Abstract
OBJECTIVE:
The objective was to evaluate the relationship between endometrial thickness on the day of human chorionic gonadotropin administration and pregnancy outcome in in vitro fertilization cycles.
DESIGN:
This was a systematic review and meta-analysis.
MATERIALS AND METHODS:
We identified 484 articles using Cochrane library, PubMed, Web of Science, and Embase searches with various key words including endometrial thickness, pregnancy, assisted reproductive technology, endometrial pattern, and in vitro fertilization. A total of 14 studies with data on endometrial thickness and outcome were selected, representing 4922 cycles (2204 pregnant and 2718 nonpregnant). The meta-analysis with a random effects model was performed using comprehensive meta-analysis software. We calculated the standardized mean difference, odds ratio (OR), and 95% confidence intervals (CIs).
RESULTS:
There was a significant difference in the mean endometrial thickness between pregnant and nonpregnant groups (P<0.001), with a standardized mean difference of 0.4 mm (95% CI 0.22–0.58). The OR for pregnancy was 1.40 (95% CI 1.24–1.58).
CONCLUSIONS:
The mean endometrial thickness was significantly higher in pregnant women compared to nonpregnant. The mean difference between two groups was <1 mm which may not be clinically meaningful. Although there may be a relationship between endometrial thickness and pregnancy, implantation potential is probably more complex than a single ultrasound measurement can determine.
KEY WORDS: Assisted reproductive technology, endometrial pattern, endometrial thickness, in vitro fertilization, pregnancy
INTRODUCTION
Assisted reproductive technology (ART) has been commonly used in infertility treatment over the past two decades. The high cost, relatively low implantation, and increased multiple pregnancy rates in in vitro fertilization (IVF) cycles have led to a need to evaluate the predictors of success in these patients. One important factor is the endometrial receptivity.[1] In addition to the embryo quality, the receptivity of the endometrium also plays a role in the implantation process.
The standard method of endometrial dating is the histological evaluation of an endometrial biopsy specimen.[2] Indeed, this technique has allowed for the demonstration of a possible asynchrony in endometrial development in the course of cycles with ovarian stimulation for IVF when embryo transfer had to be cancelled.[3–5] Obviously, the invasiveness of endometrial biopsy is not acceptable in the clinical context of ART cycles.[6] The ability to identify a receptive uterus prospectively by a noninvasive method would have an invaluable impact on treatment efficiency and success rates following ART. The need to evaluate endometrial development encouraged the use of high-resolution ultrasonography as an alternative noninvasive method of the assessment of uterine receptivity. Several sonographic parameters have been used to assess receptivity, including endometrial thickness, endometrial pattern, and endometrial and subendometrial blood flow.[6]
The effect of endometrial thickness on the pregnancy rate in ART patients has been evaluated by many authors, with controversial results.[7–16] Using abdominal ultrasound, Glissant et al. reported a significantly thicker endometrium in conception cycles compared with nonconception cycles;[17] however, several reports using abdominal sonography gave contradictory findings.[18–20] Li et al. reported no correlation between endometrial thickness measured by abdominal ultrasound and histological dating of endometrium.[21] Some authors demonstrated a higher pregnancy rate at a certain endometrial thickness,[8,9,15,16,22] while others did not show a significant correlation between endometrial thickness and pregnancy rates in IVF patients.[10,12,13] Other authors reported a threshold of <7 and/or >14 mm which was associated with a significant reduction in the implantation and pregnancy rates.[7,11]
No conclusive cut-off value of endometrial thickness has been established in order to help clinicians in counseling the couple about the outcome. The reason for such controversy could be probably due to a relatively low number of cycles for patients with both extreme ends of endometrial thicknesses. Heterogeneity of these studies such as protocols used for controlled ovarian hyperstimulation, use of different time points and routes of ultrasonographic examination (transvaginal vs. transabdominal), and differences in the statistical evaluation of the predictive value of the endometrial thickness makes them incomparable.
Despite the fact that multiple studies investigated the endometrial thickness in ART cycles, it is still unknown whether the mean endometrial thickness in successful ART cycles is significantly greater than that of failed cycles. Therefore, the aim of our study was to determine if the endometrial thickness measured on the day of hCG administration had any effect on the outcome of IVF treatment with a long gonadotropin-releasing hormone analog (GnRHa) protocol, utilizing meta-analysis of previously published studies.
MATERIALS AND METHODS
Study identification
We identified 484 articles using Cochrane library, PubMed, Web of Science, and Embase searches with different combinations of various key words including endometrial thickness, pregnancy, assisted reproductive technology, endometrial pattern, and in vitro fertilization. Initially, a total of 38 studies with data on endometrial thickness and outcome were selected. After a second review, 14 studies were selected for a systematic review representing 4922 cycles (2204 pregnant and 2718 nonpregnant). The studies were published between 1994 and 2009. Figure 1 summarizes the selection of these articles.
Figure 1.
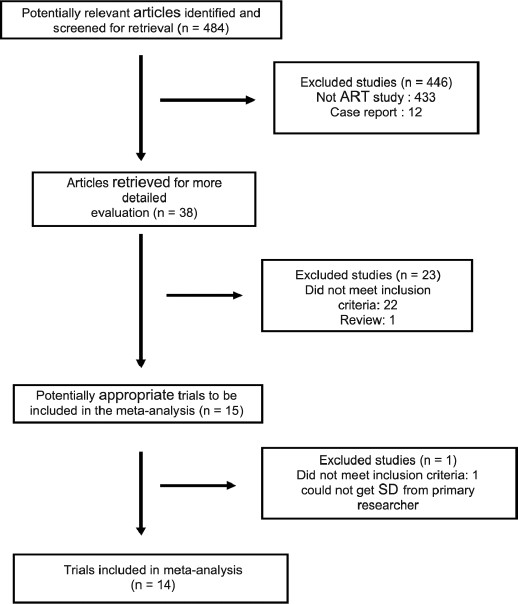
Number of selected studies and reasons for exclusion at each step of the systematic search
Inclusion criteria were as follows:
Articles in English
Measurement of endometrial thickness with transvaginal ultrasound
Measurement of endometrial thickness on the day of hCG injection
Availability of the mean of endometrial thickness on the day of hCG injection in millimeters in pregnant and nonpregnant groups
Availability of standard deviation in each group
Availability of number of cycles in each group.
