Abstract
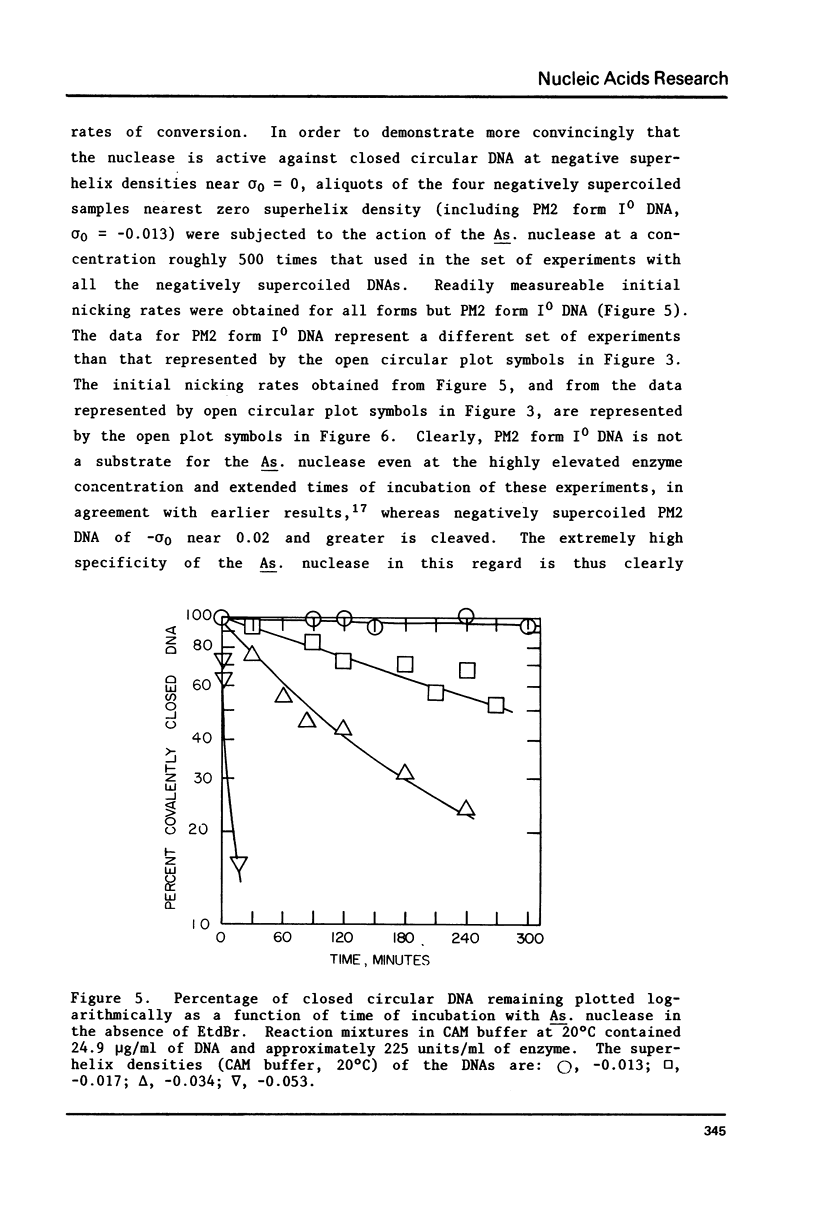
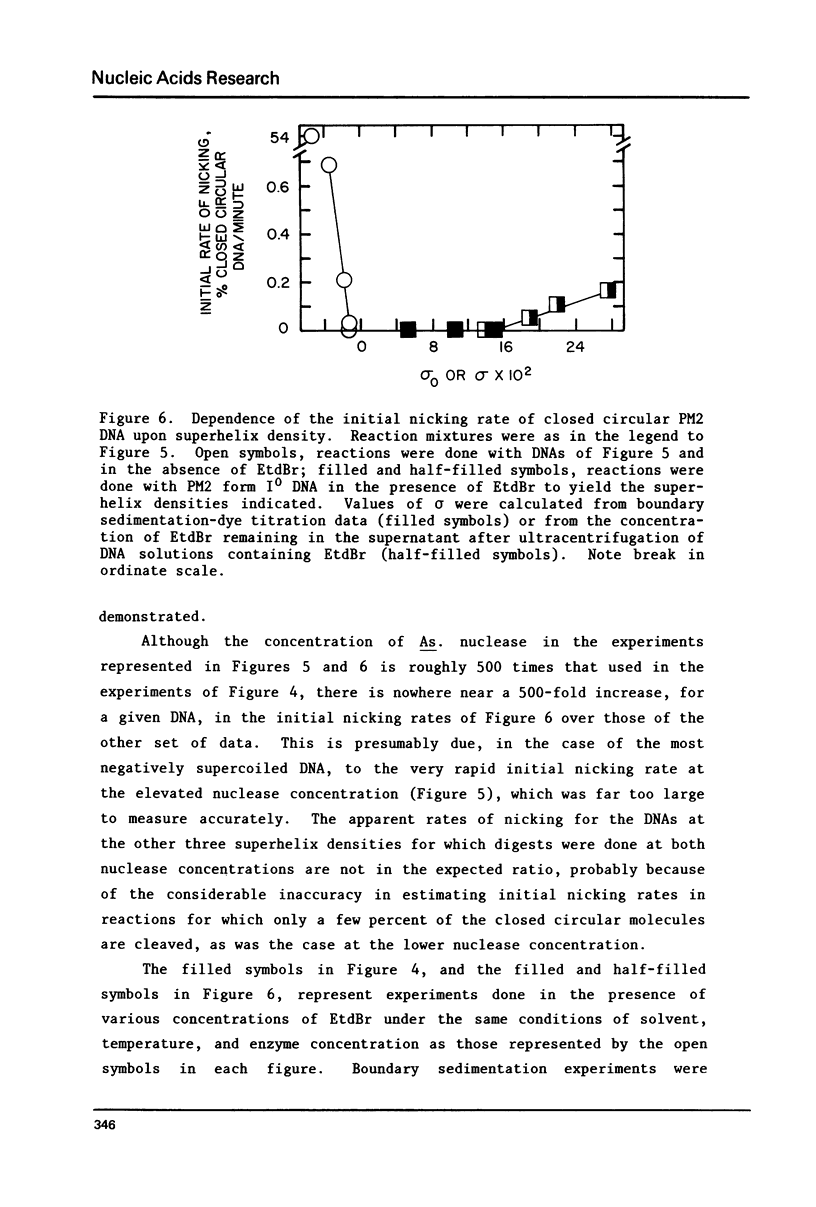
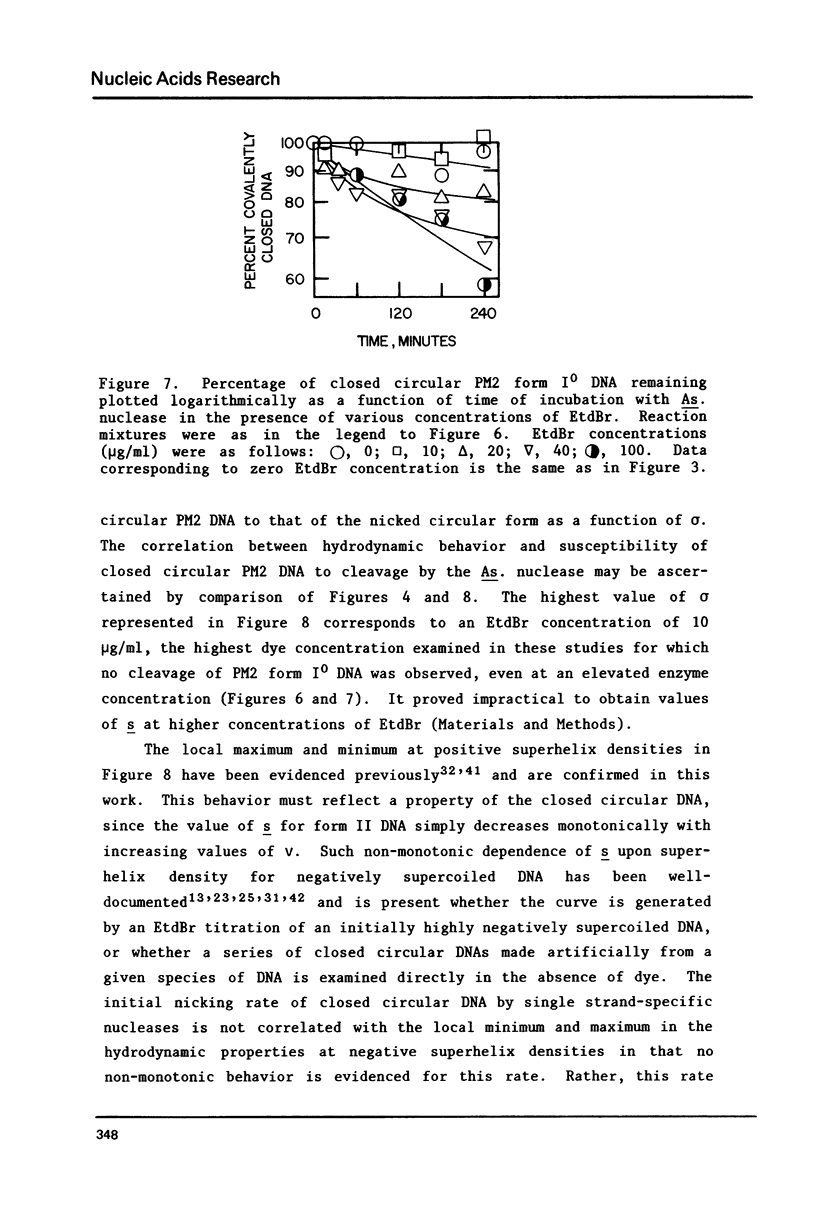
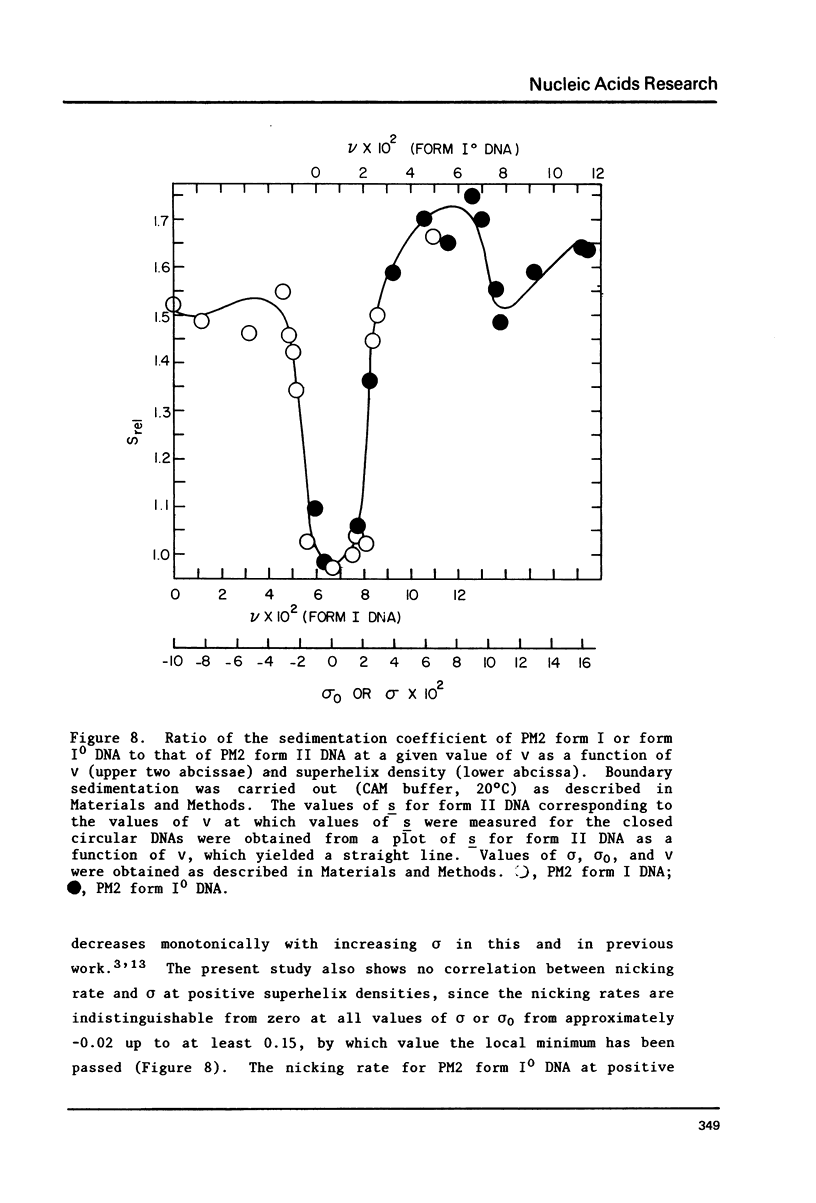
The dependence of the initial rate of introduction of the first single-chain scission (initial nicking rate) into covalently closed circular phage PM2 DNA by the single strand-specific nuclease from Alteromonas espejiana BAL 31 upon the superhelix density (sigma) of the DNA has been examined. The initial nicking rate decreases with decreasing numbers of negative superhelical turns (decreasing values of -sigma), which behavior is characteristic of other single strand-specific nucleases as reported earlier. In contrast to earlier work, the initial nicking rates of closed circular DNAs by the action of the Alteromonas nuclease have been shown to be readily measurable at values of -sigma as low as 0.02. However, even at the elevated concentrations of enzyme and extended digestion periods required to cause nicking at an appreciable rate at near-zero values of sigma, closed circular DNA containing very few superhelical turns (form IO DNA) is not cleaved at a detectable rate. When this DNA is rendered positively supercoiled by ethidium bromide (EtdBr), it is not affected by the nuclease until very high positive values of sigma are attained, at which low rates of cleavage can be detected at elevated enzyme concentrations. The effects of EtdBr on the enzyme activity have been tested and are entirely insufficient to allow the interpretation of zero nicking rates as the result of inhibition of the nuclease activity by the dye. Positively supercoiled DNA is concluded not to contain regions having significant single-stranded character until values of sigma are reached which are very much higher than the values of -sigma for which negatively supercoiled DNAs behave as if they contain unpaired or weakly paired bases.
