Abstract
Background:
Vascular proliferation in the papillary dermis is considered to be an important and probably an early feature of psoriasis. Few morphometric studies have attempted to analyze the vascular changes. However, no study was found in the available literature comparing vascular changes between psoriasis and psoriasiform dermatitis.
Materials and Methods:
Skin biopsies from 25 cases each of psoriasis and psoriasiform lesions were immunohistochemically stained for CD34 (endothelial marker). Microvessel density (MVD), microvessel length density and ratio of microvessel area to papillary dermal area were calculated using image analysis software.
Results:
Skin biopsies from psoriasis showed higher staining for CD34 on light microscopy. Using morphometric techniques, microvessel length density was significantly higher in psoriasis compared to psoriasiform lesions (P value <0.05). MVD was also higher in psoriasis, though the difference was not significant. The ratio of microvessel area to dermal area was almost similar in both the groups.
Conclusion:
Our results indicate that vascular tortuousity and dilatation is significant only in psoriatic lesions. These results may assist in automated diagnosis of skin biopsies.
Keywords: Morphometry, psoriasis, skin biopsy, vasculature
Introduction
Psoriasis, an autoimmune cutaneous disease elicited by as yet unknown antigen, is characterized by epidermal changes accompanied by markedly increased dermal vascularity.[1] The dermal vascular changes have been evaluated by electron microscopy and shown to be due to change from arterial-type vessels to venules.[2] The vascular changes may also precede the epidermal alterations, as shown in some studies.[3] Morphometric evaluation of dermal vasculature in psoriasis has shown increased endothelial and luminal volume of vessels compared to control subjects.[4] Studies utilizing native capillaroscopy have demonstrated reduction in microvessel density and length density after pulsed dye laser treatment.[5] However, none of the earlier studies have compared vascular morphology between psoriasis and psoriasiform dermatitis.
This study was aimed at evaluating the morphometric parameters of papillary dermal vessels in psoriasis and to compare these with psoriasiform lesions.
Materials and Methods
In this study, skin biopsies from 25 cases of psoriasis and 25 of psoriasiform lesions were included. The diagnosis of psoriasis was confirmed on the basis of clinical features (pink to red papules with fine silvery scales and positive Auspitz sign) in conjunction with characteristic histologic changes. The latter included: regular acanthosis with club-shaped rete ridges, suprapapillary epidermal thinning, hypogranulosis, confluent parakeratosis, spongiform pustules of Kogoj and/or Munro microabscesses. Psoriasiform lesions included in the study were: Endogenous eczema: nine cases, hyperkeratotic eczema: seven cases, seborrheic dermatitis: three cases, nummular eczema: 2 cases, and one case each of allergic contact dermatitis, irritant contact dermatitis, lichen simplex chronicus, and pityriasis rosea.
For all the cases, H- and E-stained sections were reviewed. Serial sections were stained for CD34 (BioGenex, USA) using the standard streptavidin-biotin-peroxidase method.
Using image analysis software, the number, area, and length density of microvessel in dermal papillae were calculated from the CD34-stained sections. The microvessel density was calculated as number of vessels per unit (mm2) papillary dermal area. Length density of microvessel (mm/mm2 or mm-1) was calculated by superimposition of cycloid grid on vertical sections of skin biopsy [Figure 1]. Microvessel length density is defined as length of microvessels per unit dermal area and was calculated as 2I/L, where I refer to the number of intersections and L is total length of test lines. Ratio of microvessel area to the papillary dermal area was also calculated in both the groups included.
Figure 1.