Exclusion criteria were as follows:
Studies that used clomiphene citrate in their stimulation protocols
Studies that report their data as categorical data
Studies that used crypreserved embryo transfer
Statistical analysis
The meta-analysis with random and fixed effects models was performed using comprehensive meta-analysis software version 2 (Biostat, Englewood, NJ, USA). We calculated the standardized mean difference, and odds ratio (OR) with 95% confidence intervals (CIs).
RESULTS
A total of 14 studies were selected for the systematic review representing 4922 cycles (2204 pregnant and 2718 nonpregnant). The studies were published between 1994 and 2009.
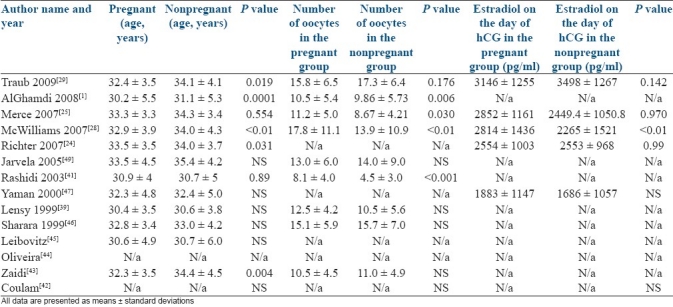
The mean age, number of oocytes retrieved, and estradiol level on the day of hCG administration for each study are presented in Table 1. Two studies did not have actual data on these parameters.
Table 1.
Age and number of oocytes retrieved and estradiol level in both groups
The mean endometrial thickness, standard deviation, and number of cycles in each study are demonstrated in Table 2. Four studies showed a statistically significant difference in the endometrial thickness between pregnant and nonpregnant groups.[1,24–26] Ten studies found no difference between two groups.
Table 2.
Author name and year, and sample size in each group
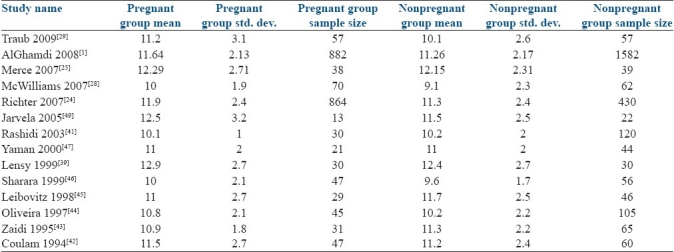
Table 3 shows the weight which was given to each study for both fixed and random effects models. Larger studies such as Al-Ghamdi and Richter were assigned 54% and 22% of the total weight in the fixed effects model, but in the random effects model these were 35% and 23%, respectively. Therefore, we chose to use the random effects model as it would allow us to avoid one or two studies skewing the results.
Table 3.
Calculated weights for each study, for mean differences in fixed and random effects models
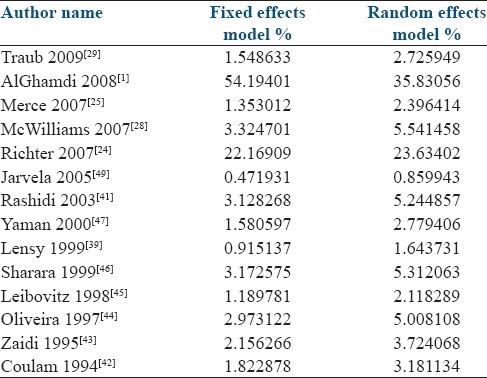
Table 4 and Figure 2 demonstrate the mean differences which were calculated for each study using the random effects models. In the random effects model, the standardized mean difference between pregnant and nonpregnant groups was 0.404 mm. The confidence interval did not include 0 (95% CI 0.226–0.582). Therefore, it was a significant increase in the endometrial thickness.
Table 4.
Differences in the mean endometrial thicknesses with 95% confidence intervals
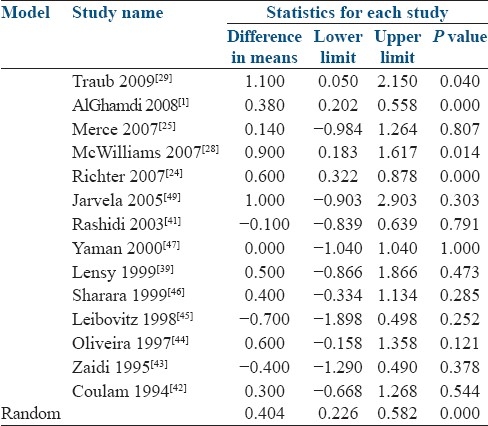
Figure 2.