Full text
PDF








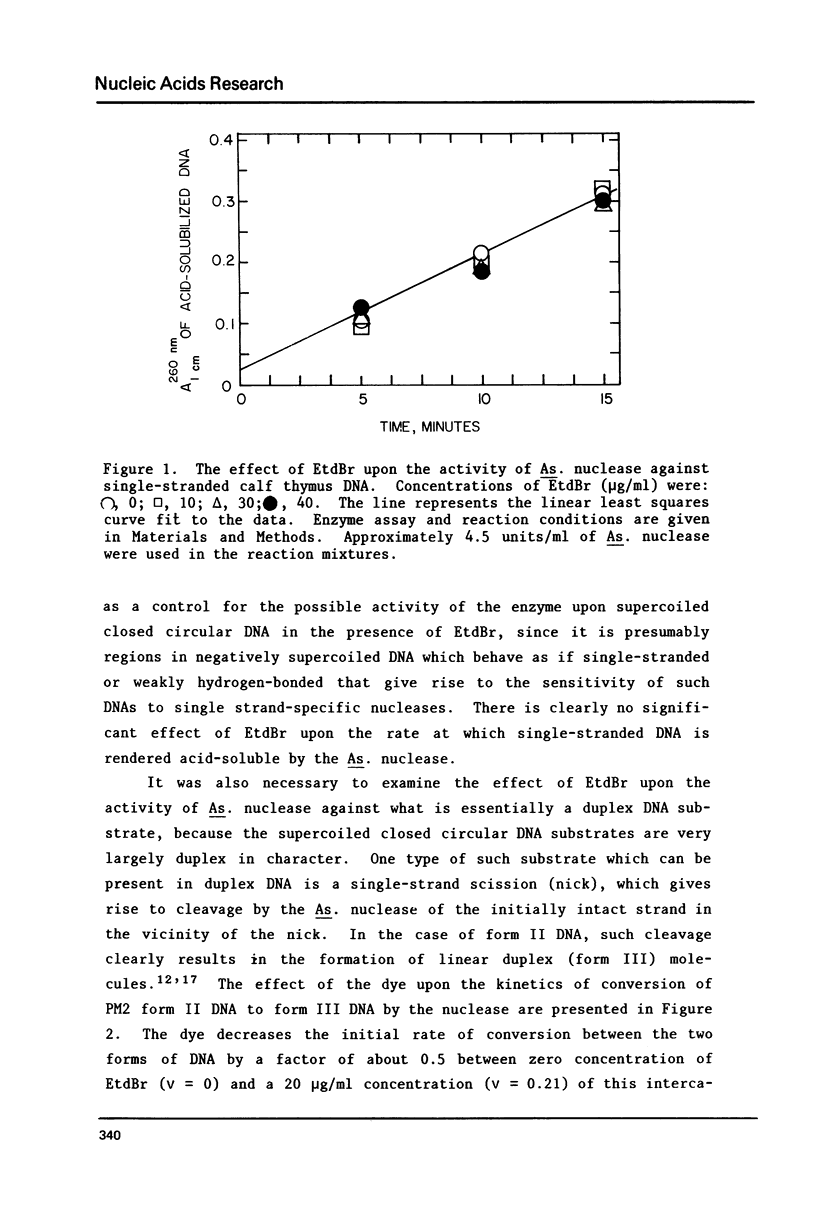
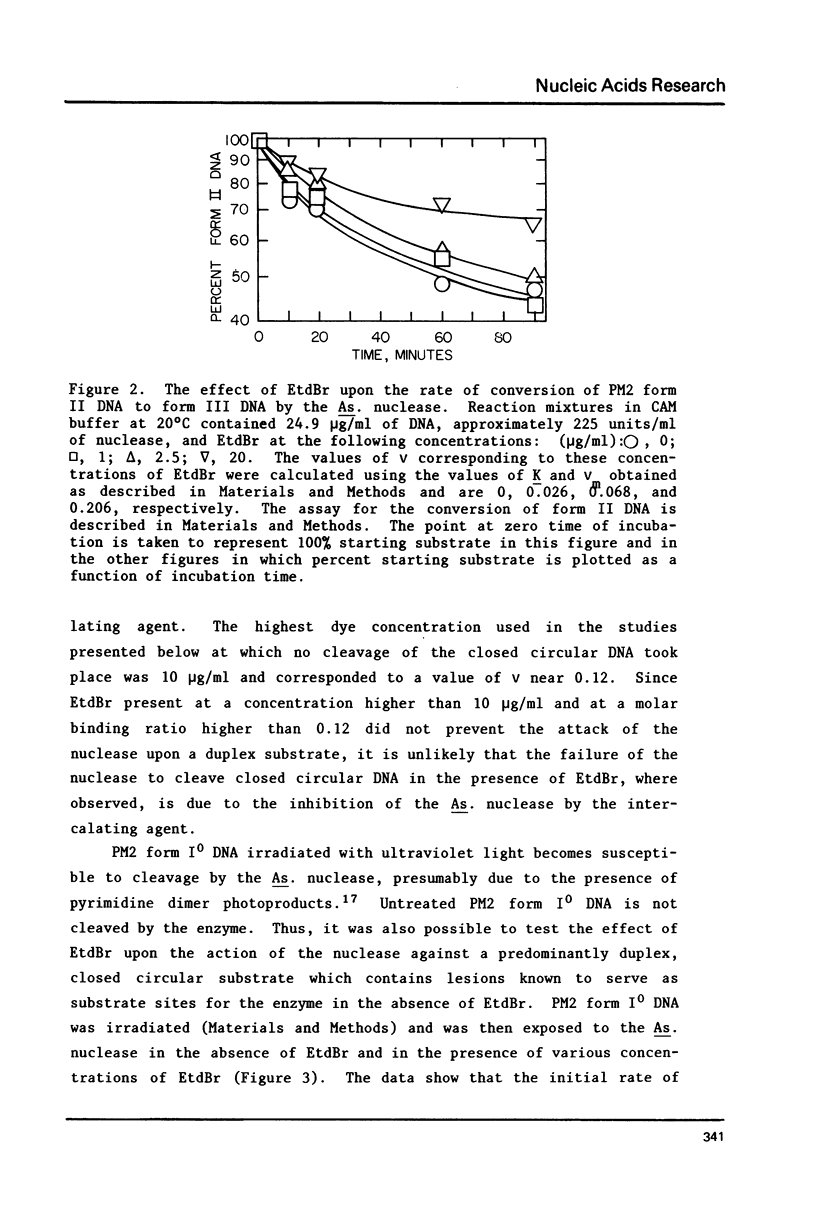
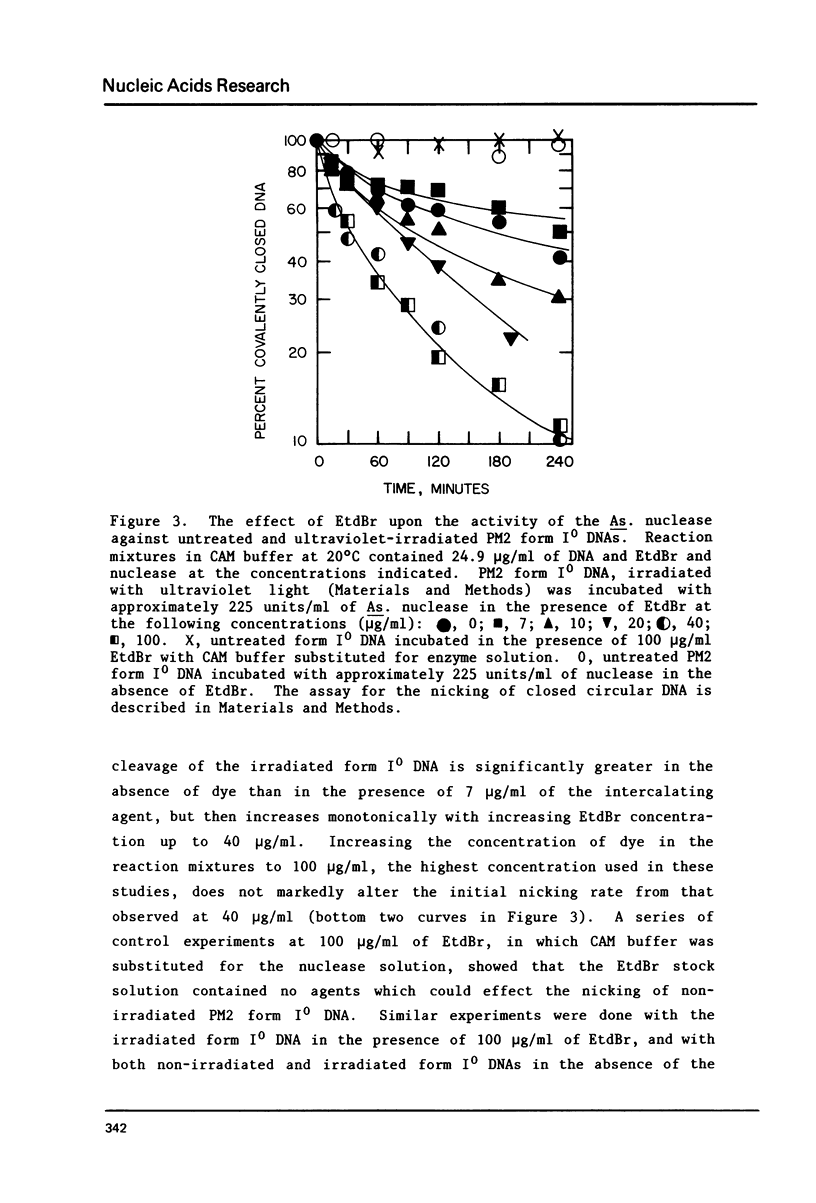

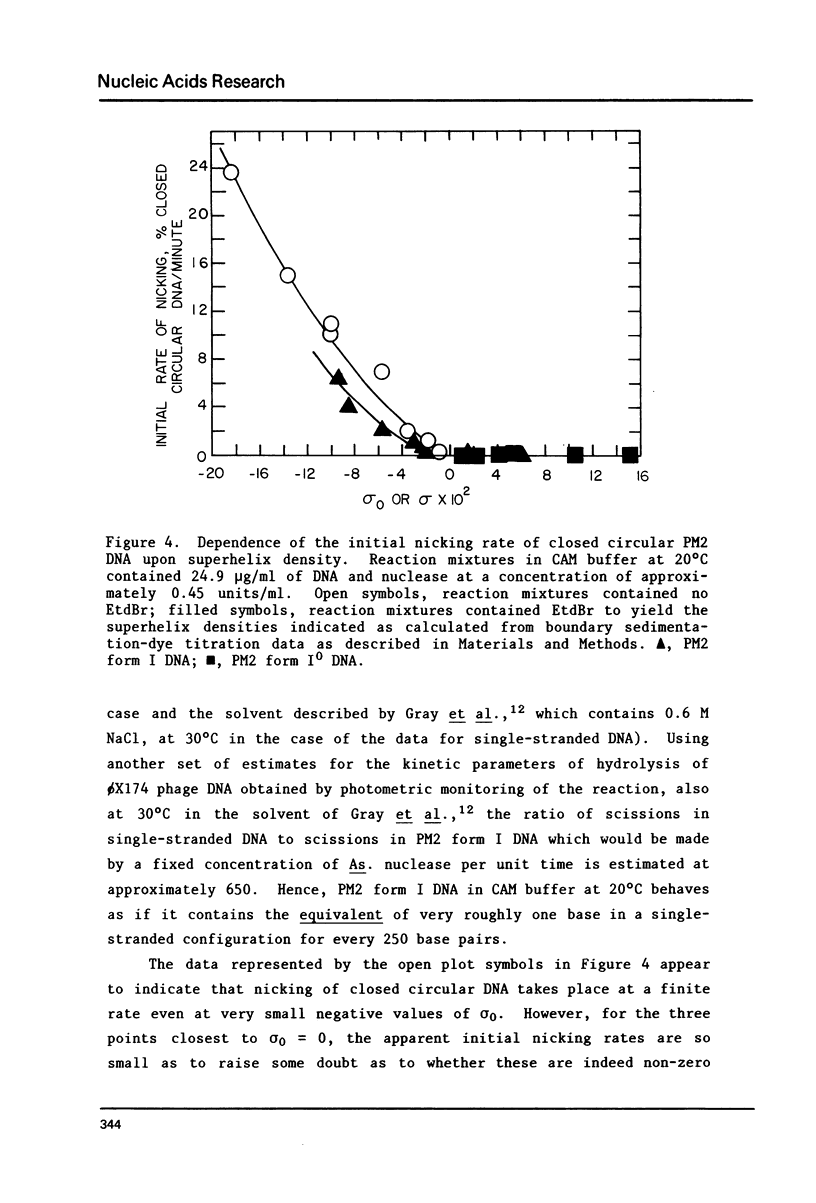









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. Interaction of closed circular DNA with intercalative dyes. II. The free energy of superhelix formation in SV40 DNA. J Mol Biol. 1970 Feb 14;47(3):419–435. doi: 10.1016/0022-2836(70)90312-8. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerman T. A., Lebowitz J. Further analysis of the altered secondary structure of superhelical DNA. Sensitivity to methylmercuric hydroxide a chemical probe for unpaired bases. J Mol Biol. 1973 Sep 25;79(3):451–470. doi: 10.1016/0022-2836(73)90398-7. [DOI] [PubMed] [Google Scholar]
- Burke R. L., Bauer W. Measurement of superhelix densities in buoyant dye/CsCl. The use of a standard other than native SV40 DNA. J Biol Chem. 1977 Jan 10;252(1):291–292. [PubMed] [Google Scholar]
- Dean W. W., Lebowitz J. Partial alteration of secondary structure in native superhelical DNA. Nat New Biol. 1971 May 5;231(18):5–8. [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Vogt V. M., Hirt B. Characterization of the single-strand-specific nuclease S1 activity on double-stranded supercoiled polyoma DNA. Eur J Biochem. 1974 Apr 16;43(3):591–600. doi: 10.1111/j.1432-1033.1974.tb03446.x. [DOI] [PubMed] [Google Scholar]
- Gray H. B., Jr, Ostrander D. A., Hodnett J. L., Legerski R. J., Robberson D. L. Extracellular nucleases of Pseudomonas BAL 31. I. Characterization of single strand-specific deoxyriboendonuclease and double-strand deoxyriboexonuclease activities. Nucleic Acids Res. 1975 Sep;2(9):1459–1492. doi: 10.1093/nar/2.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H. B., Jr, Upholt W. B., Vinograd J. A buoyant method for the determination of the superhelix density of closed circular DNA. J Mol Biol. 1971 Nov 28;62(1):1–19. doi: 10.1016/0022-2836(71)90127-6. [DOI] [PubMed] [Google Scholar]
