Abstract
Background:
Burn surgeons use autologous skin graft technique for patients, but a challenge remains for large surface wounds. Recently, a method was described which used a small piece of skin to cover a 70 times greater surface by spraying epidermal cells on injured skin. We designed a comparative study to find the best method to make an epidermal cell suspension.
Materials and Methods:
Eleven discarded skin samples were sent to our laboratory from Ghotboddin Burn Hospital, Shiraz. Each sample was sliced into four small pieces (1 cm2) and each piece was treated with a different chemical including sodium bromide (2N) and (4N), ammonium hydroxide (2N), and trypsin (0.05%) for 20 minutes. The epidermis and dermis were separated using forceps. Trypsin was added to all samples (except the trypsinized sample) to begin the intercellular detachment. Afterward, epidermis was sliced into small pieces followed by filtration and centrifugation. Cells were counted using hemocytometer. Identification of keratinocytes and melanocytes was made through immunocytochemical staining for cytokeratin and melanosome antigens, respectively.
Results:
There was a significant difference in alive cell counts comparing cells obtained from NaBr (4N) method to other methods. Considering total cell count and alive cell count, NaBr (4N) yielded the most cells. Immunocytochemical staining showed that in all methods, some cells are stained positively for cytokeratin antibody and some for melanosome antibody.
Conclusion:
Although recent papers had advised trypsin method to make a cell suspension to use for burn patients, we found that NaBr (4N) method yields more alive cells and less toxicity.
Keywords: Autologous transplantation, epidermal separation, NaBr
Introduction
The skin is often known as the largest organ of the human body and contains three primary layers: epidermis, dermis, and hypodermis. Skin performs in different functions such as protection, sensation, heat regulation, water resistance, and many others. Today, most burn surgeons use autologous skin graft technique for patients, but a challenge remains for large surface wounds and also the high risk of infections. Since 1975, epidermal cell culture to cultivate keratinocytes in vitro was introduced[1] and keratinocyte sheets have been used for more than 20 years in clinical setting,[2] but these methods had major problems. Recently, scientists have been thinking about using the patient's own epidermal cells to cover the burning aria.
For burned patients, epidermal replacement is an important step in which keratinocytes, Langerhans cells, and melanocytes are restored.[3] The first step in isolating epidermal cells is to separate it from dermis. Several methods have been reported by scientists for the separation of epidermis from dermis over the past decades. It was first reported in 1941 by Medewar et al. that elastic fibers can be rapidly and specifically dissolved by trypsin which result in separation of epidermis from dermis.[4] Then, in 1942, heat separation was introduced as a simple and reliable method by Baumberger et al. and then this method were improved by reducing the time of heating in 1982 by Kassis et al.[5,6] Some chemicals were reported to be effective to separate the epidermis from dermis such as sodium thiocyanate,[7] sodium bromide, and ammonium hydroxide.[5] Fraulin et al. established the use of an aerosolized spray to transfer epithelial cells to a wound bed in pigs.[8] In 2005, a device was introduced known as the ReCell system in which a small piece of skin biopsy is used to make a suspension of epidermal cells.[3] The cell suspension can then be sprayed onto the patients’ wound surface as a regenerative method to accelerate the healing, minimize the risk of scarring, and improve skin texture and color. This procedure can harvest enough skin cells from a 4-cm2 donor site to treat an area the size of 320 cm2 .[3] In this study, several methods that were previously used for the separation of epidermis from dermis and making an epidermal cell suspension were compared to select the best method for clinical usage. The best method was defined as a method in which the least toxicity and the most alive cells are produced.
Materials and Methods
Preparation of cell suspension
Eleven discarded skin biopsies were sent to our laboratory from Ghotboddin Burn Hospital, Shiraz, Iran. Each sample was sliced into four small pieces (1 cm2) using scalpel's blade and placed in separate petri-dishes. In this study, four different chemicals were tested: sodium bromide (2N) and (4N), ammonium hydroxide (2N), and trypsin (0.05%). The epidermis was put in 5 ml of mentioned chemicals for 20 minutes at 37°C to begin the intercellular detachment. Trypsin was quickly neutralized in trypsinized sample using 5 ml of Dulbecco's Modified Eagle's Medium (DMEM) (USA) supplemented with 10% fetal bovine serum (FBS). The three remaining samples were washed with 5 ml of phosphate buffer saline. Epidermis and dermis were separated using forceps [Figure 1] and cells were scraped from the junctional surfaces with the sterile scalpels blade to form a cloud of cell. The dermis was then discarded. Trypsin (5 ml) was added to samples treated with NaBr (2N), (4N), and NH 4 OH and placed in 37°C for 20 minutes. Trypsin was neutralized with 5 ml of DMEM-10% FBS media. Afterward, epidermis in all samples was sliced into small pieces manually using scalpel's blade. Then, the suspensions were filtered using cell strainer (BD Falcon, USA) and centrifuged at 1500 rpm for 10 minutes. The supernatant was removed and the pellet was resuspended in 1 ml of DMEM. Cells were counted using hemocytometer and number of dead and alive cells were determined using the vital stain, 1% Evans Blue Counterstain (UK), which was diluted 1: 20 with distilled water.