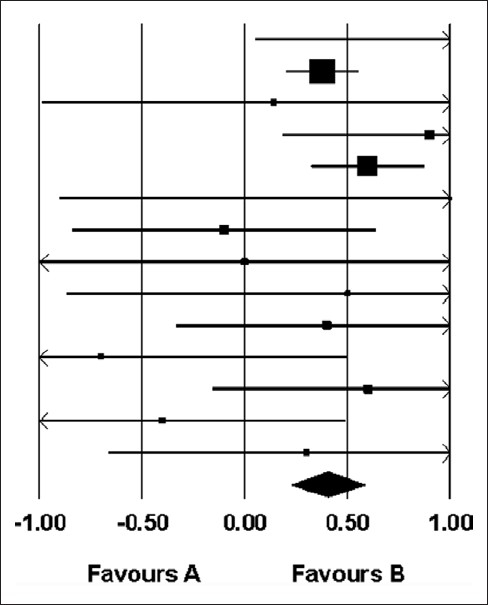
Difference in means and 95% confidence intervals
The odd ratios with 95% CI for each study and also for the random effects model are presented in Table 5 and Figure 3. The OR for pregnancy in the random effects model was 1.402 (95% CI 1.240-1.585) which was statistically significant.
Table 5.
Odds ratios with 95% confidence intervals
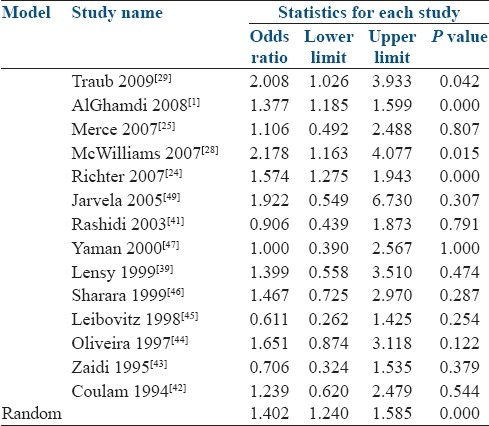
Figure 3.
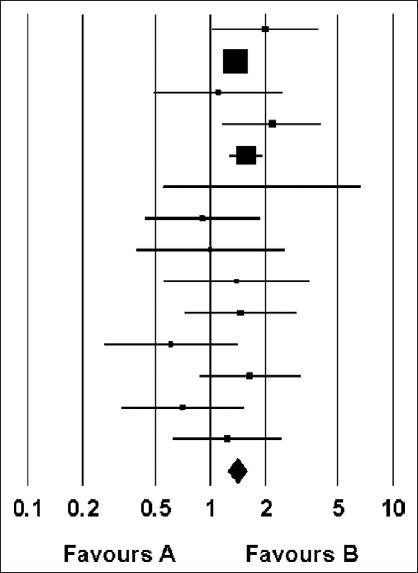
Odds ratio and 95% confidence intervals
DISCUSSION
To our best knowledge, this study is the first meta-analysis that addresses the effect of endometrial thickness on the pregnancy rate in IVF cycles with the long GnRHa protocol. Multiple studies in the literature showed that the endometrial thickness was significantly higher in pregnant women compared to nonpregnant women.[6,8,9,15–17,20,22–24,26–38] However, there are just as many studies that failed to find a significant difference.[10,12,13,18,19,25,39–70] The publication year of all these papers ranged from 1984 to 2009.
In reviewing the IVF cycles stimulated by human menopausal gonadotrophin/human chorionic gonadotrophin (HMG/hCG), Rabinowitz et al. described a daily growth of 0.5 mm starting from 3 days prior to the hCG administration up to the day of oocyte retrieval.[19] The growth continued through the luteal phase at a slower rate of 0.1 mm/day. Conception cycles were characterized by an accelerated growth compared with nonconception cycles starting 17 days after the hCG administration.[19] Imoedemhe et al. have also found a positive correlation between the endometrial thickness in the luteal phase and conception rates in IVF cycles.[23] On the other hand, Lesny et al. have reported that the maximal endometrial thickness is reached at the time of hCG injection followed by a small decrease or no increase at the time of oocyte retrieval and embryo transfer.[39]
Weisman et al. investigated the association between the endometrial thickness and the pregnancy rate by questioning whether there was a maximal value for endometrial thickness above which pregnancy was unlikely to occur.[11] They found that pregnancy rates were significantly lower above a maximum thickness of 14 mm in their patient population. Similarly, Dickey et al. reported increased biochemical pregnancy rates with an endometrial thickness >14 mm.[17] Rashidi et al. also showed no pregnancies with an endometrial thickness > 12 mm.[71] However, there are case series which reported successful pregnancies in women with an endometrial thickness ≥ 20 mm.[72,74]
A triple-layer endometrial pattern and an endometrial thickness greater than 7 mm have also been proposed as markers of endometrial receptivity but have yielded a high percentage of false-positive results.[6] However, some authors think that endometrial thickness is a distinct parameter, unrelated to the endometrial pattern on the day of hCG administration.[18,21,22,27] Several studies have evaluated the endometrial lining at different time points during the stimulation cycles. The day of hCG administration,[1,12,13,40,71] the day before hCG administration,[9,11–13,22,24,25,28,29,39,41–49,73] the day of oocyte retrieval,[13,20,26,46] and the day of embryo transfer[13,15,50,74] were used in various studies. Another factor which is also different among studies is that different treatment and stimulation protocols were applied including natural cycles with cryopreserved embryo transfer,[42,75] natural cycles with fresh embryo transfer,[40] ovarian stimulation cycles for IVF with different stimulation protocols such as long GnRHa down-regulation,[16,30,31,40,42,51–53] clomiphene citrate with HMG, short GnRHa down-regulation,[22,54] HMG only,[76] and hormone replacement therapy with oocyte donation.[19,32,42,55,77,78]
These studies used various fertility treatment regimens, endometrial thickness evaluation methods, and time points. Therefore, the study populations are extremely heterogeneous making it hard to duplicate the results. In a review by Friedler et al. published in 1996, patients also suffered from the same issues as natural cycles, fresh IVF cycles, and oocyte donation cycles with hormone replacement therapy were included.[6] Therefore, we decided to study a more homogenous study population that underwent the same type of stimulation protocol and endometrial thickness evaluation. We chose the day of hCG administration as an inclusion criteria for our systematic review, for two main reasons. First, most of the authors used that day as the preferred day for endometrial evaluation.[1,9,11–13,22,25,28,29,39,41–49,72,73] Second, that day is the best day to formulate the plan for the ongoing cycle. Among various ovarian stimulation protocols for fresh IVF cycles, the long GnRHa down-regulation protocol is internationally accepted and used by most centers as the standard of care. Therefore, we chose to analyze studies where patients underwent fresh IVF cycles with the long GnRHa protocol.