- Grossman L. I., Watson R., Vinograd J. Restricted uptake of ethidium bromide and propidium diiodide by denatured closed circular DNA in buoyant cesium chloride. J Mol Biol. 1974 Jun 25;86(2):271–283. doi: 10.1016/0022-2836(74)90018-7. [DOI] [PubMed] [Google Scholar]
- Hancock R. Interphase chromosomal deoxyribonucleoprotein isolated as a discrete structure from cultured cells. J Mol Biol. 1974 Jul 5;86(3):649–663. doi: 10.1016/0022-2836(74)90187-9. [DOI] [PubMed] [Google Scholar]
- Hearst J. E., Gray H. B., Jr Titanium centerpieces and modified temperature control system for the Spinco analytical ultracentrifuge. Anal Biochem. 1968 Jul;24(1):70–79. doi: 10.1016/0003-2697(68)90060-2. [DOI] [PubMed] [Google Scholar]
- Hinton D. M., Bode V. C. Purification of closed circular lambda deoxyribonucleic acid and its sedimentation properties as a function of Sodium chloride concentration and ethidium binding. J Biol Chem. 1975 Feb 10;250(3):1071–1079. [PubMed] [Google Scholar]
- Hodnett J. L., Legerski R. J., Gray H. B., Jr Dependence upon temperature of corrected sedimentation coefficients measured in a Beckman analytical ultracentrifuge. Anal Biochem. 1976 Oct;75(2):522–537. doi: 10.1016/0003-2697(76)90107-x. [DOI] [PubMed] [Google Scholar]
- Hsieh T. S., Wang J. C. Thermodynamic properties of superhelical DNAs. Biochemistry. 1975 Feb 11;14(3):527–535. doi: 10.1021/bi00674a011. [DOI] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R. J., Lebowitz J., Printz M. P. Unpaired bases in superhelical DNA: kinetic evidence. Nucleic Acids Res. 1974 Apr;1(4):549–558. doi: 10.1093/nar/1.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. J. DNA replication of SV40-infected cells. VII. Formation of SV40 catenated and circular dimers. J Mol Biol. 1973 Jan 10;73(2):199–212. doi: 10.1016/0022-2836(73)90323-9. [DOI] [PubMed] [Google Scholar]