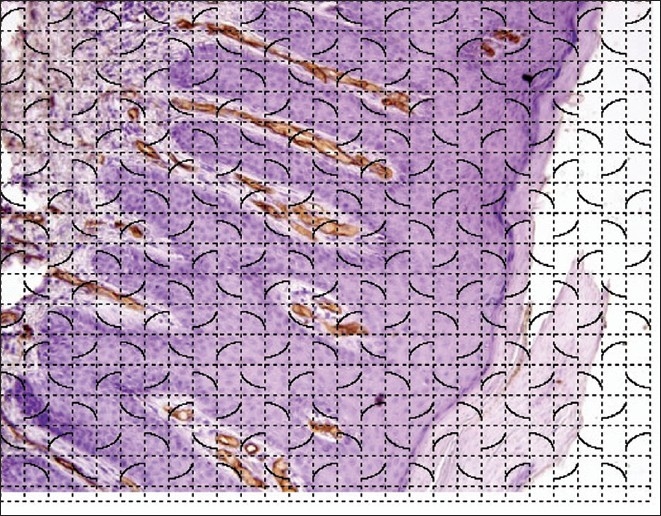
Photomicrograph showing Immunostaining for CD34 in a case of psoriasis with superimposed cycloid grid for morphometry (LSAB, ×200)
Appropriate statistical analysis was performed on the morphometric parameters between the two groups.
Results
On light microscopic evaluation of CD34-stained sections, biopsies from psoriasis showed much greater microvascular staining in the papillary dermis compared to psoriasiform dermatitis [Figure 2].
Figure 2.
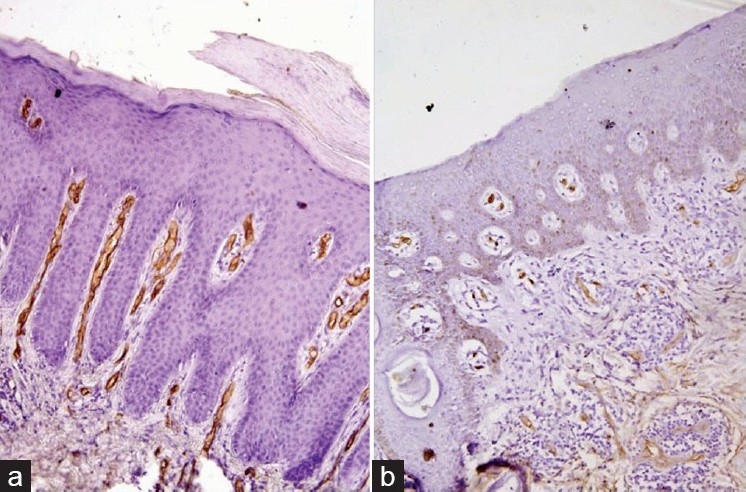
Photomicrograph demonstrating the dermal vessels in psoriasis (a, LSAB, ×200) and psoriasiform dermatitis (b, LSAB ×200)
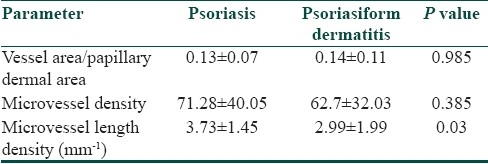
Morphometric analysis showed that length density of microvessels was significantly higher in psoriasis (3.73±1.45 mm-1) compared to psoriasiform lesions (2.99±1.99 mm- 1, P value 0.03). Microvessel density, i.e., number of vessels per unit dermal area, was also higher in psoriasis (71.28±40.05) than psoriasiform lesions (62.7±32.03), though the difference was not statistically significant. In contrast to these parameters, the ratio of microvessel area to papillary dermal area was similar in psoriasis (0.13±0.07) and psoriasiform lesions (0.14±0.11) [Table 1].
Table 1.
Comparative analysis of dermal microvasculature in psoriasis and psoriasiform dermatitis
These three morphometric vascular parameters are depicted in scatter plots [Figures 3a–c].
Figure 3.
