Abstract
Physicians and veterinarians often prescribe oxytocin to treat dystocia. However, oxytocin administration to pregnant women or animals is not without risk. In the venue of laboratory animal medicine, the use of oxytocin may present confounding variables to research. Although oxytocin has been studied extensively, many of its physiologic effects and interactions with other hormones remain unclear. Investigator concerns about adverse and confounding effects of oxytocin in their research mice prompted the current review of oxytocin and its use to treat murine dystocia. Well-controlled studies of oxytocin in dystocic mice have not been conducted. However, in humans and other animals, inconsistent and adverse effects are well-documented. Limited knowledge of the complex physiologic and molecular mechanisms of action of oxytocin and scant support for the efficacy of oxytocin in dystocic mice fail to meet the standards of evidence-based veterinary medical practice. The administration of oxytocin is contraindicated in many cases of dystocia in research mice, and its use in dystocic mice may be unfounded. A brief review of oxytocin and the physiologic mechanisms of parturition are provided to support this conclusion. Alternative treatments for murine dystocia are discussed, and a holistic approach is advocated to better serve animal welfare and to safeguard the integrity of valuable research. Laboratory animal veterinarians overseeing the development of guidelines or standard operating procedures for technician or investigator treatment of dystocic mice should understand the effects of oxytocin administration in light of relevant research.
Dystocia is a perturbation in the normal progression of labor and has complex causes including maternal reproductive tract abnormalities; large, deformed, malposed, or dead fetuses; and primary or secondary uterine inertia.58 Dystocia can be managed medically, with uterotonic (or ecbolic) agents and assisted fetal extraction, or surgically, with delivery through Cesarean section. Dystocia is a clinically heterogeneous condition; consequently, diagnosis (and the initiation of subsequent medical or surgical intervention) can depend as much on the obstetric experience of attending medical or veterinary personnel as on objective patient characteristics. According to World Health Organization guidelines,88 a graphic depiction called a partogram (or partograph) typically is used in women to track and document labor progress, including cervical dilation, contractions, and fetal presentation.63,72 Indices on the partogram aid medical decision-making during labor; the ‘alert line’ warns physicians of possible dystocia, and the ‘action line’ signals a need for prompt clinical intervention. The value of the partogram and its components has been discussed.23 Before resorting to surgical intervention, physicians generally ensure that women have experienced adequate uterine contractions for at least 4 h, with or without oxytocin administration, and possibly longer if fetal vital signs are normal and there is some progress in descent.71
Attempts have been made to define dystocia in some nonhuman animals. The Merck Veterinary Manual2 indicates that dystocia should be suspected in dogs and cats when an animal has a history of previous dystocia or reproductive tract obstruction; parturition does not occur within 24 h after the drop in rectal temperature (to less than 100 °F); strong abdominal contractions occur for 1 to 2 h without the birth of a puppy or kitten; the dam is in active labor for 1 to 2 h without delivery of subsequent puppies or kittens; the resting period during active labor exceeds 4 to 6 h; the bitch or queen exhibits obvious signs of pain; there is black, purulent, or hemorrhagic vaginal discharge; there are signs of systemic illness; or gestation is prolonged. Dystocia is an economically important problem in cattle, and dystocia scoring systems have been developed and recently discussed.46 Cattle dystocia scoring scales are ordinal and vary from a 2-point5 to 7-point system.45 Scoring systems are in use internationally, although ‘calving ease’ is quantified in some countries whereas ‘calving difficulty’ is scored in others.
Similar guidance has not been developed to define dystocia in mice. Dystocia is a common emergency health concern in laboratory mice.28 The condition was reported in laboratory mice as early as 1967,69 well before the advent of transgenic technology, and although recent information on murine dystocia incidence is unavailable, dystocia is phenotypic in many transgenic lines and a common occurrence under the intense breeding practices used to generate genetically manipulated mice.59 Although laboratory mice are primarily nocturnal, as judged by the timing of feeding and running wheel behavior,16 and labor initiation in mice has been posited to be under circadian influences,59 recent work has shown that genetics is an important determinant of murine gestational length,52 suggesting that parturition during vivaria daytime hours is not necessarily indicative of dystocia. Mice have the ability to delay parturition when stressed,20 and laboratory mice may preferentially but not exclusively deliver during periods of low human activity in the animal facility, such as overnight and on weekends.
Oxytocin is an ecbolic agent commonly used to medically treat dystocia in humans and animals. The word oxytocin was formulated from Greek words meaning ‘quick birth’ when its uterine-contracting properties were first recognized in 1906.17 As a single agent, oxytocin is the strongest known uterotonic drug.42 Treatment of dystocia varies between laboratory mouse facilities, but oxytocin is prescribed frequently, with or without other pharmacologic agents.24,25,60,80,81 At least one institution has codified the anecdotal ‘dystocia cocktail’ of synthetic oxytocin, calcium gluconate, and crystalloid fluids, with or without an analgesic such as ketoprofen.14 Genetically manipulated mice deficient in the oxytocin system have been developed and characterized.40,54,78,89 Research addressing oxytocin's physiologic consequences has revealed that the drug has a multitude of behavioral effects and therefore could confound certain types of research. Investigator concerns about adverse and confounding effects of oxytocin in their mice have prompted a review of oxytocin and its use to treat murine dystocia. Although oxytocin may be a useful pharmacologic agent in some circumstances, its administration to dystocic research mice is often contraindicated and may be unfounded. A brief review of oxytocin and the physiologic mechanisms of parturition in mice and other species is provided to support this recommendation, and alternative and holistic treatment approaches for murine dystocia are discussed. Laboratory animal veterinarians overseeing the development of guidelines or standard operating procedures for technician or investigator treatment of dystocic mice will benefit from a reexamination of oxytocin administration in light of relevant research.