- Johnson P. H., Laskowski M., Sr Mung bean nuclease I. II. Resistance of double stranded deoxyribonucleic acid and susceptibility of regions rich in adenosine and thymidine to enzymatic hydrolysis. J Biol Chem. 1970 Feb 25;245(4):891–898. [PubMed] [Google Scholar]
- Kato A. C., Bartok K., Fraser M. J., Denhardt D. T. Sensitivity of superhelical DNA to a single-strand specific endonuclease. Biochim Biophys Acta. 1973 Apr 21;308(7):68–78. doi: 10.1016/0005-2787(73)90123-8. [DOI] [PubMed] [Google Scholar]
- Lebowitz J., Chaudhuri A. K., Gonenne A., Kitos G. Carbodiimide modification of superhelical PM2 DNA: considerations regarding reaction at unpaired bases and the unwinding of superhelical DNA with chemical probes. Nucleic Acids Res. 1977 Jun;4(6):1695–1711. doi: 10.1093/nar/4.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz J., Garon C. G., Chen M. C., Salzman N. P. Chemical modification of simian virus 40 DNA by reaction with a water-soluble carbodiimide. J Virol. 1976 Apr;18(1):205–210. doi: 10.1128/jvi.18.1.205-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Gray H. B., Jr A sedimentation velocity method for the separation of complementary strands of DNA. Biochim Biophys Acta. 1976 Aug 18;442(2):129–141. doi: 10.1016/0005-2787(76)90483-4. [DOI] [PubMed] [Google Scholar]
- Legerski R. J., Gray H. B., Jr, Robberson D. L. A sensitive endonuclease probe for lesions in deoxyribonucleic acid helix structure produced by carcinogenic or mutagenic agents. J Biol Chem. 1977 Dec 10;252(23):8740–8746. [PubMed] [Google Scholar]