Using more homogenous study population enabled us to detect a significant difference in endometrial thicknesses between pregnant and non-pregnant groups. On the other hand, this limits the generalization of our findings. Also, we could not identify a cut-off value for endometrial thickness in our study, as studies we analyzed did not report any linear data of endometrial thickness.
Calculating endometrial volume could be an option to find differences which could be meaningful clinically. Some authors actually used endometrial volume instead of endometrial thickness for their evaluation.[47,58,79] However, more studies on endometrial volume are needed before reaching any conclusions.
In summary, a continuing use of transvaginal ultrasound to evaluate endometrial thickness and the changes occurring during ovarian stimulation can aid providers in counselling patients and predicting IVF success. It is unclear if the improved IVF success is the result of a more responsive endometrial lining or the responsiveness of the endometrial lining is only a marker of a better hormonal stimulation of the ovary with downstream effects on the endometrium. It is important to note that the correlation between endometrial thickness and pregnancy outcomes described here does not necessarily imply a causal relationship; also it is our limitation that in these studies, we cannot indentify if the endometrial thickness was taken into consideration before making the decision for hCG administration or not. The relationship may merely result from a correlation with some other confounding factors that are directly responsible for differences in receptivity such as blood flow or some other underlying machinery responsible for cyclic endometrial development. Therefore, even if the treatment protocols resulting in significant improvements in endometrial thickness are identified, such therapies may not necessarily have any clinical benefits in terms of pregnancy rates.[24]
Finally, in our systematic review, the mean endometrial thickness is significantly higher in pregnant women compared to non-pregnant. The difference between two groups is <1 mm which may not be clinically meaningful. Although there may be a relationship between endometrial thickness and pregnancy, the implantation potential is probably more complex than a single ultrasound measurement can determine.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Al-Ghamdi A, Coskum S, Al-Hassan S, Al-Rejjal R, Awartani K. The correlation between endometrial thickness and outcome of in vitro fertilization and embryo transfer (IVF-ET) outcome. Reprod Biol Endocrinol. 2008;6:37–41. doi: 10.1186/1477-7827-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frydman R, Testart J, Giacomini P, Imbert MC, Martin E, Nahoul K. Hormonal and histological study of the luteal phase in women following aspiration of the preovulatory follicle. Fertil Steril. 1982;38:312–7. doi: 10.1016/s0015-0282(16)46512-x. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JJ, Debache C, Pigeau F, Mandelbaum J, Plachot M, de Brux J. Sequential use of clomiphene citrate, human menopausal gonadotropin and human chorionic gonadotropin in human in vitro fertilization. II. Study of luteal adequacy following aspiration of the preovulatory follicles. Fertil Steril. 1984;42:360–5. doi: 10.1016/s0015-0282(16)48073-8. [DOI] [PubMed] [Google Scholar]
- 4.Garcia JE, Acosta AA, Hsiu JG, Jones HW., Jr Advanced endometrial maturation after ovulation induction with human menopausal gonadotropin/human chorionic gonadotropin for in vitro fertilization. Fertil Steril. 1984;41:31–5. doi: 10.1016/s0015-0282(16)47536-9. [DOI] [PubMed] [Google Scholar]
- 5.Friedler S, Schenker JG, Herman A, Lewin A. The role of ultrasonography in the evaluation of endometrial receptivity following assisted reproductive treatments: A critical review. Hum Reprod Update. 1996;2:323–35. doi: 10.1093/humupd/2.4.323. [DOI] [PubMed] [Google Scholar]
- 6.Forrest TS, Elyaderani MK, Kuilenburg MI, Bewtra C, Kable WT, Sullivan P, et al. Cyclic endometrial changes: US assessment with histologic correlation. Radiology. 1988;167:233–7. doi: 10.1148/radiology.167.1.3279455. [DOI] [PubMed] [Google Scholar]
- 7.Isaacs JD, Jr, Wells CS, Williams DB, Odem RR, Gast MJ, Strickler RC. Endometrial thickness is a valid monitoring parameter in cycles of ovulation induction with menotropins alone. Fertil Steril. 1996;65:262–6. doi: 10.1016/s0015-0282(16)58082-0. [DOI] [PubMed] [Google Scholar]
- 8.Noyes N, Liu HC, Sultan K, Schattman G, Rosenwaks Z. Endometrial thickness appears to be a significant factor in embryo implantation in in-vitro fertilization. Hum Reprod. 1995;10:919–22. doi: 10.1093/oxfordjournals.humrep.a136061. [DOI] [PubMed] [Google Scholar]