Endogenous Oxytocin and Reproduction
Oxytocin is a 9-amino-acid neurotransmitter and hormone made primarily by the pituitary gland and, variably, by reproductive tissues. It was the first hormone to be sequenced (in 195321) and subsequently was synthesized by the same group. Oxytocin is structurally similar to the nonapeptide vasopressin (antidiuretic hormone), which regulates the body's water balance; linkage between the oxytocin and vasopressin genes has been described in humans and mice.31,68 Oxytocin currently is known to have only one receptor. Peptide oxytocin antagonists such as Atosiban (Ferring Pharmaceuticals, Saint-Prex, Sweden), a tocolytic drug which is used clinically to delay premature delivery in humans, also have affinity for one of several vasopressin receptors.44 There are sex- and species-associated differences in oxytocin receptor location, and these differences may help to explain diverse responses to oxytocin.41,74 For example, female animals and women make more oxytocin and have more numerous receptors than do male. Oxytocin is catabolized by the kidneys and liver, and its circulating half-life in humans is a brief 5 min. Metabolic clearance increases during pregnancy mainly due to the formation of oxytocinase in placental tissue.79
Although the role of oxytocin in parturition in humans and animals has been extensively studied, it has not been characterized definitively (please see reference 7 for review). Reliably determining oxytocin levels during labor has proven difficult due to the hormone's short half life and pulsatile release. In addition, assays currently available to measure oxytocin are crossreactive with vasopressin and related compounds. Little is known about endogenous oxytocin's roles in the 3 stages of labor: cervical dilation, fetal expulsion, and delivery of the placenta. Synthetic oxytocin (e.g., Pitocin [Pfizer, New York, NY]) routinely is used clinically to induce uterine contractions either at the beginning of labor or to augment labor that has stalled, but the details of how exactly oxytocin exerts these effects have not been worked out. The complex interplay between oxytocin, prostaglandins, and steroid hormones appears to be important. Oxytocin increases intracellular calcium in uterine myofibrils, precipitating contraction.38 But oxytocin also appears to stimulate uterine production of prostaglandins, which are themselves uterotonic.7 Studies in litter-bearing animals such as rats have identified brief dramatic increases in plasma oxytocin associated with synchronized bursts of hypothalamic electrical activity coincident with cervical distension by each pup (the Ferguson Reflex).33,76 These results indicate a role for oxytocin in the expulsive phase of labor, but more research is needed to determine how oxytocin expedites labor and its importance in parturition across species.
Comparing Parturition between Species
One of the reasons that oxytocin's function in labor is so difficult to determine is that parturition is induced and regulated differently in different species. The relative importance of steroid hormones, prostaglandins, and oxytocin in labor initiation and progression vary widely, even among species that are closely related. Generally speaking, progesterone is associated with pregnancy and myometrial quiescence, whereas estrogen is thought to precipitate myometrial activity (labor) by inciting prostaglandin and oxytocin synthesis. In addition, estrogen is important in increasing the number and affinity of oxytocin receptors systemically and locally in the uterus and breast, variably facilitating delivery and milk letdown.61,65,76 But estrogens are not essential for parturition in all species. Estrogen receptor antagonists such as tamoxifen (such as Nolvadex [AstraZeneca, Wilmington, DE]) delay but do not abolish labor in mice and rats.26 Similarly, estrogens are not vital to the timing and process of human labor. Decreased maternal plasma progesterone and an increased estrogen:progesterone ratio is a defining feature of term gestation in mice, rats, and several other species.49 Exogenous progesterone administration can be used to delay labor in mice.32 In contrast, maternal levels of progesterone do not fall at term in humans or guinea pigs, leading some authors to propose guinea pigs as a more appropriate model for human parturition.49
Systemic (endocrine) oxytocin alone does not normally induce preterm labor in any species. Even at term, oxytocin does not reliably induce labor unless a tissue-damaging procedure (such as an amniotomy in humans) is performed also.37 Pharmacologic inhibition of oxytocin delays but does not inhibit labor in sheep, rats, guinea pigs, humans, and outbred mice,4,20,29 suggesting that oxytocin is involved in parturition across species but that it may not be essential to the process in any of them. Discovery of oxytocin gene expression in the uteri of humans and rats has generated interest in paracrine oxytocin as an initiator of labor,48 but studies remain inconclusive. There is no evidence for a paracrine oxytocin system in the mouse uterus,48,51 but oxytocin receptors increase near the end of pregnancy in many species, including mice.34
The inflammatory process is implicated in parturition, and the interaction between oxytocin and prostaglandins may be important. The induction of oxytocin receptors in the rat can be inhibited by prostaglandin blockade, with subsequent prolongation of gestation and dystocia.11,12 In addition to directly inducing uterine contraction, prostaglandins act to soften the cervix, an event essential for vaginal delivery.85 Mounting evidence suggests that prostaglandins are involved more directly than is oxytocin in the initiation of labor in many species.37 In the mouse, the luteolytic effects of prostaglandin F2α appear to be instrumental in progesterone withdrawal and the initiation of parturition.28,50
Parturition is a physiologically complex process that is regulated differently across species (see Table 1 in reference 49 for a summary). Despite voluminous research over many years, labor and the mechanism of action of oxytocin in labor are not well characterized in animals or humans. Clearly oxytocin alone does not induce parturition. The best evidence indicates that oxytocin is important in the expulsive phase of labor in some species (for example, rats); therefore the timing of exogenous oxytocin administration is likely important. In any case, mice appear to differ from humans and other species in key mechanisms of labor initiation and regulation, such that extrapolating medical treatment for dystocia from other species to mice may be inappropriate.