- Legerski R. J., Hodnett J. L., Gray H. B., Jr Extracellular nucleases of pseudomonas BAL 31. III. Use of the double-strand deoxyriboexonuclease activity as the basis of a convenient method for the mapping of fragments of DNA produced by cleavage with restriction enzymes. Nucleic Acids Res. 1978 May;5(5):1445–1464. doi: 10.1093/nar/5.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. On the degree of unwinding of the DNA helix by ethidium. II. Studies by electron microscopy. Biochim Biophys Acta. 1975 Jul 23;395(4):401–412. [PubMed] [Google Scholar]
- Méchali M., de Recondo A. M., Girard M. Action of the S1 endonuclease from Aspergillus oryzae on simian virus 40 supercoiled component I DNA. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1306–1320. doi: 10.1016/0006-291x(73)91130-3. [DOI] [PubMed] [Google Scholar]
- Müller-Oswald U., Ruppen R., Baumann U., Angst J. Persönlichkeitsaspekte jugendlicher Drogenkonsumenten. Eine repräsentative Umfrage an 6315 neunzehnjährigen Zürchern. Arch Psychiatr Nervenkr (1970) 1973 Jul 20;217(3):207–222. doi: 10.1007/BF02552836. [DOI] [PubMed] [Google Scholar]
- Ostrander D. A., Gray H. B., Jr, Robberson D. L. Catenanes of closed circular intracellular PM2 phage DNA. Biochim Biophys Acta. 1974 May 31;349(3):296–304. doi: 10.1016/0005-2787(74)90117-8. [DOI] [PubMed] [Google Scholar]
- Ostrander D. A., Gray H. B., Jr Sedimentation and intrinsic viscosity behavior of PM2 bacteriophage DNA in alkaline solution. Biopolymers. 1973 Jun;12(6):1387–1419. doi: 10.1002/bip.1973.360120614. [DOI] [PubMed] [Google Scholar]
- Ostrander D. A., Gray H. B., Jr Superhelix density heterogeneity in closed circular intracellular PM2 DNA. Biopolymers. 1974 May;13(5):955–975. doi: 10.1002/bip.1974.360130511. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Morgan A. R. The sense of naturally occurring superhelices and the unwinding angle of intercalated ethidium. J Mol Biol. 1975 Jan 5;91(1):1–13. doi: 10.1016/0022-2836(75)90368-x. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P. Mechanism of ethidium bromide inhibition of RNA polymerase. J Mol Biol. 1973 Aug 25;78(4):703–714. doi: 10.1016/0022-2836(73)90290-8. [DOI] [PubMed] [Google Scholar]
