Abstract
The effect of chronic daily orogastric gavage with water (5 mL/kg) on behavior and physiology was evaluated in male Sprague–Dawley rats. Treatment groups included: unmanipulated control, restraint control, dry gavage, and gavage, with all rats singly housed (n = 9 or 10 per group). In addition, a group of pair-housed rats (n = 18) was included to determine whether social housing affected response to gavage. Weekly body weights and food consumption were recorded as well as use of a nylon chew toy for enrichment. Feces were collected biweekly at the end of the light and dark phases for fecal corticoid metabolite determinations. After 28 d of treatment, animals underwent conditioned place preference testing to evaluate sensitivity to motivational properties of the anxiolytic drug chlordiazepoxide (5.6 mg/kg SC). Brain and paired adrenal gland weights were collected at necropsy. Week 2 total fecal corticosterone levels were elevated in all groups and attributed to a fire alarm accidentally tripped during building renovations. No differences occurred in body weight or food consumption between any groups. All groups used a nylon chew toy given for enrichment and demonstrated mild preference for the drug-associated chamber. Fecal weights and corticoid metabolite levels were similar between all groups at week 4 and showed normal diurnal variation. No biologically significant variations were noted in brain or paired adrenal gland to body weight ratios. We conclude that orogastric gavage of aqueous solutions at 5 mL/kg does not negatively affect the welfare of laboratory rats acclimated to handling.
In an effort to refine procedures with laboratory animals, considerable recent interest has focused on evaluating whether routine procedures are distressing to subjects or induce chronic stress. Orogastric gavage is a common technique used in rodent and nonrodent toxicology and pharmacology studies. When performed by skilled personnel, oral gavage is a rapid and efficient means of accurately delivering substances into the stomach. Although specific materials, such as oily vehicles, and high dose volumes (for example, 40 mg/kg) can induce adverse physiologic responses in rats after gavage,4 whether repeated orogastric gavage with aqueous materials induces chronic adverse stress in rats, impairing animal wellbeing is unknown.
Physiologic stress in rats can be measured in numerous ways. An increase in the relative adrenal gland:body weight ratio occurs in chronically stressed rats; however, this technique is invasive and can be applied only at necropsy.24 Heart rate and blood pressure alterations from baseline can be used to monitor short-term stress. However, whether these changes have a chronic adverse effect on animal wellbeing can be difficult to determine, given that changes are often transient, lasting less than an hour, and similar alterations in heart rate and blood pressure are often noted when performing routine husbandry procedures, such as cage changes.19 The combined use of physiologic tests to assess long-term changes in hypothalamic–pituitary–adrenal axis function and behavioral tests designed to measure anxiety has been suggested as more useful for assessing the chronic effect of a particular procedure in rats than is a single test alone.12 Fecal corticoid metabolite measurements have become an increasingly popular noninvasive method of evaluating physiologic stress responses in rodents, given that at least 85% of secreted plasma corticosterone and active metabolites are eliminated in the feces.23 Fecal levels reflect hormonal states occurring 6 to 8 h previously, eliminating variation due to handling during blood collection and minimizing variability induced by the hourly circadian fluctuations seen when evaluating plasma corticosterone levels. Samples are readily collected with minimal disruption of animal activity by placing animals in clean cages and collecting all fecal pellets eliminated during the 12-h dark and light phases over a 24-h interval. This procedure has been used to evaluate the responses of rats to routine husbandry procedures.7
Conditioned place preference testing is used in rodents to evaluate the motivational valence of various stimuli, including drugs. In this test, animals learn an association between environmental stimuli (typically a visually distinct chamber within the test apparatus) and the effects of a drug administered by the experimenter; subsequently and in a drug-free state, they emit an unlearned behavior that is motivated or directed by these stimuli.6 Drugs of abuse such as cocaine and heroin produce clear preferences,2 but when the drug is a benzodiazepine, place preference is typically much weaker.18 For example, chlordiazepoxide does not produce a strong preference in normal rats,13,21 but its motivational valence may be altered by stress and anxiety.11 Therefore, the current study explored whether the weak conditioned place preference induced by chlordiazepoxide (5.6 mg/kg SC) could be modulated by gavage and housing conditions. Rats experiencing anxiety due to acute or chronic stress would be expected to show stronger preference for chambers in which anxiolytic drugs are administered, compared with control animals, which may show only mild preference for the drug-associated chambers.