Figure 1.
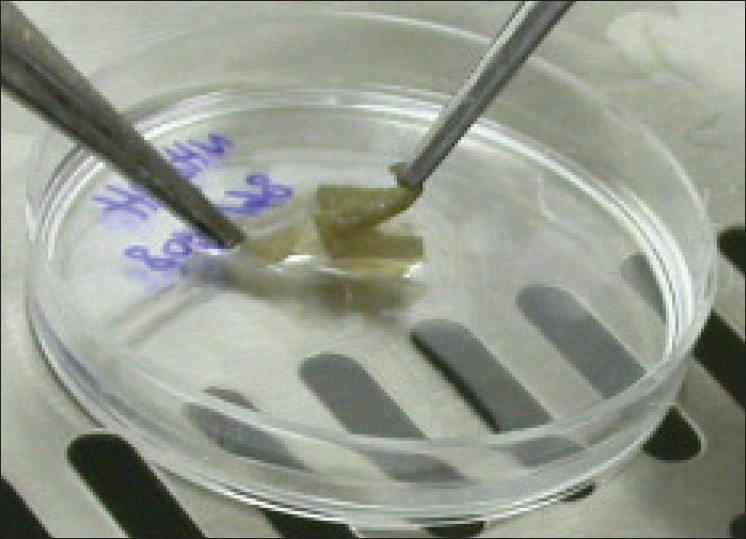
Separation of epidermis from dermis using the forceps. The top layer (gray) is the epidermis and the bottom layer (white) is the dermis
Immunocytochemistry
For each method, two immunocytochemistry slides were prepared using the cell centrifuge. HMB-45 and cytokeratin antibodies were used to identify the presence of melanocytes and keratinocytes, respectively, for all methods.
Results
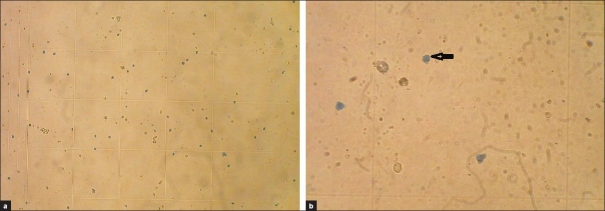
All 44 (11 skin biopsies and each were divided into four pieces) samples were counted in four different fields of hemocytometer by two experts [Figure 2a] and the means were used for the analysis. Cells with blue cytoplasm represent dead cells as they cannot exit vital stain due to lack of active pumps [Figure 2b]. We also counted the alive cells to compare with the dead cells. These cells contain the active pumps and the shapes of them are like an empty circle (shown by arrow in Figure 2b).
Figure 2.
(a) Epidermal cell suspension cells that are stained with 1% Evans blue stain with 100× magnification. (b) Dead cells are stained blue (arrow) with 200× magnification
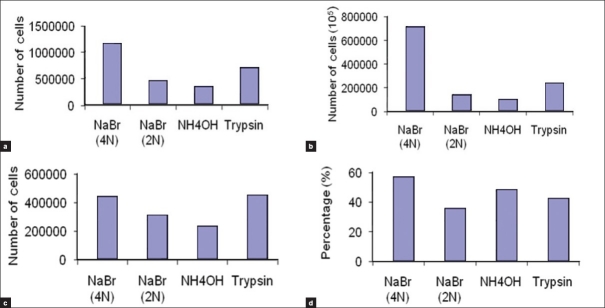
The results of counting the total, dead, and alive cells are shown in histogram [Figure 3]. Data were also analyzed using SPSS software version 11.5. Friedman two-way analysis of variance test was used to compare all methods generally and Wilcoxon matched-pairs signed-ranks test was used to compare each pair of methods (data not shown).
Figure 3.
Histogram of the different methods to obtain the best method for preparation of epidermal cell suspension. (a) Total cell counts. (b) Alive cell counts. (c) Dead cell counts. (d) Percentage of alive cells
Generally, there was no significant difference between total cell counts, dead cell counts, and percent of alive cells obtained from four different methods. But there was a significant difference between alive cell counts obtained from NaBr (4N) method compared with other methods, as it is shown in Table 1. While comparing NaBr (4N) method to each of NaBr (2N), NH4OH, and trypsin methods, P values were 0.008, 0.169, and 0.182, respectively, that all of them are significant. Considering total cell count and alive cell count, NaBr (4N) yielded the most cells and trypsin method was after that. On the other hand, trypsin method produced the most dead cell counts, although the differences were insignificant. The last parameter which is alive cell percentage shows no significant difference between all methods, though NaBr 4N and NH4OH had the greatest percentages, respectively. While using the NH4OH method, the epidermis color changed to dark and under microscopic field yielded a percent of large squamous cells with high cytoplasm to nucleus ratio. These cells were rarely seen with other methods. Other cells obtained by this method were small round cells similar to the cells obtained with other methods.