- Salzman N. P., Lebowitz J., Chen M., Sebring E., Garon C. F. Properties of replicating SV40 DNA molecules and mapping unpaired regions in SV40 DNA I. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):209–218. doi: 10.1101/sqb.1974.039.01.027. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Gray H. B., Jr, Vinograd J. Sedimentation velocity behavior of closed circular SV40 DNA as a function of superhelix density, ionic strength, counterion and temperature. J Mol Biol. 1971 Nov 28;62(1):21–38. doi: 10.1016/0022-2836(71)90128-8. [DOI] [PubMed] [Google Scholar]
- Upholt W. B. Superhelix densities of circular DNA's: a generalized equation for their determination by the bouyant method. Science. 1977 Mar 4;195(4281):891–891. doi: 10.1126/science.190680. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interactions between twisted DNAs and enzymes: the effects of superhelical turns. J Mol Biol. 1974 Aug 25;87(4):797–816. doi: 10.1016/0022-2836(74)90085-0. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Variation of the average rotation angle of the DNA helix and the superhelical turns of covalently closed cyclic lambda DNA. J Mol Biol. 1969 Jul 14;43(1):25–39. doi: 10.1016/0022-2836(69)90076-x. [DOI] [PubMed] [Google Scholar]
- Watson R., Bauer W., Vinograd J. An optical system for the photography of fluorescent bands in preparative ultracentrifuge tubes. Anal Biochem. 1971 Nov;44(1):200–206. doi: 10.1016/0003-2697(71)90361-7. [DOI] [PubMed] [Google Scholar]
- Woodworth-Gutai M., Lebowitz J. Introduction of interrupted secondary structure in supercoiled DNA as a function of superhelix density: consideration of hairpin structures in superhelical DNA. J Virol. 1976 Apr;18(1):195–204. doi: 10.1128/jvi.18.1.195-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



