Abstract
Background:
Tinea corporis is a common superficial dermatophytosis seen in tropical countries. Newer molecules are constantly being introduced for its treatment. Topical clotrimazole is in vogue as the treatment for this condition for a long time. Amorolfine is a comparatively recently introduced drug for topical use in this condition.
Aims:
To assess the effectivity and safety of amorolfine 0.25% cream in patients with tinea corporis, in comparison to clotrimazole 1% cream.
Materials and Methods:
Patients presenting with symptoms of tinea corporis were mycologically confirmed for the presence of fungal hyphae. They were randomly divided into two groups: one group received amorolfine and the other received clotrimazole. Treatment duration was for 4 weeks and study duration was for 8 weeks. Evaluation was carried out using the standard clinical parameters on day 1, day 14, day 28 and a follow-up on day 56. Adverse effects were also recorded. Data entry was done in Excel datasheet and analyzed with Epiinfo 2002. Chi-square test and t-test were used according to the type of data.
Results:
The patients of the two groups were matched at baseline in respect to their demographic profile. Analysis of collected data showed significant improvement in both the groups, suggesting that both the drugs were effective agents in tinea corporis infection. Between-groups comparison of mycological cure rate and clinical improvement showed no significant difference.
Conclusion:
Amorolfine 0.25% cream is found to be safe and effective, like clotrimazole, when used topically in tinea corporis.
Keywords: Amorolfine, clotrimazole, tinea corporis, topical antifungal agents
Introduction
Superficial dermatophytosis of the skin in the form of tinea corporis is a very common infection seen in clinical practice. Fungal infections are common in hot and humid climate of tropical countries like India. Dermatophytes are fungi that infect epidermis of the skin, hair and nail due to colonization in the keratinized layers.[1] The most common dermatophytes that cause tinea corporis are Trichophyton rubrum, Trichophyton mentagrophytes, Microsporum canis and Trichophyton tonsurans. Typical infections have an annular appearance that is commonly referred to as “ringworm.”
A survey conducted by the World Health Organization on the prevalence of dermatophytic infection has shown that 20% of people presenting for clinical advice are suffering from cutaneous fungal infections worldwide.[2]
Both topical and systemic therapies may be used to treat dermatophyte infections. Topical therapy is generally effective for uncomplicated tinea corporis of small areas and of short duration.[3] Any topical agent used for superficial fungal infections has to have broad-spectrum activity, high mycological cure rate, convenient dosing, low incidence of side effects and low cost. The imidazole antifungal agent, clotrimazole, has been widely used topically for the treatment of superficial dermatophytosis for over 25 years[4,5] with satisfaction by patients and physicians. There is as yet no report of resistance to this drug.
There has always been a search for better antifungals, both systemic and topical. Some of the recently introduced molecules are used exclusively for topical purposes, some are systemic and a few others are used both as systemic and topical agents. Recently, some of the antifungal agents that have been in use as a systemic agent or in a lacquer form for tinea unguium have been introduced in the market in a topical form for use in tinea corporis.
Amorolfine, a morpholine derivative, is the first of a new class of antifungal drugs. The mechanism of action is inhibition of ergosterol biosynthesis in the fungal cell membrane. Alteration in the membrane sterol content leads to changes in membrane permeability and disruption of key fungal metabolic processes.[6,7] There are studies regarding topical use of amorolfine in the form of nail lacquer in onychomycosis.[8,9] Amorolfine in cream formulation has been studied for its safety and efficacy in dermatomycoses,[10] although data are rare, particularly from our country. Thus, amorolfine has been seen to possess antifungal properties, but data regarding effectivity of this drug in tinea corporis are limited. A topical formulation of amorolfine as 0.25% cream has been recently introduced in the Indian market for use in tinea corporis. This study aims to assess the effectivity and safety of amorolfine 0.25% cream as compared to clotrimazole 1% cream as a topical antifungal agent in dermatophytic infection.
Materials and Methods
Screening of patients and recruitment were carried out at the dermatology outdoor clinic of School of Tropical Medicine, Kolkata, which is a tertiary care hospital. Altogether, 150 adult patients of either sex in the age group 18–65 years with a clinical diagnosis of mild to moderate grades of dermatophytosis, who satisfied the inclusion and exclusion criteria and consented to participate, were selected for the study. Necessary ethical clearance was obtained from the institutional ethical committee.