- 9.Rinaldi L, Lisi F, Floccari A, Lisi R, Pepe G, Fishel S. Endometrial thickness as a predictor of pregnancy after in-vitro fertilization but not after intracytoplasmic sperm injection. Hum Reprod. 1996;11:1538–41. doi: 10.1093/oxfordjournals.humrep.a019434. [DOI] [PubMed] [Google Scholar]
- 10.Yuval Y, Lipitz S, Dor J, Achiron R. The relationships between endometrial thickness, and blood flow and pregnancy rates in in-vitro fertilization. Hum Reprod. 1999;14:1067–71. doi: 10.1093/humrep/14.4.1067. [DOI] [PubMed] [Google Scholar]
- 11.Weissman A, Gotlieb L, Casper RF. The detrimental effect of increased endometrial thickness on implantation and pregnancy rates and outcome in an in vitro fertilization program. Fertil Steril. 1999;71:147–9. doi: 10.1016/s0015-0282(98)00413-0. [DOI] [PubMed] [Google Scholar]
- 12.De Geyter C, Schmitter M, De Geyter M, Nieschlag E, Holzgreve W, Schneider HP. Prospective evaluation of the ultrasound appearance of the endometrium in a cohort of 1,186 infertile women. Fertil Steril. 2000;73:106–13. doi: 10.1016/s0015-0282(99)00484-7. [DOI] [PubMed] [Google Scholar]
- 13.Bassil S. Changes in endometrial thickness, thickness, length and pattern in predicting pregnancy outcome during ovarian stimulation in in vitro fertilization. Ultrasound Obstet Gynecol. 2001;18:258–63. doi: 10.1046/j.1469-0705.2001.00502.x. [DOI] [PubMed] [Google Scholar]
- 14.Schild RL, Knobloch C, Dorn C, Fimmers R, Ven H van der, Hansmann M. Endometrial receptivity in an in vitro fertilization program as assessed by spiral artery blood flow, endometrial thickness, endometrial volume, and uterine artery blood flow. Fertil Sterill. 2001;75:361–6. doi: 10.1016/s0015-0282(00)01695-2. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs P, Matyas S, Boda K, Kaali SG. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod. 2003;18:2337–41. doi: 10.1093/humrep/deg461. [DOI] [PubMed] [Google Scholar]
- 16.Check JH, Nowroozi K, Choe J, Dietterich C. Influence of endometrial thickness and echo patterns on pregnancy rates during in vitro fertilization. Fertil Steril. 1991;56:1173–5. doi: 10.1016/s0015-0282(16)54736-0. [DOI] [PubMed] [Google Scholar]
- 17.Glissant A, de Mouzon J, Frydman R. Ultrasound study of the endometrium during in vitro fertilization cycles. Fertil Steril. 1985;44:786–90. doi: 10.1016/s0015-0282(16)49038-2. [DOI] [PubMed] [Google Scholar]
- 18.Fleischer AC, Herbert CM, Sacks GA, Wentz AC, Entman SS, James AE., Jr Sonography of the endometrium during conception and nonconception cycles of in vitro fertilization and embryo transfer. Fertil Steril. 1986;46:442–7. doi: 10.1016/s0015-0282(16)49583-x. [DOI] [PubMed] [Google Scholar]
- 19.Rabinowitz R, Laufer N, Lewin A, Navot D, Bar I, Margalioth EJ, et al. The value of ultrasonographic endometrial measurement in the prediction of pregnancy following in-vitro fertilization. Fertil Steril. 1986;45:824–8. doi: 10.1016/s0015-0282(16)49400-8. [DOI] [PubMed] [Google Scholar]
- 20.Welker BJ, Gembruch U, Diedrich K, Al-Hasani S, Krebs D. Transvaginal sonography of the endometrium during ovum pick up in stimulated cycles for in vitro fertilization. J Ultrasound Med. 1989;8:549–53. doi: 10.7863/jum.1989.8.10.549. [DOI] [PubMed] [Google Scholar]
- 21.Li TC, Nutall L, Klentzeris L, Cooke ID. How well does ultrasonographic measurement of endometrial thickness predict the results of histological dating? Hum Reprod. 1992;7:1–5. doi: 10.1093/oxfordjournals.humrep.a137538. [DOI] [PubMed] [Google Scholar]
- 22.Dickey RP, Olar TT, Curole DN, Taylor SN, Rye PH. Endometrial pattern and thickness associated with pregnancy outcomeafter assisted reproduction technologies. Hum Reprod. 1992;7:418–21. doi: 10.1093/oxfordjournals.humrep.a137661. [DOI] [PubMed] [Google Scholar]
- 23.Imoedemhe DA, Shaw RW, Kirkland A, Chan R. Ultrasound measurement of endometrial thickness on different ovarian stimulation regimens during in vitro fertilization. Hum Reprod. 1987;2:545–7. doi: 10.1093/oxfordjournals.humrep.a136586. [DOI] [PubMed] [Google Scholar]
- 24.Richter KS, Bugge KR, Bromer JG, Levy MJ. Relationship between endometrial thickness and embryo implantation, based on 1294 cycles of in vitro fertilization with transfer of two balstocyst-stage embryos. Fertil Streril. 2007;87:53–9. doi: 10.1016/j.fertnstert.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 25.Merce LT, Barco MJ, Bau S, Troyano J. Are endometrial parameters by three-dimensional ultrasound and power Doppler angiography related to in vitro fertilization/embryo transfer outcome? Fertil Stril. 2008;89:111–7. doi: 10.1016/j.fertnstert.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Gonen Y, Casper RF. Prediction of implantation by the sonographic appearance of the endometrium during controlled ovarian stimulation for in vitro fertilization (IVF) J In Vitro Fert Embryo Transf. 1990;7:146–52. doi: 10.1007/BF01135678. [DOI] [PubMed] [Google Scholar]