Genetically Manipulated Mice
The creation and study of genetically engineered oxytocin and oxytocin-receptor knockout mice has cast further doubt on the importance of oxytocin in murine parturition. Using homologous recombination, one research group eliminated the part of the oxytocin gene hypothesized to help with the packaging and transport of oxytocin.89 Surprisingly, the resulting knockout female mice (Oxt−/−) carried and delivered their young normally. Although milk was present in the mammary glands of knockout dams and pups were observed attempting to suckle, no milk was observed in the stomachs of offspring, suggesting failure of milk ejection but not milk synthesis.15 Unassisted offspring perished from starvation within 24 h, but intraperitoneal administration of oxytocin to postpartum dams 12 h after delivery induced milk letdown and rescued the phenotype. Another group created a similar mouse the same year with identical results.54 The authors injected Oxt−/− dams with oxytocin every 2 to 6 h for several days after delivery and noted milk in the stomachs of mouse pups soon after initiating treatment.54
Mice that are null for the oxytocin receptor (Oxtr−/−) deliver normally but fail to eject milk, just like oxytocin-deficient dams.78 In 2008, 2 lines of mice were created by using the Cre–Lox system; one line was null for the oxytocin receptor gene, and the other line was a conditional knockout.40 The conditional knockout mice have low levels of oxytocin receptor binding in select forebrain regions (for example, the hippocampus), whereas receptor binding in other brain regions and peripheral tissues is normal. Conditional oxytocin receptor knockout mice carry and deliver their young normally, as do other oxytocin knockouts. However, in contrast to total oxytocin and oxytocin receptor knockouts, conditional oxytocin receptor knockout mice are able to nurse productively. This finding suggests functionality of both central and peripheral (mammary) oxytocin signaling pathways for milk ejection but not for parturition.
Other genetically engineered mice have been created to explore what is essential to parturition in the mouse. Lending further support to the importance of prostaglandins is the finding that labor does not occur in mice in which genes encoding the prostaglandin F receptor are disrupted.75 Similarly, deletion of the gene for cyclooxgenase 1, responsible for the synthesis of prostaglandin F2α, prevents parturition.30 Oxytocin receptor expression does not increase at term in these mice, suggesting that oxytocin is involved also, but that prostaglandins, and not oxytocin, are the key regulator of murine parturition. It is possible that there is physiologic redundancy in the oxytocin activating system in the mouse and that related gene systems such as vasopressin are involved in parturition.9 In the knockout mice studied so far, deletion of the oxytocin system occurs before development of networks controlling reproductive functions. This outcome does not preclude the possibility of compensation by another physiologic mechanism in these animals. Selection for redundancy in physiologic mechanisms favoring successful parturition would not be unexpected. Interestingly, mice null for both oxytocin and cyclooxygenase 1 initiate labor on the day expected for normal mice, but labor is prolonged in some animals.30
Other Physiologic Effects of Oxytocin
Oxytocin has a diverse array of physiologic effects and therefore its clinical use in dystocic mice could confound certain types of research. Oxytocin's influences are widely and increasingly studied by the neurobehavioral community. A PubMed keyword search for oxytocin produced more than 20,000 hits, with approximately 650 from 2010 publications alone. Not surprisingly, the oxytocin system appears to be important in behaviors associated with reproduction. Detailed behavioral studies of Oxt−/− and Oxtr−/− postpartum mice showed that fewer knockout female mice retrieve pups when scattered, that those that do retrieve pups gather fewer pups, and that knockout female mice groom themselves and their pups less than do wildtype female mice.55,78 A recent study in women showed an association between low prepartal plasma oxytocin levels and increased risk for postpartum depression.73 In addition to its function in reproductive behaviors, oxytocin has been implicated in social memory, affiliation, aggression, and learning and memory and has been shown to have anxiolytic and antidepressant effects in humans and in animals.41,53 Interestingly, the ‘Bruce effect’ was perturbed in one line of oxytocin knockout female mice. Separated female mice aborted if reexposed to either their mate or a novel male mouse, suggesting that oxytocin is vital to mate recognition.83 In addition, oxytocin administration accelerated pair bonding in monogamous prairie voles (Microtus ochragaster), and administration of oxytocin receptor antagonists eliminated mating-induced pair bonding in female voles.35,84 Because of these significant and increasingly recognized behavioral effects, neurobehavioral investigators at the National Institute of Neurological Disorders and Stroke routinely ask that oxytocin be eliminated from treatment of their dystocic mice.
Deleterious Effects of Oxytocin in Reproduction
Although oxytocin is a useful pharmacologic agent in some circumstances and has been lauded as the ‘love drug’ for its behavioral effects, adverse outcomes have been associated with its clinical use as an ecbolic agent. Accurate dosing is important for efficacious clinical use, but recommendations for oxytocin administration are controversial in humans and animals. The potency of synthetic oxytocin is standardized according to its vasopressor activity in poultry and is expressed in USP Posterior Pituitary Units, for which 1 unit (U) approximates 2.0 to 2.2 mg of pure hormone. Oxytocin for veterinary use is typically available in 20-U/mL concentrations. Anecdotal dosages recommended for dystocic mice vary from 0.1 U8,60 to 1 U10,57 administered subcutaneously. As a peptide hormone, oxytocin is rapidly destroyed by the stomach and is not effective when given orally; buccal and nasal administration is efficacious. Intraperitoneal administration is contraindicated because it is technically difficult in gravid mice. In some mouse dystocia cases, oxytocin administration is repeated when labor does not progress, but generally speaking, lack of treatment response within a few hours precipitates euthanasia or, in valuable animals, Cesarean section. Although the 1.0-U dose for mice has been published,10,57 a controlled study of oxytocin dosing in mice could not be identified. One group has reported that low-dose oxytocin actually delays mouse labor by maintaining the corpus luteum.34 To induce or advance labor in women, synthetic oxytocin generally is administered as a continuous infusion of 1 to 6 mU/h, or 0.18 to 1.08 U every 3 h,47,67 roughly equivalent to the dosing range for mice.8,10,57,60,80 The efficacy of continuous low- compared with continuous high-dose oxytocin in women has been discussed.47,67 Although low doses sometimes were able to establish active labor and achieve vaginal delivery, high-dose treatment was more often associated with these outcomes, although high-dose oxytocin significantly increased the incidence of Cesarean section due to fetal distress. Serial, daily, low-dose injection of oxytocin has been advocated in horses due to its wide safety margin.82 Clearly, more detailed analysis of dosing levels is warranted in both humans and animals.