The objectives of the current study were to compare the effects of chronic orogastric gavage and the components of the technique, including restraint for gavage and placing the needle as though to gavage (dry gavage), on the behavior and physiology of singly housed male Sprague–Dawley rats, by using body weight, food consumption, biweekly fecal corticosterone levels, conditioned place preference effects to chlordiazepoxide, and selected organ:body weight ratios. In addition, because of reports indicating that pair-housed rats may experience less stress to various procedures than do singly housed animals, paired gavaged rats were included as a treatment group. Because all rats were housed in relatively barren cages for the study, the use of a nylon chew toy by the rats was evaluated also.
We expect that the results of this study will have direct application for refinement of animal use in toxicology and pharmacology studies by providing insight into what constitutes potentially distressful procedures for laboratory rats.
Materials and Methods
Animals.
Male (age, 32 to 35 d old Sprague–Dawley (CD IGS) rats were obtained from Charles River Laboratories (St Constant, Quebec, Canada). Vendor surveillance records indicated that the rats were free of known bacterial, viral, and parasitic pathogens, including pneumonia virus of mice, sialodacryoadenitis virus, Kilham rat virus, Toolan H1 virus, rat parvovirus, reovirus 3, Theiler murine encephalomyelitis virus, lymphocytic choriomeningitis virus, Bordetella bronchiseptica, Corynebacterium kutscheri, Helicobacter hepaticus, H. bilis, Clostridium piliforme, Salmonella spp., Streptobacillus moniliformis, Streptococcus pneumoniae, Pseudomonas aeruginosa, Streptococci β hemolytica, Klebsiella pneumoniae, Pasturella spp., Mycoplasma pulmonis, and common ecto- and endoparasites. Upon receipt, the 60 rats were randomized into study groups by body weight and housed singly (except for paired rats in group 5) in polycarbonate cages (approximately 48 × 27 × 20 cm) with wire lids and corncob bedding, on a 12:12-h reversed light:dark cycle (lights on at 1900), and at constant temperature (22 ± 4 °C) and relative humidity (30% to 70%). Food (Teklad Global 2014, Harlan, Indianapolis, IN) and water were provided ad libitum. Rats were provided with a 20-g plastic bone toy (Nylabone Products, Neptune, NJ) for chewing and given 1 wk to acclimate to the facility and the reversed light cycle. During this week, rats were picked up and held gently, during which time they could freely explore the gloved hands of the researcher, for approximately 30 s between 1200 and 1500 for each of 3 afternoons, to habituate the rats to human handling. At the beginning of the dark phase of collection at week 2, the facility fire alarm was set off inadvertently by a building renovation contractor, and remained audible for approximately 25 min. The alarm bell sounded at approximately 90 decibels, and the nearest bell was located immediately outside the rat housing room. The University of Guelph Animal Care Committee reviewed and approved the study protocol and the facility, and all procedures are in compliance with the Animals for Research Act of Ontario20 and the Guidelines of the Canadian Council on Animal Care.5
Experimental design.
Rats were allocated into 5 treatment groups (groups 1 through 4, n = 9 or 10 per group; group 5, n = 18) to assess each step of the orogastric gavage procedure, including unmanipulated control (group 1), restraint for gavage control (group 2), dry gavage control (group 3), gavage with 5 mL/kg of tap water (group 4), and pair-housed gavaged (group 5) groups. Although 60 animals were purchased for study, prior to study initiation, 2 rats (1 each from groups 2 and group 5) were withdrawn because of unthriftiness. Because one of these rats was in group 5, the animal's partner also was withdrawn from the study. Restraint or gavage procedures were conducted daily between 0900 and 1000 for 28 d. Restraint consisted of grasping and firmly immobilizing the rat with the head and body held vertically as though for oral gavage and holding for a count of 2 s (based on the maximal time required to gavage any single rat) before releasing the rat back into the cage. A 5-cm curved stainless steel gavage needle with 3-mm ball tip was used for gavaged rats and was wiped between animals. Personnel performing oral gavage were experienced in the procedure and refreshed their skills by practicing the technique on 2 training colony rats as often as twice daily during the week prior to study initiation. Body weight and food consumption were recorded weekly, and chew toy weights were recorded at pretest and study termination. Total feces produced over a 24-h interval (12-h light- and dark-phase samples were collected separately) were collected from each cage at the end of the acclimation (pretest) period, during week 2, and at the end of week 4, to measure fecal corticoid metabolite production. Fecal samples were weighed and stored at −20 °C before analysis. At the conclusion of the study, rats were euthanized by CO2 inhalation, and the weights of the brain and paired adrenal glands were recorded.