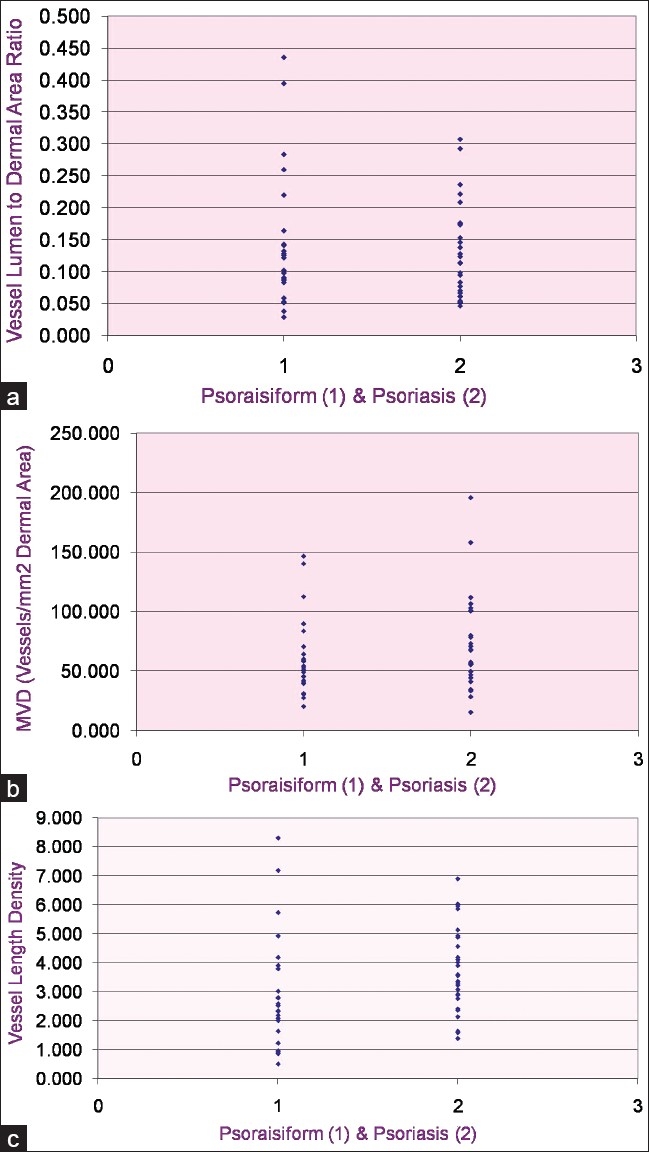
(a) Scatter plots of vessel area per unit papillary dermal area; (b) microvessel density; and (c) length density in psoriasis and psoriasiform dermatitis
Discussion
Psoriasis is an autoimmune disease affecting people of all ages, though there is a tendency for onset in early adulthood in patients with genetic transmission.[6] Apart from epidermal changes, skin biopsies from patients with psoriasis demonstrate alterations in papillary dermis such as edema and dilated tortuous capillaries. These dilated capillaries are the source of bleeding points generated by gentle scraping of skin (Auspitz sign).[1] Few ultrastructural studies have shown that dermal capillaries in psoriasis have a wider lumen, bridged fenestrations with edematous areas in endothelial cytoplasm.[2] Some authors have reported that these vascular morphologic changes precede the visible epidermal hyperplasia.[3] These changes also correlate with enhanced cutaneous blood flow, including in the perilesional clinically unaffected skin.[7] It has been proposed that vascular changes in the papillary dermis, including enhanced expression of adhesion molecules, permit leukocyte adhesion and assist in the establishment of inflammatory response.[8]
Few earlier studies have evaluated the morphometric characteristics of dermal vessels in psoriatic skin. Barton et al., in their study, demonstrated higher endothelial volume and luminal volume in the lesional psoriatic skin compared to uninvolved skin of patients with psoriasis as well as control subjects. The authors suggested that increase in these parameters was partly due to the increase in number of vascular profiles.[4] In another study, computerized image analysis was performed to demonstrate the great degree of expansion of the vascular plexus in papillary dermis. The same study also showed significant endothelial proliferation in the papillary dermis of active psoriatic plaques.[9]
This study aimed at evaluating the vascular changes in psoriasis vis-á-vis psoriasiform dermatitis. This comparison has not been performed in the available literature till date, to the best of our knowledge. We found a significantly higher length density of vessels in psoriasis compared to psoriasiform lesions (P<0.05). Microvessel density was also higher in psoriasis, though the difference did not reach statistical significance. These results indicate that though there is some vascular proliferation in response to inflammation in psoriasiform dermatitis, the tortuosity, and elongation of vessels reflected by length density is significant in psoriatic lesions only. With the advancing automation in diagnosis, these morphometric techniques might assist in differentiation between psoriasis and psoriasiform lesions, especially in early lesions of psoriasis.