No matter the dose, patient evaluation before and during treatment is important, and inappropriate oxytocin administration can have deleterious consequences. Because of oxytocin's potent uterotonic effects,42 the drug should not be used to treat dystocia where obstruction of the birth canal is a prominent feature. Oxytocin administration in these cases can lead to tetanic uterine contractions, placental separation, uterine rupture, and hemorrhage.58 In addition, oxytocin can have potent hemodynamic effects, causing profound maternal vasodilation and hypotension.39,77 A recent study in women concluded that bolus oxytocin induced chest pain, transient profound tachycardia, hypotension, and myocardial ischemia.77 Patients dosed with oxytocin may experience decreased cardiac output due to an inability to increase stroke volume.39 In addition, due to the antidiuretic effects of the hormone, water intoxication with convulsions can occur,57 and patients receiving oxytocin may be at risk for pulmonary edema.19 Careful attention to fluid balance should be paid to patients receiving oxytocin. Clearly, the indiscriminate use of oxytocin can have severe consequences. Notwithstanding, a recent retrospective study concluded that oxytocin administration was undertaken in an unstructured manner in women, with insufficient dosage in some women but unnecessary treatment in others.70
Pharmacologic Treatment of Mouse Dystocia
Laboratory personnel treating dystocic mice generally are working without the benefit of diagnostic information (for example, imaging or blood work) to fully characterize the clinical condition of their patients and to rule out obstructive dystocia. Oxytocin administration may be dictated by ‘one size fits all’ standard operating procedures, which do not take individual characteristics into account. Veterinarians may or may not be consulted. When dystocic mice are found, attending personnel often have no idea how long they have been laboring. If significant time has elapsed, other factors such as fetal death (falling cortisol), inflammation, sepsis, and aberrant immune responses may be in effect, none of which can be corrected with oxytocin. Further, ‘dystocia cocktails’ in rodents often include a nonsteroidal antiinflammatory drug such as ketoprofen. Although the provision of pain relief is appropriate in cases of true dystocia, most commonly used nonsteroidal antiinflammatory drugs inhibit cyclooxygenase, which is responsible for producing prostaglandins which are essential to successful parturition in the mouse.30,75,86 Nonsteroidal antiinflammatory drugs are tocolytic and therefore contraindicated in dystocic mice, and alternative analgesic choices should be explored.
Few alternative agents to treat dystocia medically are available. Calcium's gluconate salt is often coadministered with oxytocin,14,24,60 because ionic calcium is necessary for the contraction of muscle, including myometrial smooth muscle. Furthermore, because cardiac arrhythmias due to contraction of heart muscle are a potentially serious side effect, intravenous calcium gluconate is often administered to larger animals attendant to serum calcium concentrations and coincident with frequent auscultation, neither of which are commonly gleaned in mice. Therefore, calcium administration should be undertaken carefully and avoided in mice used in studies of the cardiovascular system. Although oxytocin has been hypothesized to increase the frequency of myometrial contraction, calcium increases contraction strength,18 and because of this attribute, administration of calcium 10 to 15 min before oxytocin dosing sometimes is advocated.24,60 Both exogenous calcium and oxytocin appear to facilitate calcium-dependent myometrial contraction, suggesting overlapping and complimentary effects.43 The exact mechanism of myometrial calcium regulation and the source of the increased calcium (intracellular or extracellular) is unknown.66 Calcium and other alternatives to oxytocin use in mice need to be explored in controlled studies. Prostaglandins such as misoprostol (marketed as Cytotec [Pfizer, New York, NY]) induce luteolysis and strengthen uterine contractions, and are effective as abortifacients or labor-inducing agents in rodents and humans.13,87 In addition, cytokines such as interleukins 1 and 6 have been shown to be effective in inducing and controlling mouse labor.62,64 If oxytocin use is elected in murine research colonies, animals should be evaluated carefully before administration, veterinary staff should be consulted, and personnel should be circumspect in administering it to animals used in endocrine and neurochemical studies and those undergoing behavioral assays.
Breeding Colony Management and Veterinary Nursing Care
The clinical management of large colonies of breeding rodents should entail proactive information gathering and prestudy planning. A simple system of querying investigators about their breeding colonies and asking, in particular, if any lines seem to have problems with parturition or nursing will alert the veterinary staff to cages that should be observed more vigilantly. Various lines of mice, including those discussed following (see also Table 1 in reference 59), will be at risk for dystocia. Perusing vendor websites or performing literature searches on unfamiliar mouse lines may reveal reproductive challenges of which the investigator is unaware. In addition, conditional knockout or knockin mice treated with tamoxifen or RU486 to induce gene expression are at risk for dystocia.22,26 The IACUC should take an active role in the oversight of the breeding of genetically manipulated animals by requiring prospective gathering of information when new studies are proposed so that monitoring and action plans can be in place in advance of experimental work. In some cases, a line may be uncharacterized, or it may develop a novel phenotype under specific study conditions. Investigators have a responsibility to bring health concerns such as dystocia to the attention of the veterinary staff so that animals receive prompt care. If investigators believe that dystocia incidence is remarkable in a particular line of mice, they should monitor and document dystocia cases per number of breeding pairs and report these data to the IACUC in the annual report for the animal study proposal covering the breeding of that particular line. Intervention and endpoint plans for dystocia then can be cooperatively developed for inclusion in the animal study proposal.36
Whether vivarium or investigative personnel oversee breeding colonies, day-to-day management practices dramatically affect reproductive success. Limiting numbers of litters for female breeders will improve fertility rates and decrease colony dystocia incidence due to reproductive pathology such as uterine mineralization (and uterine adenomyosis or cystic endometrial hyperplasia in older female mice). Cystic endometrial hyperplasia is a frequent finding (as high as 100% incidence in some studies) in aged female mice and may be associated with secondary bacterial pyometra with Klebsiella oxytoca and other agents,27,56 leading to peripartum complications such as preterm birth or dystocia. In humans, recent work has shown that dystocia is more common in women whose close female relatives experienced difficult labor6 and that dystocia likely has a multiple-loci genetic component.3 Inbreeding has consistently unfavorable effects on dystocia incidence in cattle1,90 and, although unproven, may increase colony dystocia incidence in mice. Euthanasia of even mildly affected mice may be the preferred option, because animals with dystocic tendencies then are removed from the breeding pool. In addition, investigators experiencing increased dystocia in their colonies should be counseled to backcross their lines or to rederive congenic mice.