Conditioned place preference.
The place conditioning apparatus was made of gray PVC and comprised a smaller (23 × 30 × 26 cm) compartment between 2 large (30 × 40 × 26 cm) compartments. Removable inserts, with or without archway openings (10 × 10 cm), formed the inner walls of the large compartments and thus the center compartment. The 2 large compartments differed in visual cues: one was completely gray whereas the other had one white wall and a 10-cm white stripe along the top of 2 gray walls. The entire apparatus was covered by black wire mesh to allow automated video tracking of animals by using EthoVision (version 3, Noldus, Wageningen, The Netherlands).
The experiment consisted of 3 phases: habituation, conditioning, and test. For the habituation phase, inserts with openings were used, and rats were allowed access to the entire apparatus for 20 min. During this phase, spontaneous preference for the compartment to be paired with the drug was measured. The day after habituation, the phase of conditioning began. Inserts with openings were replaced with solid inserts to fully separate the compartments. Rats received 6 conditioning sessions in 6 consecutive days. On the first day, each rat received chlordiazepoxide HCl (5.6 mg/kg SC; Sigma-Aldrich, St Louis, MO) or the equivalent volume of saline (vehicle) and was confined in 1 of the 2 large compartments for 30 min. The following day, the same rat received a vehicle (or chlordiazepoxide) injection and was confined in the other large compartment for 30 min. In total, each rat received 3 drug–compartment and 3 vehicle–compartment pairings. The specific compartment chosen to be associated with chlordiazepoxide and the order of vehicle or drug injections were counterbalanced across subjects. The last phase included the test of conditioned place preference, which occurred the day after the last conditioning session. For this test, the solid inserts were replaced by those with the openings, and rats were given access to all 3 compartments for 20 min under drug-free conditions.
Total fecal corticoid analyses.
For fecal corticoid determination, all feces produced during a light or dark period over 24 h were collected and weighed at pretest, week 5, and week 10 of the study. Samples were frozen at −20 °C until extracted. Extraction followed a previously described technique.9 Briefly, samples were dried for 2 h at 30 °C, weighed, and pulverized, and a 0.2-g sample was removed for extraction. To the fecal sample, 0.8 mL water and 5 mL dichloromethane were added, and samples were vortexed for 30 s in 5-s pulses. Samples were centrifuged for 15 min at 1690 × g. The dichloromethane (bottom) fraction was transferred and washed with 1 mL 0.1M NaOH by vortexing for 10 s, followed by centrifugation for 10 min at 1690 × g. The dichloromethane fraction was transferred and washed twice with water, centrifuged, and again transferred to a fresh tube. Of the final dichloromethane fraction, 1 mL was transferred, evaporated to dryness under N2 for approximately 15 min, and stored at –20 °C until analyzed. Samples were resuspended in 1 mL 95% ethanol, vortexed, and diluted 1:25 with kit assay buffer. Cortisol concentration was determined by using the Correlate EIA Kit (Assay Designs, Ann Arbor, MI) according to manufacturer's instructions. ELISA plates were read at 405 nm on a plate reader (PowerWave XS, BioTek, Winooski, VT). Concentration was determined as percentage bound by using a standard curve ranging from 32 to 20,000 pg/mL (kit sensitivity is 27 pg/mL). Values were expressed based on the total feces collected over a time period and as nanograms corticosterone per gram of feces. The assay kit has 28.6% crossreactivity with deoxycorticosterone and desoxycorticosterone, metabolites of corticosterone. Therefore, the values measured and reported largely represent corticosterone and these metabolites. Although the term ‘fecal corticoid metabolites’ more accurately reflects the assay outcome, the term ‘total corticosterone’ has been used in figures for the sake of brevity.