Those who had uncontrolled diabetes, were HIV infected or suffering from concomitant bacterial infection were excluded from the study. Pregnant or lactating mothers and female patients of the reproductive age group practicing unreliable methods of contraception were excluded from the study. Those who received systemic and/or topical antifungal agents during the last 1 month were also excluded. Those who had negative skin scraping for fungus from a clinically suspected lesion on baseline visit were also excluded from the study.
The patients were divided into three groups randomly. Each group was allocated one topical antifungal, namely, clotrimazole, amorolfine and another systemic antifungal molecule newly introduced for topical use, which is to be published later.
Study design
This was a randomized, controlled trial with three parallel treatment arms. The selected 150 patients were randomly offered three antifungal creams of which two are the experimenting drugs of the present study, namely, amorolfine and clotrimazole. The patients were instructed to apply the allocated cream twice daily for 4 weeks. Though amorolfine has been recommended by the manufacturer for once daily use, we have recommended its use in the study patients twice daily for the purpose of blinding the evaluation of its effectivity and adverse effects.
The patients were scheduled for examination by the investigator four times during the trial. After the baseline visit, patients were asked to report again on day 14, day 28 and on day 56 for follow-up to look for any relapse. We tried to single blind the study by engaging an investigator for evaluating the patients during selection and follow-up, but was kept blinded regarding the molecule used by the patient.
Study drugs
Amorolfine 0.25% cream and clotrimazole 1% cream were dispensed to the patients according to randomization. The first application was supervised and the patient was advised to use the medication thereafter as per the study schedule.
Patients enrolled for the study were not permitted to concomitantly use any antifungal other than the trial drug or any other topical medication. Systemic antifungals and corticosteroids were also not permitted. No systemic antihistamine was given. They were asked to discontinue the drug and report at the earliest if any discomfort was felt.
Assessment of effectivity and safety
Each subject was required to attend the clinic on four occasions during the study. In the first visit, the patient was screened, which also served as the baseline visit if the patient was not receiving any interacting drug. Otherwise, a separate baseline visit was advised after appropriate wash-out period on withdrawal of the interacting drug. At screening, a thorough medical history was taken and clinical examination of the potential subjects was done to assess their suitability for participation in the study. Informed consent was obtained. Skin scrapings were collected, treated with 10% KOH and examined under the microscope for determination of fungal elements. Routine blood examination was done to rule out diabetes or any other co-morbid condition in selected cases. The study medication was dispensed to the subject following randomization, provided all inclusion and exclusion criteria were satisfied. The patients were instructed to apply the cream thinly to the affected area twice daily. Each patient was asked to maintain a trial diary.
The second visit was on day 14 when the patient was clinically examined and compliance determined from the trial diary. Adverse events, if any, were recorded. Mycological examination was repeated. A second dose of the study medication was dispensed if necessary.
During the third visit on day 28, clinical assessment was repeated, compliance determined, adverse events, if any, were recorded and mycological examination was also done. The patients were instructed not to apply any medication thereafter.
The end-of-trial visit was 4 weeks thereafter, i.e. on day 56 from inclusion of the subject, to record relapse, if any. A repeat clinical evaluation was carried out and mycological examination was done from the treated area.
The clinical improvement was assessed based on effectivity parameters for evaluation of signs and symptoms, which were itching, erythema and scaling. These parameters were assessed on a pre-determined 4-point scale as absent, mild, moderate and severe.
The mycological cure rate was studied in patients of both the groups. Absence of fungal elements in the skin scraping material constituted mycological cure.
The physician's global clinical assessment of effectivity and tolerability was graded on a 4-point scale as poor, satisfactory, good and excellent.
The patient's assessment regarding effectivity and acceptability of treatment was similarly recorded on a 4-point scale as poor, satisfactory, good and excellent.
Statistical analysis
Effectivity data were evaluated for subjects who reported for the follow-up visit at the end of 4 weeks. Data entry was done in Microsoft Excel Sheet and analysis was carried out in Epiinfo 2002. Chi-square test and t-test were used according to the type of data.
Results
Among the selected patients, after randomization, 48 were given amorolfine cream and 51 received clotrimazole cream.
Comparison of mean age between the groups did not show any significant difference (P=0.84). Comparison of male to female ratio in the study population between the two groups also failed to show any significant difference (P=0.50). The groups were thus matched in respect to their baseline demographic profile [Table 1].
Table 1.