Table 1.
Comparison of the P values of different used methods
![]()
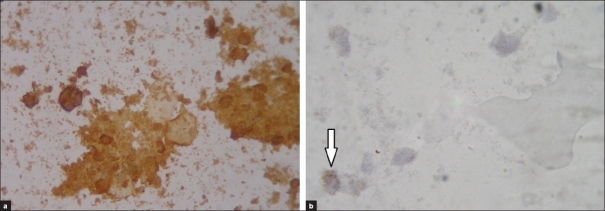
Immunocytochemical staining showed that in all methods, some cells are stained positively for cytokeratin antibody and some for melanosome antibody. Keratinocytes are the dominant cells in the epidermal cell suspension but a few melanocytes also exist in suspension, as shown in Figure 4. Regarding this test, there was no difference between four different methods as all of them yielded keratinocytes as the dominant cells of suspension and melanocytes that were less than 5% of the cells in suspension.
Figure 4.
(a) Identification of the keratinocytes using immunocytochemistry. Cells that are stained positive for cytokeratin antibody are keratinocytes (brown cells). (b) Identification of the melanocytes using immunocytochemistry. Only one melanocyte is observed in this figure (shown by arrow), which is positively stained for HMB-45 antibody (brown cell)
Conclusion
Burns are among the worst traumatic problems in our society that can be caused by heat, cold, electricity, chemicals, light, radiation, or friction. Burns can be minor or can present a life-threatening emergency, depending on the heat's intensity and length of exposure to the skin. Twenty years ago, burns covering half of the body frequently ended in death, although today, patients with burns covering over 90% of their body can survive. Despite all of the progresses that have been made in treatment of burn patients, it still has high mortality and morbidity. Keratinocyte cell culture and keratinocyte sheets introduced in 1975 provided new hopes for burn patients. However, these cells undergo phenotypic changes associated with contact inhibition and their surface proteins become disabled by enzymes applied in cell culture; so, the epidermis formed by these sheets is unstable and vulnerable to blistering with minor trauma. In adition, making these sheets is time consuming and expensive.[9] ReCell procedure uses trypsin method to make the cell suspension but in this paper, we showed that trypsin method is not the method of choice. Instead, we concluded that NaBr 4N can be used as the best method to make the cell suspension because it yields the most alive cells. The suspension produced by this method contains necessary cells required to form a normal epidermis, means keratinocytes and melanocytes. Also, there is no obvious change in color or morphology of the cells treated with NaBr 4N. So, we conclude that this method can be substituted with the previously used trypsin method in clinical conditions, but more investigations are needed for evaluation of any minor effect that NaBr 4N may have on cells in molecular level. Now, we are working on clinical effects of this method and the previously used trypsin method.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
References
- 1.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell. 1975;6:331–43. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 2.Gallico GG, O’Conner NE, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448–51. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 3.Currie LJ, Martin RJ, Sharpe JR, James SE. A comparison of keratinocyte cell sprays with and without fibrin glue. Burns. 2003;29:677–85. doi: 10.1016/s0305-4179(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 4.Gravante G, Di Fede MC, Araco A, Grimaldi M, Angelis B, Arpino A, et al. A randomized trial comparing ReCellW system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns. 2007;33:966–72. doi: 10.1016/j.burns.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Medewar PB. Sheets of pure epidermal from human skin. Nature. 1941;148:783. [Google Scholar]
- 6.Baumberger JP, Suntzeff V, Dowdry EV. Methods for separation of epidermis from dermis and some physiologic and chemical properties of isolated epidermis. J Natl Cancer Inst. 1942;2:413–23. [Google Scholar]
- 7.Kassis V, Sondergaard J. Heat-separation of normal human skin for epidermal and dermal prostaglandin analysis. Arch Dermatol Res. 1982;273:301–6. doi: 10.1007/BF00409259. [DOI] [PubMed] [Google Scholar]
- 8.Diaz LA, Heaphy MR, Calvanico NJ, Tomasi TB, Jordan RE. Separation of epidermis from dermis with sodium thyiocyanate. J Invest Dermatol. 1977;68:36–8. doi: 10.1111/1523-1747.ep12485156. [DOI] [PubMed] [Google Scholar]
- 9.Fraulin FO, Bahoric A, Harrop AR, Hiruki T, Clarke HM. Autotransplantation of epithelial cells in the pig via an aerosol vehicle. J Burn Care Rehabil. 1998;19:337–45. doi: 10.1097/00004630-199807000-00012. [DOI] [PubMed] [Google Scholar]