- 27.Check JH, Dietterich C, Graziano V, Lurie D, Choe JK. Effect of maximal endometrial thickness on outcome after frozen embryo transfer. Fertil Stril. 2004;81:1399–400. doi: 10.1016/j.fertnstert.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 28.McWilliams GD, Frattarelli JL. Changes in measured endometrial thickness predict in vitro fertilization success. Fertil Steril. 2007;88:74–81. doi: 10.1016/j.fertnstert.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 29.Traub ML, Van Arsdale A, Pal L, Jindal S, Santoro N. Endometrial thickness, Caucasian ethnicity, and age predict clinical pregnancy following fresh balstocyst embryo transfer: A retrospective cohort. Reprod Biol Endocrinol. 2009;7:33. doi: 10.1186/1477-7827-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sher G, Dodge S, Maassarani G, Knutzen V, Zouves C, Feinman M. Management of suboptimal sonographic endometrial patterns in patients undergoing in-vitro fertilization and embryo transfer. Hum Reprod. 1993;8:347–9. doi: 10.1093/oxfordjournals.humrep.a138049. [DOI] [PubMed] [Google Scholar]
- 31.Check JH, Lurie D, Dietterich C, Callan C, Baker A. Adverse effect of a homogenous hyperechogenic endometrial sonographic pattern, despite adequate endometrial thickness on pregnancy rates following in vitro fertilization. Hum Reprod. 1993;8:1293–6. doi: 10.1093/oxfordjournals.humrep.a138244. [DOI] [PubMed] [Google Scholar]
- 32.Bustillo M, Krysa LW, Coulam CB. Uterine receptivity in an oocyte donation programme. Hum Reprod. 1995;10:442–5. doi: 10.1093/oxfordjournals.humrep.a135959. [DOI] [PubMed] [Google Scholar]
- 33.Sher G, Herbert C, Maassarani G, Jacobs MH. Assessment of the late proliferative phase endometrium by ultrasonography in patients undergoing in-vitro fertilization and embryo transfer (IVF/ET) Hum Reprod. 1991;6:232–7. doi: 10.1093/oxfordjournals.humrep.a137312. [DOI] [PubMed] [Google Scholar]
- 34.Smith B, Porter R, Ahuja K, Craft I. Ultrasonic assessment of endometrial changes in stimulated cycles in an in vitro fertilization and embryo transfer program. J In Vitro Fertil Embryo Transf. 1984;1:233–8. doi: 10.1007/BF01131622. [DOI] [PubMed] [Google Scholar]
- 35.Bergh C, Hillensjo T, Nilsson L. Sonographic evaluation of the endometrium in in vitro fertilization IVF cycles.A way to predict pregnancy? Acta Obstet Gynecol Scand. 1992;71:624–8. doi: 10.3109/00016349209006231. [DOI] [PubMed] [Google Scholar]
- 36.Bohrer MK, Hock DL, Rhoads GG, Kemmann E. Sonographic assessment of endometrial pattern and thickness in patients treated with human menopausal gonadotropins. Fertil Steril. 1996;66:244–7. doi: 10.1016/s0015-0282(16)58447-7. [DOI] [PubMed] [Google Scholar]
- 37.Remohi J, Ardiles G, Garcia-Velasco JA, Gaitan P, Simon C, Pellicer A. Endometrial thickness and serum oestradiol concentrations as predictors of outcome in oocyte donation. Hum Reprod. 1997;12:2271–6. doi: 10.1093/humrep/12.10.2271. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Chen CH, Confino E, Barnes R, Milad M, Kazer RR. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2005;83:336–40. doi: 10.1016/j.fertnstert.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Lesny P, Killick SR, Tetlow RL, Manton DJ, Robinson J, Maguiness SD. Ultrasound evaluation of the uterine zonal anatomy during in-vitro fertilization and embryo transfer. Hum Reprod. 1999;14:1593–8. doi: 10.1093/humrep/14.6.1593. [DOI] [PubMed] [Google Scholar]
- 40.Ueno J, Oehninger S, Brzyski RG, Acosta AA, Philput CB, Muasher SJ. Ultrasonographic appearance of the endometrium in natural and stimulated in-vitro fertilization cycles and its correlation with outcome. Hum Reprod. 1991;6:901–4. doi: 10.1093/oxfordjournals.humrep.a137455. [DOI] [PubMed] [Google Scholar]
- 41.Rashidi BH, Sadeghi M, Jafarabadi M, Tehrani Nejad ES. Relationships between pregnancy rates following in vitro fertilization or intracytoplasmic sperm injection and endometrial thickness and pattern. Eur J Obstet Gynecol Reprod Biol. 2005;120:179–84. doi: 10.1016/j.ejogrb.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Coulam CB, Bustillo M, Soenksen DM, Britten S. Ultrasonographic predictors of implantation after assisted reproduction. Fertil Steril. 1994;62:1004–10. doi: 10.1016/s0015-0282(16)57065-4. [DOI] [PubMed] [Google Scholar]
- 43.Zaidi J, Campbell S, Pittrof R, Tan SL. Endometrial thickness, morphology, vascular penetration and velocimetry in predicting implantation in an in vitro fertilization program. Ultrasound Obstet Gynecol. 1995;6:191–8. doi: 10.1046/j.1469-0705.1995.06030191.x. [DOI] [PubMed] [Google Scholar]
- 44.Oliveira JB, Baruffi RL, Mauri AL, Petersen CG, Borges MC, Franco JG., Jr Endometrial ultrasonography as a predictor of pregnancy in an in-vitro fertilization programme after ovarian stimulation and gonadotrophin releasing hormone and gonadotrophins. Hum Reprod. 1997;12:2515–8. doi: 10.1093/humrep/12.11.2515. [DOI] [PubMed] [Google Scholar]