Baseline demographic profile in the two groups

Of the 51 patients who used clotrimazole cream, 6 did not turn up for follow-up on day 14 or their compliance was not satisfactory; 3 patients in this group were not considered suitable for evaluation on day 28 for similar reasons. In the amorolfine group, we lost four patients on day 14 and six on day 28.
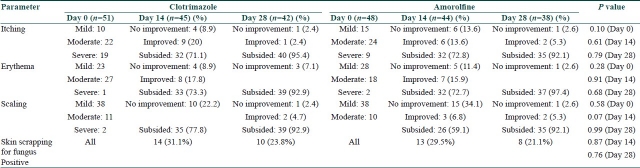
With either drug, there was clinical improvement in all the effectivity parameters on day 14, with further improvement continuing till day 28. In the clotrimazole group, itching subsided in 71.1 and 95.4% of the patients, erythema was absent in 73.3 and 92.9% of the patients, and scaling subsided in 77.8 and 92.9% of the patients on days 14 and 28, respectively. Similar results were seen in the amorolfine group where itching subsided in 72.8 and 92.1% of the patients, erythema was absent in 72.7 and 97.4% of the patients and scaling was absent in 59.1 and 92.1% of the patients on days 14 and 28, respectively [Table 2]. This indicates continuous reduction in patient's suffering with both the drugs. Hence, both the drugs have good effectivity as topical antifungal agents.
Table 2.
Distribution of subjects according to parameters at different points of time in the two treatment groups
Between-groups comparison of the primary effectivity parameters at different study points showed no significant difference in any of the parameters between the two groups at any point of time (P>0.05) [Table 2]. Thus, it can be inferred that both the drugs provided effective relief from the signs and symptoms of tinea corporis and there was no difference in effectivity between them.
Skin smear for fungus was positive at baseline in all cases in both the groups as per the inclusion criteria. After 14 days of treatment with clotrimazole, skin smear was positive in 14 (31.1%) patients; thus, a mycological cure of 68.9% was achieved. On day 28, the cure rate reached was 76.2%. In the amorolfine group, the mycological cure rate was 70.5% on day 14 and 78.9% on day 28. These differences between two groups were not statistically significant [Table 2].
Between-groups comparison of physician's assessment of effectivity at the end of the study did not show any significant difference (P=0.91). Also, no significant difference could be detected between the groups in the patient's assessment of effectivity and tolerability (0.82) [Table 3].
Table 3.
Global assessment of physician and patients at the end of study

Only three patients in the clotrimazole group and six in the amorolfine group attended for follow-up on day 56, i.e. 4 weeks after stoppage of treatment. Neither these patients had any sign of activity of the disease nor were the skin scrapings from old sites found positive for fungus at that point.
One patient in the clotrimazole group showed increased erythema on day 14. The patient continued application without improvement up to day 28. Barring this patient, none experienced any untoward effect with the medications. Thus, both the molecules were tolerated well.
Discussion
Tinea corporis is a superficial fungal infection, significantly prevalent in the people belonging to the socioeconomic strata that our tertiary care hospital caters to. Dermatophytes belonging to the genera Trichophyton, Epidermophyton and Microsporum are responsible for most of the superficial fungal infections. These infections occur in both healthy and immunocompromised patients. Early recognition and treatment is essential to reduce morbidity and possibility of transmission. The most common dermatophytes that cause tinea corporis are T. rubrum, T. mentagrophytes, M. canis and T. tonsurans.
Mycoses have significant negative social, psychological, occupational and health effects. Treatment of dermatophytosis is not merely for esthetic reasons. Persistent infections can compromise the quality of life to a remarkable extent.
Both topical and systemic therapies are used to treat dermatophyte infections depending upon the site involved and type and extent of infection. Topical therapy is effective for uncomplicated tinea corporis of mild to moderate grades. Topical agents used in these infections are the imidazoles, allylamines, tolnafnate and ciclopirox. The imidazole antifungal agent, clotrimazole, has been widely used topically for the treatment of superficial dermatophytosis for years together.[3,4] There is as yet no report of resistance to this drug. So, we selected this drug to compare the effectivity and safety of topical amorolfine cream (0.25%), which is a comparatively new drug for topical use in uncomplicated cases of tinea corporis infection.