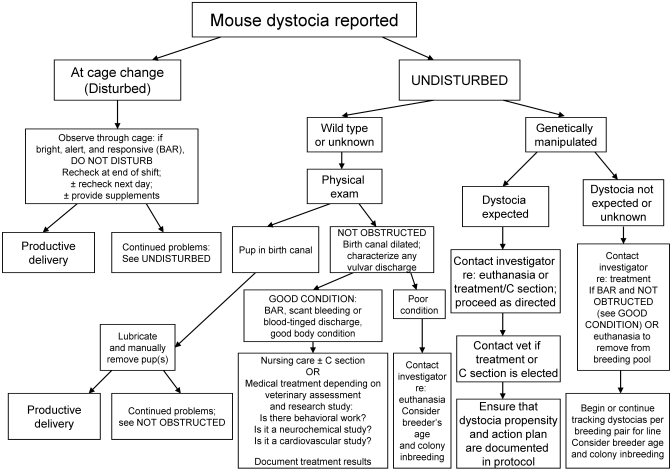
When dystocia cases are encountered in mice that cannot be euthanized, simple veterinary nursing steps may have positive benefit without potential confounding effects from pharmaceutical treatment. Like many other species, mice interrupt labor when stressed, for example, by cage change,20 and minimizing disturbance to the cage and judicious supportive care with fluids, high-calorie supplements, and thermal support sometimes can be sufficient to achieve vaginal delivery by animals that are in good condition or disrupted during parturition. Furthermore, if possible, adding material to the cage for nest building seems to provide beneficial effect. If labor does not progress in mice left undisturbed, physical exam may reveal a pup lodged in the birth canal; in these cases, generous lubrication and careful manual extraction may allow productive labor to resume. Pharmacologic treatment may be a suitable option in some cases of mouse dystocia. Controlled studies of medical treatment options need to be performed. The available evidence does not support the use of oxytocin in dystocic mice, and it certainly should not be the first-line or automatic course of action when cases of mouse dystocia present. Any medical management of dystocia in mice and other animals is appropriate only if the dam is in good condition, the cervix is dilated, and there is no evidence of obstruction or fetal distress. Veterinary advice should be sought before the initiation of pharmacologic or surgical treatment of mouse dystocia. A holistic approach to managing reproductive emergencies in research mice (Figure 1) best serves the welfare of the animals and the integrity of the research.
Figure 1.
Decision tree for management of mouse dystocia.
Conclusion
Although a widely and historically used drug, oxytocin is contraindicated in many cases of murine dystocia, and its use in mice may be unfounded. Endogenous oxytocin is not necessary for parturition in mice, and limited evidence supports its efficacy as an ecbolic agent in this species. The drug can cause pain and distress if administered without indication, and its suitability may be difficult to discern in the limited pretreatment evaluations of research mice. Finally, the numerous behavioral effects of oxytocin constitute a potent research variable. Dystocic research mice should be managed by using a comprehensive approach that considers the context of presentation, the genetic signalment of the animal, and the focus of the protocol to which the mouse is assigned. Animal evaluation by veterinary personnel should be implicit in any pharmacologic or surgical treatment of dystocic animals. As clinicians pursuing evidence-based medicine, guardians of animal welfare, and scientific support staff charged with limiting research variables, laboratory animal veterinarians should avoid automatic treatment of dystocic mice with oxytocin.
Acknowledgments
Thank you to Dr Judith Davis for significantly improving this manuscript with several astute reviews. I appreciate Dr James O'Malley's support for the completion of this project. Thank you to Clint Narver for encouraging my writing.
The views expressed in this article are solely those of the author and do not reflect the views of the National Institutes of Health or the United States Government.