- 45.Leibovitz Z, Grinin V, Robia R, Degani S, Shapiro I, Tal J, et al. Assessment of endometrial receptivity for gestation in patients undergoing in vitro fertilization, using endometrial thickness and the endometrium-myometrium relative echogenecity coefficient. Ultrasound Obstet Gynecol. 1999;14:194–9. doi: 10.1046/j.1469-0705.1999.14030194.x. [DOI] [PubMed] [Google Scholar]
- 46.Sharara FI, Lim J, McClamrock HD. Endometrial pattern on the day of oocyte retrieval is more predictive of implantation success than the pattern or thickness on the day of hCG administration. J Assist Reprod Genet. 1999;16:523–8. doi: 10.1023/A:1020545120256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yaman C, Ebner T, Sommergruber M, Polz W, Tews G. Role of three dimensional ultrasonographic measurement of endometrium volume as a predictor of pregnancy outcome in an IVF-ET program: A preliminary study. Fertil Steril. 2000;74:797–801. doi: 10.1016/s0015-0282(00)01493-x. [DOI] [PubMed] [Google Scholar]
- 48.Dietterich C, Check JH, Choe JK, Nazari A, Lurie D. Increased endometrial thickness on the day of human chorionic gonadotropin injection does not adversely affect pregnancy or implantation rates following in vitro fertilization-embryo transfer. Fertil Steril. 2002;77:781–6. doi: 10.1016/s0015-0282(01)03276-9. [DOI] [PubMed] [Google Scholar]
- 49.Järvelä IY, Sladkevicius P, Kelly S, Ojha K, Campbell S, Nargung G. Evaluation of endometrial receptivity during in-vitro fertilization using three-dimensional power Doppler ultrasound. Ultrasound Obstet Gynecol. 2005;26:765–9. doi: 10.1002/uog.2628. [DOI] [PubMed] [Google Scholar]
- 50.Puerto B, Creus M, Carmona F, Civico S, Vanrell JA, Balasch J. Ultrasonography as a predictor of embryo implantation after in vitro fertilization: A controlled study. Fertil Steril. 2003;79:1015–22. doi: 10.1016/s0015-0282(02)04854-9. [DOI] [PubMed] [Google Scholar]
- 51.Serafini P, Batzofin J, Nelson J, Olive D. Sonographic uterine predictors of pregnancy in women undergoing ovulation induction for assisted reproductive treatments. Fertil Steril. 1994;62:815–22. doi: 10.1016/s0015-0282(16)57010-1. [DOI] [PubMed] [Google Scholar]
- 52.Khalifa G, Brzyski RG, Oehninger S, Acosta A, Muasher SJ. Sonographic appearance of the endometrium: The predictive value for the outcome of in-vitro fertilization in stimulated cycles. Hum Reprod. 1992;7:677–80. doi: 10.1093/oxfordjournals.humrep.a137718. [DOI] [PubMed] [Google Scholar]
- 53.Oliveira JB, Baruffi RL, Mauri AL, Petersen CG, Campos MS, Franco JG, Jr, et al. Endometrial ultrasonography as a predictor of pregnancy in an in-vitro fertilization programme. Hum Reprod. 1993;8:1312–5. doi: 10.1093/oxfordjournals.humrep.a138248. [DOI] [PubMed] [Google Scholar]
- 54.Eichler C, Krampl E, Reichel V, Zegermacher G, Obruca A, Strohmer H, et al. The relevance of endometrial thickness and echo patterns for the success of in vitro fertilization evaluated in 148 patients. J Assist Reprod Genet. 1993;10:223–7. doi: 10.1007/BF01239226. [DOI] [PubMed] [Google Scholar]
- 55.Check JH, Nowroozi K, Choe L, Lurie D, Dietterich C. The effect of endometrial thickness and echo pattern on in vitro fertilization outcome in a donor oocyte embryo transfer cycle. Fertil Steril. 1993;59:72–5. doi: 10.1016/s0015-0282(16)55617-9. [DOI] [PubMed] [Google Scholar]
- 56.Fleischer AC, Herbert CM, Hill GA, Kepple DM, Worrell JA. Transvaginal sonography of the endometrium during induced cycles. J Ultrasound Med. 1991;10:93–5. doi: 10.7863/jum.1991.10.2.93. [DOI] [PubMed] [Google Scholar]
- 57.Mardesic T, Müller P, Zetová L, Miková M, Stroufová A. Factors affecting the results of in vitro fertilization-III. The effect of the height and properties of the endometrium in the ultrasound image on the probability of implantation. Ceska Gynekol. 1995;60:3–7. [PubMed] [Google Scholar]
- 58.Schild RL, Indefrei D, Eschweiler S, Van der Ven H, Fimmers R, Hansmann M. Three-dimensional endometrial volume calculation and pregnancy rate in an in-vitro fertilization programme. Hum Reprod. 1999;14:1255–8. doi: 10.1093/humrep/14.5.1255. [DOI] [PubMed] [Google Scholar]
- 59.Csemiczky G, Wramsby H, Johannisson E, Landgren BM. Endometrial evaluation is not predictive for in vitro fertilization treatment. J Assist Reprod Genet. 1999;16:113–6. doi: 10.1023/A:1022594729197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.IJland MM, Hoogland HJ, Dunselman GA, Lo CR, Evers JL. Endometrial wave direction switch and the outcome of in vitro fertilization. Fertil Steril. 1999;71:476–81. doi: 10.1016/s0015-0282(98)00501-9. [DOI] [PubMed] [Google Scholar]
- 61.Fanchin R, Righini C, Ayoubi JM, Olivennes F, de Ziegler D, Frydman R. New look at endometrial echogenicity: Objective computer-assisted measurements predict endometrial receptivity in in vitro fertilization-embryo transfer. Fertil Steril. 2000;74:274–81. doi: 10.1016/s0015-0282(00)00643-9. [DOI] [PubMed] [Google Scholar]