The vascular proliferation in psoriatic skin can also be utilized for antiangiogenic therapies. Systemic therapy for psoriasis, such as methotrexate, cyclosporine, and TNF antagonists exert their therapeutic effect by immune modulation as well as interference with proangiogenic mediators.[10] In addition, several molecules such as neovastat (inhibitor of endothelial cell proliferation) and inhibitors of VEGF receptors are undergoing clinical trials.[11,12] Hern et al., performed native capillaroscopy and showed a significant reduction in the microvessel density, image area fraction, length, density and vessel image width after pulsed dye laser treatment of plaque psoriasis.[5] Hence, the superficial microvascular bed in psoriatic skin is a legitimate and easily accessible target for antiangiogenic therapy.
Conclusion
The present study demonstrates significant difference in the papillary dermal vasculature between psoriatic skin and psoriasiform dermatitis. These results, which need to be confirmed in further larger studies, hold promise in the era of automation in diagnostic modalities.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, popular and squamous diseases. In: Elder DE, Elenitsas R, Johnson BL, Murphy GF, editors. Lever's Histopathology of the Skin. 9th ed. Lippincott Williams and Wilkins: Philadelphia; 2005. pp. 184–91. [Google Scholar]
- 2.Braverman IM, Yen A. Ultrastructure of the human dermal microcirculation: II, The capillary loops of the dermal papillae. J Invest Dermatol. 1977;68:44–52. doi: 10.1111/1523-1747.ep12485165. [DOI] [PubMed] [Google Scholar]
- 3.Kulka JP. Microcirculatory impairment as a factor in inflammatory tissue damage. Ann NY Acad Sci. 1964;116:1018–44. doi: 10.1111/j.1749-6632.1964.tb52565.x. [DOI] [PubMed] [Google Scholar]
- 4.Barton SP, Abdullah MS, Marks R. Quantification of microvascular changes in the skin in patients with psoriasis. Br J Dermatol. 1992;126:569–74. doi: 10.1111/j.1365-2133.1992.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 5.Hern S, Stanton AW, Mellor RH, Harland CC, Levick JR, Mortimer PS. In vivo quantification of the structural abnormalities in psoriatic microvessels before and after pulsed dye laser treatment. Br J Dermatol. 2005;152:505–11. doi: 10.1111/j.1365-2133.2005.06435.x. [DOI] [PubMed] [Google Scholar]
- 6.Lebwohl N. Psoriasis. Lancet. 2003;361:1197–204. doi: 10.1016/S0140-6736(03)12954-6. [DOI] [PubMed] [Google Scholar]
- 7.Goodfield M, Hull SM, Holland D, Roberts G, Wood E, Reid S, et al. Investigations of the ‘active’ edge of plaque psoriasis: Vascular proliferation precedes changes in epidermal keratin. Br J Dermatol. 1994;131:808–13. doi: 10.1111/j.1365-2133.1994.tb08582.x. [DOI] [PubMed] [Google Scholar]
- 8.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 9.Creamer D, Allen MH, Sousa A, Poston R, Barker JN. Localization of endothelial proliferation and microvascular expansion in active plaque psoriasis. Br J Dermatol. 1997;136:859–65. [PubMed] [Google Scholar]
- 10.Heidenreich R, Röcken M, Ghoreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Path. 2009;90:232–48. doi: 10.1111/j.1365-2613.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faivre S, Delbado C, Vera K, Robert C, Lozahic S, Lassau N, et al. Safety, pharmacokinetics and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 12.Sauder DN, Dekoven J, Champagne P, Croteau D, Dupont E. Neovastat (AE-941), an inhibitor of angiogenesis: Randomized phase I/II clinical trial results in patients with plaque psoriasis. J Am Acad Dermatol. 2002;47:535–41. doi: 10.1067/mjd.2002.124702. [DOI] [PubMed] [Google Scholar]