References
- 1.Adamec V, Cassell BG, Smith EP, Pearson RE. 2006. Effects of inbreeding in the dam on dystocia and stillbirths in US Holsteins. J Dairy Sci 89:307–314 [DOI] [PubMed] [Google Scholar]
- 2.Aiello SE. 1998. The Merck veterinary manual, 8th ed. Whitehouse Station (NJ): Merck. [Google Scholar]
- 3.Algovik M, Kivinen K, Peterson H, Westgren M, Kere J. 2010. Genetic evidence of multiple loci in dystocia–difficult labor. BMC Med Genet 11:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonijevic IA, Douglas AJ, Dye S, Bicknell RJ, Leng G, Russell JA. 1995. Oxytocin antagonists delay the initiation of parturition and prolong its active phase in rats. J Endocrinol 145:97–103 [DOI] [PubMed] [Google Scholar]
- 5.Bar-Anan R, Heiman M, Ron M, Weller JI. 1987. Comparison of proven sires from 5 Holstein–Friesian strains in high-yield Israeli dairy herds. Livest Prod Sci 17:305–322 [Google Scholar]
- 6.Berg-Lekas ML, Hogberg U, Winkvist A. 1998. Familial occurrence of dystocia. Am J Obstet Gynecol 179:117–121 [DOI] [PubMed] [Google Scholar]
- 7.Blanks AM, Thornton S. 2003. The role of oxytocin in parturition. BJOG 110:46–51 [DOI] [PubMed] [Google Scholar]
- 8.Brower M. 2006. Practitioner's guide to pocket pet and rabbit theriogenology. Theriogenology 66:618–623 [DOI] [PubMed] [Google Scholar]
- 9.Caldwell HK, Young WS., 3rd. 2006. Oxytocin and vasopressin:genetics and behavioral implications, p 573–607. In: Lim R. Neuroactive proteins and peptides. New York (NY): Springer. [Google Scholar]
- 10.Carpenter J, Mashima T, Rupiper D. 2004. Exotic animal formulary, 3rd ed. Philadelphia (PA): Saunders. [Google Scholar]
- 11.Chan WY. 1980. The separate uterotonic and prostaglandin-releasing actions of oxytocin. Evidence and comparison with angiotensin and methacholine in the isolated rat uterus. J Pharmacol Exp Ther 213:575–579 [PubMed] [Google Scholar]
- 12.Chan WY, Chen DL. 1992. Myometrial oxytocin receptors and prostaglandin in the parturition process in the rat. Biol Reprod 46:58–64 [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Cao L, Gu ZP. 1997. [Effect of gossypol in combination with misoprostol on termination of early pregnancy in rats and mice] Yao Xue Xue Bao 32:801–807 [Article in Chinese] [PubMed] [Google Scholar]
- 14.Cornell University. [Internet]. CARE. 610.01. Mouse dystocia., [Cited 13 May 2011]. Available at: http://www.research.cornell.edu/care/documents/ACUPs/ACUP610.pdf
- 15.Crawley J. 2000. What's wrong with my mouse? New York (NY): Wiley–Liss. [Google Scholar]
- 16.Daan S, Spoelstra K, Albrecht U, Schmutz I, Daan M, Daan B, Rienks F, Poletaeva I, Dell'Omo G, Vyssotski A, Lipp HP. 2011. Lab mice in the field: unorthodox daily activity and effects of a dysfunctional circadian clock allele. J Biol Rhythms 26:118–129 [DOI] [PubMed] [Google Scholar]
- 17.Dale HH. 1906. On some physiological actions of ergot. J Physiol 34:163–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson A. 1998. Uterine monitoring during pregnancy, p 123–125. In: Proceedings of the Annual Meeting of the Society for Theriogenology. Montgomery (AL): Society for Theriogenology. [Google Scholar]
- 19.Dogdu O, Yarlioglues M, Inanc T, Ardic I, Zencir C, Kaya MG. 2011. Fatal pulmonary oedema following oxytocin administration in a pregnant woman with acute myocardial infarction. Cardiovasc Toxicol 11:74–77 [DOI] [PubMed] [Google Scholar]
- 20.Douglas AJ, Leng G, Russell JA. 2002. The importance of oxytocin mechanisms in the control of mouse parturition. Reproduction 123:543–552 [DOI] [PubMed] [Google Scholar]
- 21.Du Vigneaud V, Ressler C, Trippett S. 1953b. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem 205:949–957 [PubMed] [Google Scholar]
- 22.Dudley DJ, Branch DW, Edwin SS, Mitchell MD. 1996. Induction of preterm birth in mice by RU486. Biol Reprod 55:992–995 [DOI] [PubMed] [Google Scholar]
- 23.Dujardin B, De Schampheleire I, Sene H, Ndiaye F. 1992. Value of the alert and action lines on the partogram. Lancet 339:1336–1338 [DOI] [PubMed] [Google Scholar]
- 24.Duke University and Medical Center. [Internet]. Dystocia (difficult birth). [Cited 20 May 2011]. Available at: http://vetmed.duhs.duke.edu/AnimalDiseases.html
- 25.Dyson M. [Internet]. Oxytocin to induce parturition in mice summary. COMPMED Listserv archives. Item no. 050850 Date 2006-08-01 Time 11:50 Lines 142 Subject Oxytocin to induce parturition in mice summary. [Cited 17 November 2010]
- 26.Fang X, Wong S, Mitchell BF. 1996. Relationships among sex steroids, oxytocin, and their receptors in the rat uterus during late gestation and at parturition. Endocrinology 137:3213–3219 [DOI] [PubMed] [Google Scholar]
- 27.Fox JG, Barthold SW, Davissen MT, Newcomer CE, Quimby FW, Smith AL. 2007. The mouse in biomedical research, vol 2, 2nd ed: diseases. Burlington (MA): Academic Press. [Google Scholar]
- 28.Fox JG, Barthold SW, Davissen MT, Newcomer CE, Quimby FW, Smith AL. 2007. The mouse in biomedical research, vol 3, 2nd ed: normative biology, husbandry, and models. Burlington (MA): Academic Press. [Google Scholar]
- 29.Goodwin TM, Paul R, Silver H, Spellacy W, Parsons M, Chez R, Hayashi R, Valenzuela G, Creasy GW, Merriman R. 1994. The effect of the oxytocin antagonist atosiban on preterm uterine activity in the human. Am J Obstet Gynecol 170:474–478 [DOI] [PubMed] [Google Scholar]