Amorolfine is a new class of antifungal agents having a mechanism of action different from the imidazoles. It is a phenylpropyl morpholine derivative and acts by inhibiting ergosterol biosynthesis in the fungal cell membrane. Alterations in membrane sterol content lead to changes in membrane permeability and disruption of fungal metabolic processes.[5,6] It acts on two different enzymes involved in sterol biosynthesis, which results in depletion of ergosterol. This dual mechanism of action makes amorolfine a potent fungistatic and fungicidal agent. In vitro studies have demonstrated that at concentrations of 0.1–100 μg/ml, topical amorolfine induces varying degrees of damage to the nuclear, mitochondrial and plasma membranes of both T mentagrophytes and Candida albicans.[11] An in vitro study has shown topical amorolfine to have the lowest minimum inhibitory concentration (MIC) against various strains of dermatophytes as compared to other topical antifungal agents.[12]
A comparative study has also shown amorolfine to have more effective fungistatic and fungicidal action than bifonazole and cyclopirox olamine in an in vitro study with T. rubrum collected from onychomycosis samples.[13] Amorolfine used in combination with other antifungal agents like ketoconazole, terbinafine, itraconazole and fluconazole has been seen to cause increase in fungistatic activity against T. mentagrophytes.[14] There are multiple studies to demonstrate the efficacy of topical amorolfine in onychomycosis.[8,9,15] A clinical study demonstrated comparable effectivity of amorolfine and bifonazole in the treatment of dermatomycosis.[10]
This study compared the effectivity and safety of topical amorolfine in cases of mild to moderate grades of tinea corporis, to the conventional topical preparation of clotrimazole. The results show that both the drugs are equally effective in bringing about clinical cure, with no significant difference at any point of follow-up.
With clotrimazole, itching subsided in 71.1% of the patients on day 14 and in 95.4% of the patients on day 28. Clinical improvement was seen also in erythema and scaling on day 14, with further improvement on day 28. Scaling persisted in 7.10% patients in this group even on day 28. This can be justified as scaling is a sign of healing. Thus, with clotrimazole, gradual clinical improvement was seen continuing up to 4 weeks.
With amorolfine, itching subsided in 72.8% of the patients on day 14 and in 92.1% of the patients on day 28. Erythema subsided in 72.7% of the patients on day 14 and still further in 97.4% of the patients on day 28. Scaling persisted in 7.9% of the patients on day 28. Thus, amorolfine is also seen to be effective as a topical antifungal in tinea corporis and the clinical improvement here also continued after day 14.
Between-groups comparisons of the effectivity parameters for clinical improvement showed no significant difference in any of the parameters at baseline, suggesting that the intensity of the disease process was similar in both the groups [Table 2]. Similar comparisons on the days of evaluation, i.e. day 14 and day 28, failed to show any significant difference between the groups.
Thus, it can be inferred that both the drugs provided effective relief from the signs and symptoms of tinea corporis though there was no statistically detectable difference in effectivity between them.
The mycological cure rate did not show any significant difference between the two groups either on day 14 or on day 28. With clotrimazole, mycological cure rate achieved on day 28 was 76.2%. This is at par with the result of a previous study done with clotrimazole 1% cream.[16] The amorolfine group showed a mycological cure rate of 78.9% on day 28. This is close to the results obtained in a comparative study of amorolfine cream, done with different concentrations of the drug used topically.[10]
One patient in the clotrimazole group had increased erythema on day 14, which persisted on day 28. This may be considered as an adverse event with the use of clotrimazole. With amorolfine, none of the patients experienced any local adverse event. No systemic adverse event was reported in any patient. Hence, both the drugs were seen to be safe for topical use. The physician as well as the patients were satisfied with the outcome of either drug.
Since the number of patients returning for follow-up 4 weeks after stoppage of treatment was very low and none of them showed any sign of the disease, we did not include them in commenting on the chance of relapse with either molecule.
Though both the drugs showed good results against dermatophytosis, we failed to find any difference between them. This may be due to less number of study subjects. Also, we had a limitation that our study was not double blinded though we tried to single blind it by making the evaluations blinded.
