.1995.tb04419.x. [DOI] [PubMed] [Google Scholar]
- 39.Schaumburg-Lever G. Update on immunoelectron microscopy in diagnostic dermatopathology and cutaneous biology. Dermatol Clin. 1999;17:691–704. doi: 10.1016/s0733-8635(05)70116-9. [DOI] [PubMed] [Google Scholar]
- 40.Yun WJ, Lee DW, Chang SE, Yoon GS, Huh JR, Won CH, et al. Role of CD4CD25FOXP3 regulatory T cells in psoriasis. Ann Dermatol. 2010;22:397–403. doi: 10.5021/ad.2010.22.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Zhang G, Xiao R, Chen H, Wen H. Expression of iNOS and HIF-1α with angiogenesis in affected skin biopsies from patients with psoriasis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35:952–7. doi: 10.3969/j.issn.1672-7347.2010.09.009. [Article in Chinese] (Abstract) [DOI] [PubMed] [Google Scholar]
- 42.Wetterberg J, Taher C, Azmitia E, El-Nour H. Time-dependent modulation of Serotonin and its receptors 1A and 2A expression in allergic contact dermatitis. J Eur Acad Dermatol Venereol. 2011;25:1200–5. doi: 10.1111/j.1468-3083.2010.03952.x. [DOI] [PubMed] [Google Scholar]
- 43.Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006;244:176–84. doi: 10.1097/01.sla.0000218080.94145.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bibeau F, Boissière-Michot F, Sabourin JC, Gourgou-Bourgade S, Radal M, Penault- Llorca F, et al. Assessment of epidermal growth factor receptor (EGFR) expression in primary colorectal carcinomas and their related metastases on tissue sections and tissue microarray. Virchows Arch. 2006;449:281–7. doi: 10.1007/s00428-006-0247-9. (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]