- 30.Gross GA, Imamura T, Luedke CE, Vogt SK, Olson LM, Nelson DM, Sadovsky Y, Muglia LJ. 1998. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci USA 95:11875–11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hara Y, Battey J, Gainer H. 1990. Structure of mouse vasopressin and oxytocin genes. Brain Res Mol Brain Res 8:319–324 [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto H, Eto T, Endo K, Itai G, Kamisako T, Suemizu H, Ito M. 2010. Comparative study of doses of exogenous progesterone administration needed to delay parturition in Jcl:MCH(ICR) mice. Exp Anim 59:521–524 [DOI] [PubMed] [Google Scholar]
- 33.Higuchi T, Tadokoro Y, Honda K, Negoro H. 1986. Detailed analysis of blood oxytocin levels during suckling and parturition in the rat. J Endocrinol 110:251–256 [DOI] [PubMed] [Google Scholar]
- 34.Imamura T, Leudke CE, Vogt SK, Muglia LJ. 2000. Oxytocin modulates the onset of murine parturition by competing ovarian and uterine effects. Am J Physiol Regul Integr Comp Physiol 279:R1061–R1067 [DOI] [PubMed] [Google Scholar]
- 35.Insel TR, Hulihan TJ. 1995. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci 109:782–789 [DOI] [PubMed] [Google Scholar]
- 36. Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 37.Jenkin G, Young IR. 2004. Mechanisms responsible for parturition: the use of experimental models. Anim Reprod Sci 82-83:567–581 [DOI] [PubMed] [Google Scholar]
- 38.Klonoff DC, Karam JH. 1995. Hypothalamic and pituitary hormones, p 561–77. In: Katzung BG. Basic and clinical pharmacology. Norwalk (CT): Appleton and Lange. [Google Scholar]
- 39.Langesaeter E, Roseland LA, Stubhaug A. 2011. Haemodynamic effects of oxytocin in women with severe preeclampsia. Int J Obstet Anesth 20:26–29 [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd 2008. A conditional knockout line of the oxytocin receptor. Endocrinology 149:3256–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd 2009. Oxytocin: the great facilitator of life. Prog Neurobiol 88:127–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefebvre DL, Giaid A, Bennett H, Lariviere R, Zingg HH. 1992. Oxytocin gene expression in the rat uterus. Science 256:1553–1555 [DOI] [PubMed] [Google Scholar]
- 43.Ludwig M, Leng G. 2006. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7:126–136 [DOI] [PubMed] [Google Scholar]
- 44.Manning M, Cheng LL, Klis WA, Stoev S, Przybylski J, Bankowski K, Sawyer WH, Barberis C, Chan WY. 1995. Advances in the design of selective antagonists, potential tocolytics, and radioiodinated ligands for oxytocin receptors. Adv Exp Med Biol 395:559–583 [PubMed] [Google Scholar]
- 45.McClintock SE. 2004. A genetic evaluation of dystocia in Australian Holstein Friesian cattle. [Dissertation]. Melbourne (Australia): University of Melbourne. [Google Scholar]
- 46.Mee JF. 2008. Prevalence and risk factors for dystocia in dairy cattle: a review. Vet J 176:93–101 [DOI] [PubMed] [Google Scholar]
- 47.Mercer B, Pilgrim P, Sibai B. 1991. Labor induction with continuous low-dose oxytocin infusion: a randomized trial. Obstet Gynecol 77:659–663 [PubMed] [Google Scholar]
- 48.Mitchell BF, Fang X, Wong S. 1998. Oxytocin: a paracrine hormone in the regulation of parturition? Rev Reprod 3:113–122 [DOI] [PubMed] [Google Scholar]
- 49.Mitchell B F, Taggart Michael J. 2009. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 297:R525–R545 [DOI] [PubMed] [Google Scholar]
- 50.Muglia LJ. 2000. Genetic analysis of fetal development and parturition control in the mouse. Pediatr Res 47:437–443 [DOI] [PubMed] [Google Scholar]
- 51.Murphy D, Ho MY. 1995. Oxytocin transgenic mice. Adv Exp Med Biol 395:67–78 [PubMed] [Google Scholar]
- 52.Murray SA, Morgan JL, Kane C, Sharma Y, Heffner CS, Lake J, Donahue LR. 2010. Mouse gestation length is genetically determined. PLoS ONE 5:e12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neumann ID. 2008. Brain oxytocin: a key regulator of emotional and social behaviors in both males and females. J Neuroendocrinol 20:858–865 [DOI] [PubMed] [Google Scholar]
- 54.Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. 1996. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA 93:11699–11704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pedersen CA, Vadlamundi SV, Boccia ML, Amico JA. 2006. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav 5:274–281 [DOI] [PubMed] [Google Scholar]
- 56.Percy DH, Barthhold SW. 2007. Pathology of laboratory rodents and rabbits. Ames (IA): Blackwell Publishing. [Google Scholar]
- 57.Plumb DC. 2005. Oxytocin, p 849–853. In: Plumb's veterinary drug handbook. Stockholm (WS): Pharma Vet. [Google Scholar]
- 58.Pretzer SD. 2008. Medical management of canine and feline dystocia. Theriogenology 70:332–336 [DOI] [PubMed] [Google Scholar]
- 59.Ratajczak CK, Muglia LJ. 2008. Insights into parturition biology from genetically altered mice. Pediatr Res 64:581–589 [DOI] [PubMed] [Google Scholar]
- 60.Rice University. [Internet]. Dystocia (difficult birth). [Cited 29 March 2011]. Available at: http://research.rice.edu/services/arf/diseasePopup.cfm?diseaseID=9
- 61.Richter ON, Kubler K, Schmolling J, Kupka M, Reinsberg J, Ulrich U, van der Ven H, Wardelmann E, van der Ven K. 2004. Oxytocin receptor gene expression of estrogen-stimulated human myometrium in extracorporeally perfused nonpregnant uteri. Mol Hum Reprod 10:339–346 [DOI] [PubMed] [Google Scholar]
- 62.Robertson SA, Christians I, Dorian CL, Zaragoza DB, Care AS, Banks AM, Olson DM. 2010. Interleukin 6 is an essential determinant of on-time parturition in the mouse. Endocrinology 151:3996–4006 [DOI] [PubMed] [Google Scholar]
- 63.Rocha IM, de Oliveira SM, Schneck CA, Riesco ML, da Costa AS. 2009. [The partogram as an instrument to analyze care during labor and delivery] Rev Esc Enferm USP 43:880–888 [Article in Portuguese] [DOI] [PubMed] [Google Scholar]