It can be thus concluded that amorolfine on topical use is comparable in effectivity to clotrimazole in the treatment of mild to moderate grades of dermatophytosis. It is also safe and well tolerated.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatricks’ Dermatology in General Medicine. 17th ed. Vol. 2. New York: McGraw Hill; 2008. Fungal diseases; pp. 1807–10. [Google Scholar]
- 2.Marques SA, Robles AM, Tortorano AM, Tuculet MA, Negroni R, Mendes RP. Mycoses associated with AIDS in the Third world. Med Mycol. 2000;38:269–79. [PubMed] [Google Scholar]
- 3.Calvin OM, Thomas JL. Eczema, Psoriasis, cutaneous infections, acne and other common skin disorders. In: Fauci, Braunwald, Kasper, Hauser, Longo, Jameson, Loscalzo, editors. Harrison's Principles of Internal Medicine. 17th ed. Vol. 1. New York: McGraw-Hill; 2008. p. 318. [Google Scholar]
- 4.Stary A, Soeltz-Szoets J, Ziegler C, Kinghorn GR, Roy RB. Comparison of the efficacy and safety of oral fluconazole and topical clotrimazole in patients with Candida balanitis. Genitourin Med. 1996;72:98–102. doi: 10.1136/sti.72.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sobel JD, Brooker D, Stein GE, Thomason JL, Wermeling DP, Bradley B. Single oral dose fluconazole compared with conventional clotrimazole topical therapy of Candida vaginitis. Am J Obst Gynaecol. 1995;172:1263–8. doi: 10.1016/0002-9378(95)91490-0. [DOI] [PubMed] [Google Scholar]
- 6.Haria M, Bryson MH. Amorolfine. Drugs. 1995;49:103–20. doi: 10.2165/00003495-199549010-00008. [DOI] [PubMed] [Google Scholar]
- 7.Polak A. Mode of action studies. In: Ryley JF, editor. In: Handbook of Experimental Pharmacology. Chemotherapy of Fungal Diseases. Vol. 96. Berlin, Heidelberg: Springer Verlag; 1990. pp. 152–82. [Google Scholar]
- 8.Lecha M, Alsina M, Rodriquez JM, Erenchun FR, Mirada A, Rossi AB. An open-labelled, multicenter study of the combination of amorolfine nail lacquer and oral itraconazole compared with oral itraconazole alone in the treatment of severe toenail onychomycosis. Curr Ther Res. 2002;63:366–79. [Google Scholar]
- 9.Lecha M. Amorolfine and itraconazole combination for severe toenail onychomyxosis; results of an open randomized trial in Spain. Br J Dermatol. 2001;145:21–6. [PubMed] [Google Scholar]
- 10.Nolting S, Semig G, Friedrich HK, Dietz M, Reckers R, Bergstraesser M. Double-blind comparison of amorolfine and bifonazole in the treatment of dermatomycoses. Clin Exp Dermatol. 1992;17(Suppl 1):56–60. doi: 10.1111/j.1365-2230.1992.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 11.Müller J, Polak-Wyss A, Melchinger W. Influence of amorolfine on the morphology of Candida albicans and Trichophyton mentagrophytes. Clin Exp Dermatol. 1992;17(Suppl 1):18–25. doi: 10.1111/j.1365-2230.1992.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 12.Favre B, Hofbauer B, Hildering KS, Ryder NS. Comparison of in vitro activities of 17 antifungal drugs against a panel of 20 dermatophytes by using a microdilution assay. J Clin Microbiol. 2003;41:4817–9. doi: 10.1128/JCM.41.10.4817-4819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaller M, Borelli C, Berger U, Schimdt S, Weindl G, Jackel A. Susceptibility testing of amorolfine, bifonazole and ciclopiroxolamine against Trichophyton rubrum in an in vitro model of dermatophyte nail infection. Med Mycol. 2009;47:753–8. doi: 10.3109/13693780802577892. [DOI] [PubMed] [Google Scholar]
- 14.Polak A. Combination of amorolfine with various antifungal drugs in dermatophytosis. Mycoses. 1993;36:43–9. doi: 10.1111/j.1439-0507.1993.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 15.Haria M, Bryson HM. Amorolfine. A review of its pharmacological properties and therapeutic potential in the treatment of onychomycosis and other superficial fungal infections. Drugs. 1995;49:103–20. doi: 10.2165/00003495-199549010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Barnetson RS, Marley J, Bullen M, Brookman S, Cowen P, Ellis D, et al. Comparison of one week of oral terbinafine (250 mg/day) with four weeks of treatment with clotrimazole 1% cream in interdigital tinea pedis. Br J Deramatol. 1998;139:675–8. doi: 10.1046/j.1365-2133.1998.02466.x. [DOI] [PubMed] [Google Scholar]