- 62.Contart P, Baruffi RL, Coelho J, Mauri AL, Petersen C, Franco Júnior JG. Power Doppler endometrial evaluation as a method for the prognosis of embryo implantation in an ICSI program. J Assist Reprod Genet. 2000;17:329–34. doi: 10.1023/A:1009405128160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kupesic S, Bekavac I, Bjelos D, Kurjak A. Assessment of endometrial receptivity by transvaginal color Doppler and three-dimensional power Doppler ultrasonography in patients undergoing in vitro fertilization procedures. J Ultrasound Med. 2001;20:125–34. doi: 10.7863/jum.2001.20.2.125. [DOI] [PubMed] [Google Scholar]
- 64.Noyes N, Hampton BS, Berkeley A, Licciardi F, Grifo J, Krey L. Factors useful in predicting the success of oocyte donation: A 3-year retrospective analysis. Fertil Steril. 2001;76:92–7. doi: 10.1016/s0015-0282(01)01823-4. [DOI] [PubMed] [Google Scholar]
- 65.Maugey-Laulom B, Commenges-Ducos M, Jullien V, Papaxanthos-Roche A, Scotet V, Commenges D. Endometrial vascularity and ongoing pregnancy after IVF. Eur J Obstet Gynecol Reprod Biol. 2002;104:137–43. doi: 10.1016/s0301-2115(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 66.Zenke U, Chetkowski RJ. Transfer and uterine factors are the major recipient-related determinants of success with donor eggs. Fertil Steril. 2004;82:850–6. doi: 10.1016/j.fertnstert.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 67.Laasch C, Puscheck E. Cumulative embryo score, not endometrial thickness, is best for pregnancy prediction in IVF. J Assist Reprod Genet. 2004;21:47–50. doi: 10.1023/B:JARG.0000025937.43936.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chien LW, Lee WS, Au HK, Tzeng CR. Assessment of changes in utero-ovarian arterial impedance during the peri-implantation period by Doppler sonography in women undergoing assisted reproduction. Ultrasound Obstet Gynecol. 2004;23:496–500. doi: 10.1002/uog.975. [DOI] [PubMed] [Google Scholar]
- 69.Ng EH, Chan CC, Tang OS, Yeung WS, Ho PC. The role of endometrial and subendometrial vascularity measured by three-dimensional power Doppler ultrasound in the prediction of pregnancy during frozen-thawed embryo transfer cycles. Hum Reprod. 2006;21:1612–7. doi: 10.1093/humrep/dei502. [DOI] [PubMed] [Google Scholar]
- 70.Chen MJ, Yang JH, Peng FH, Chen SU, Ho HN, Yang YS. Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J Assist Reprod Genet. 2006;23:337–42. doi: 10.1007/s10815-006-9053-1. [DOI] [PubMed] [Google Scholar]
- 71.Gonen Y, Casper R.F, Jacobson W, Blankier J. Endometrial thickness and growth during ovrian stimulation: A possible predictor of implantation in in vitro fertilization. Fertil steril. 1989;52:446–50. doi: 10.1016/s0015-0282(16)60916-0. [DOI] [PubMed] [Google Scholar]
- 72.Quintero RB, Sharara FI, Milki AA. Successful pregnancies in the setting of exaggerated endometrial thickness. Fertil Steril. 2004;82:215–7. doi: 10.1016/j.fertnstert.2004.02.099. [DOI] [PubMed] [Google Scholar]
- 73.Basir GS, O WS, So WW, Ng EH, Ho PC. Evaluation of cycle-to-cycle variation of endometrial responsiveness using transvaginal sonography in women undergoing assisted reproduction. Ultrasound Obstet Gynecol. 2002;19:484–9. doi: 10.1046/j.1469-0705.2002.00685.x. [DOI] [PubMed] [Google Scholar]
- 74.Strohmer H, Obruca A, Radnevk M, Feichtinger W. Relationship of the individual uterine size and the endometrial thickness in stimulated cycles. Fertil Steril. 1994;61:972–5. doi: 10.1016/s0015-0282(16)56716-8. [DOI] [PubMed] [Google Scholar]
- 75.Al-Shawaf T, Yang D, al-Magid Y, Seaton A, Iketubosin F, Craft I. Ultrasonic monitoring during replacement of frozen/thawed embryos in natural and hormone replacement cycles. Hum Reprod. 1993;8:2068–74. doi: 10.1093/oxfordjournals.humrep.a137983. [DOI] [PubMed] [Google Scholar]
- 76.Abdalla HI, Brooks AA, Johnson MR, Kirkland A, Thomas A, Studd JW, et al. Endometrial thickness: A predictor of implantation in ovum recipients? Hum Reprod. 1994;9:363–5. doi: 10.1093/oxfordjournals.humrep.a138509. [DOI] [PubMed] [Google Scholar]
- 77.Shapiro H, Cowell C, Casper RF. The use of vaginal ultrasound for monitoring endometrial preparation in a donor oocytes program. Fertil Steril. 1993;59:1055–8. doi: 10.1016/s0015-0282(16)55927-5. [DOI] [PubMed] [Google Scholar]
- 78.Alam V, Bernardini L, Gonzales J, Asch RH, Balmaceda JP. A prospective study of echographic endometrial characteristics and pregnancy rates during hormonal replacement cycles. J Assist Reprod Genet. 1993;10:215–9. doi: 10.1007/BF01239224. [DOI] [PubMed] [Google Scholar]
- 79.Raga F, Bonilla-Musoles F, Casañ EM, Klein O, Bonilla F. Assessment of endometrial volume by three-dimensional ultrasound prior to embryo transfer: Clues to endometrial receptivity. Hum Reprod. 1999;14:2851–4. doi: 10.1093/humrep/14.11.2851. [DOI] [PubMed] [Google Scholar]