- 64.Romero R, Mazor M, Tartakovsky B. 1991. Systemic administration of interleukin 1 induces preterm parturition in mice. Am J Obstet Gynecol 165:969–971 [DOI] [PubMed] [Google Scholar]
- 65.Rosenblatt JS, Mayer AD, Giordano AL. 1988. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology 13:29–46 [DOI] [PubMed] [Google Scholar]
- 66.Ruttner Z, Ivanics T, Slaaf DW, Reneman RS, Toth A, Ligeti L. 2002 doi: 10.1152/ajpheart.1998.275.5.H1652. In vivo monitoring of intracellular free calcium changes during uterine activation by prostaglandin F2α and oxytocin. J Soc Gynecol Investig 9:294–298. [DOI] [PubMed] [Google Scholar]
- 67.Satin AJ, Leveno KJ, Sherman ML, Brewster DS, Cunningham FG. 1992. High- versus low-dose oxytocin for labor stimulation. Obstet Gynecol 80:111–116 [PubMed] [Google Scholar]
- 68.Sausville E, Carney D, Battey J. 1985. The human vasopressin gene is linked to the oxytocin gene and is selectively expressed in a cultured lung cancer line. J Biol Chem 260:10236–10241 [PubMed] [Google Scholar]
- 69.Seamer J, Chesterman FC. 1967. A survey of disease in laboratory animals. Lab Anim 1:117–139 [Google Scholar]
- 70.Selin L, Almstrom E, Wallin G, Berg M. 2009. Use and abuse of oxytocin for augmentation of labor. Acta Obstet Gynecol Scand 88:1352–1357 [DOI] [PubMed] [Google Scholar]
- 71.Shields SG, Ratcliffe SD, Fontaine P, Leeman L. 2007. Dystocia in nulliparous women. Am Fam Physician 75:1671–1678 [PubMed] [Google Scholar]
- 72.Sizer AR, Evans J, Bailey SM, Wiener J. 2000. A second-stage partogram. Obstet Gynecol 96:678–683 [DOI] [PubMed] [Google Scholar]
- 73.Skrundz M, Bolten M, Nast I, Helhammer DH, Meinlschmidt G. 2011. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology 36:1886–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soloff MS. 1982. Oxytocin receptors and mammary myoepithelial cells. J Dairy Sci 65:326–337 [DOI] [PubMed] [Google Scholar]
- 75.Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S. 1997. Failure of parturition in mice lacking the prostaglandin F receptor. Science 277:681–683 [DOI] [PubMed] [Google Scholar]
- 76.Summerlee AJ. 1981. Extracellular recordings from oxytocin neurons during the expulsive phase of birth in unanesthetized rats. J Physiol 321:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Svanstrom MC, Biber B, Hanes M, Johansson G, Naslund U, Balfors EM. 2008. Signs of myocardial ischemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during Caesarean section. Br J Anaesth 100:683–689 [DOI] [PubMed] [Google Scholar]
- 78.Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. 2005. Pervasive social deficits, but normal parturition, in oxytocin-receptor–deficient mice. Proc Natl Acad Sci USA 102:16096–16101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thornton S, Davison J, Baylis PH. 1990. Effect of human pregnancy on metabolic clearance rate of oxytocin. Am J Physiol 259:R21–R24 [DOI] [PubMed] [Google Scholar]
- 80. Transgenic Listserv Archives. [Internet]. Dystocias in FVB transgenics. [Cited 29 March 2011]. Available at: http://www3.imperial.ac.uk/pls/portallive/docs/1/7245906.DOC.Date 2003-08-20 Subject: dystocias in FVB transgenics.
- 81. University of Virginia Center for Comparative Medicine Animal User Manual. [Internet]. Common mouse ailments-dystocia. [Cited 20 May 2011]. Available at: http;//www.medicine.virginia.edu/research/institutes-and-programs/ccm/documents/webfiles/Barrier-Training-Manual-ver-2.2.pdf Page 18.
- 82.Villani M, Romano G. 2008. Induction of parturition with daily low-dose oxytocin injections in pregnant mares at term: clinical applications and limitations. Reprod Domest Anim 43:481–483 [DOI] [PubMed] [Google Scholar]
- 83.Wersinger SR, Temple JL, Caldwell HK, Young WS., 3rd 2008. Inactivation of the oxytocin and the vasopressin (Avp) 1b receptor genes, but not the Avp 1a receptor gene, differentially impairs the Bruce effect in laboratory mice (Mus musculus). Endocrinology 149:116–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams JR, Insel TR, Harbaugh CR, Carter CS. 1994. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster). J Neuroendocrinol 6:247–250 [DOI] [PubMed] [Google Scholar]
- 85.Wilson T, Liggins GC, Whittaker DJ. 1988. Oxtocin stimulates the release of arachidonic acid and prostaglandin F2α from human decidual cells. Prostaglandins 35:771–780 [DOI] [PubMed] [Google Scholar]
- 86.Winchester SK, Imamura T, Gross GA, Muglia LM, Vogt SK, Wright J, Watanabe K, Tai HH, Muglia LJ. 2002. Coordinate regulation of prostaglandin metabolism for induction of parturition in mice. Endocrinology 143:2593–2598 [DOI] [PubMed] [Google Scholar]
- 87.Wing DA, Rahall A, Jones MM, Goodwin TM, Paul RH. 1995. Misoprostol: an effective agent for cervical ripening and labor induction. Am J Obstet Gynecol 172:1811–1816 [DOI] [PubMed] [Google Scholar]
- 88. World Health Organization Maternal and Child Health Unit. 1988. The partograph: a managerial tool for the prevention of prolonged labor. Geneva (Switzerland): World Health Organization Maternal and Child Health Unit, Division of Family Health. [Google Scholar]
- 89.Young WS, 3rd, Shephard E, Amico J, Henninghausen L, Wagner KU, LaMarca ME, McKinney C, Ginns EI. 1996. Deficiency in mouse oxytocin prevents milk ejection but not fertility or parturition. J Neuroendocrinol 8:847–853 [DOI] [PubMed] [Google Scholar]
- 90.Zaborski D, Grzesiak W, Szatkowska I, Dybus A, Muszynska M, Jedrzejczak M. 2009. Factors affecting dystocia in cattle. Reprod Domest Anim 44:540–551 [DOI] [PubMed] [Google Scholar]