All samples were run in duplicate, and samples from different test periods were randomized to ELISA plates. The intraassay coefficient of variation was 3%, and the interassay coefficient of variation was 10%. A linear regression performed on the standard concentration to percentage corticoid bound curve demonstrated excellent linearity (R2 ≥ 0.97).
Statistical methods.
Descriptive results are expressed as mean ± SE. For comparing fecal corticosterone levels on different days, between-group comparisons were analyzed by using 2-factor mixed-design ANOVA. A probability level of 0.05 or less was considered significant. Body weight and organ weight ratios and nylon bone usage were evaluated by single-factor ANOVA followed by a post hoc Tukey–Kramer test.25 For conditioned place preference results, a preference score (percentage) was calculated by dividing the time spent in the chlordiazepoxide-paired chamber by the total time spent in the 2 large compartments. Preference scores at habituation and at test were compared between groups by using 2-factor mixed-design ANOVA with an α value of 0.05.
Results
Clinical signs.
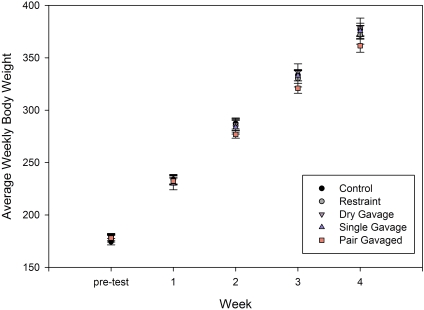
All rats continued to grow and gain weight over the 4 wk of the study, with no significant differences in total body weights (Figure 1), weekly food consumption (data not shown), or rate of body weight gain (data not shown) between rats in any of the groups by the end of week 4. No adverse events were noted after restraint or orogastric gavage, and rats appeared to acclimate rapidly to the procedures. Chew toys were used by 7 to 9 animals in all single-housed groups and by 8 of 9 pairs (Figure 2), with 10% to 20% of nylon bone weight loss from pretest (Table 1) and a mean bone weight of 16.2 ± 0.5 g after week 4. There were no significant differences in the amount of bone chewed between treatment groups (F[4,44] = 2.58, P = 0.58).
Figure 1.
Weekly body weights (mean ± SE) of male Sprague–Dawley rats throughout the gavage study.
Figure 2.

Typical nylon chew toy usage by a male Sprague–Dawley rat after 4-wk gavage study. Normal unused toy is smooth and shiny.
Table 1.
Weight (g; mean ± SE) and use of nylon chew toy by male Sprague–Dawley rats during 4-wk gavage study
| No. of chew toys | ||||
| Treatment group | Chew toy weight after week 4 (g) | Provided | Not used | |
| Control | 16.0 ± 1.6 | 10 | 3 | |
| Restraint only | 17.9 ± 0.5 | 10 | 3 | |
| Dry gavage | 15.6 ± 1.2 | 10 | 2 | |
| Gavage—single-housed | 16.5 ± 1.0 | 10 | 3 | |
| Gavage—pair-housed | 15.2 ± 1.5 | 9 | 1 | |
Mean pretest chew toy weight was 20 g for all groups.
Conditioned place preference test.
Figure 3 represents changes in preference scores from habituation to test in the various groups. The ANOVA demonstrated a significant effect of test (F[1, 53] = 10.07, P < 0.01) but no effect of group and no significant interaction. Therefore, as a result of conditioning, there was an overall significant increase in preference for the chlordiazepoxide-paired compartment, but this effect was equivalent in the various groups. In addition, considering that a preference score of 50% represents no preference, Figure 3 reveals that chlordiazepoxide-associated conditioned place preference was relatively weak across all groups.
Figure 3.
Changes in conditioned place preference scores from habituation to test in male Sprague–Dawley rats after 4-wk gavage study. *, Time spent in drug-paired compartment is significantly (P < 0.05) greater than that in the saline-paired compartment.
Fecal corticosterone levels.
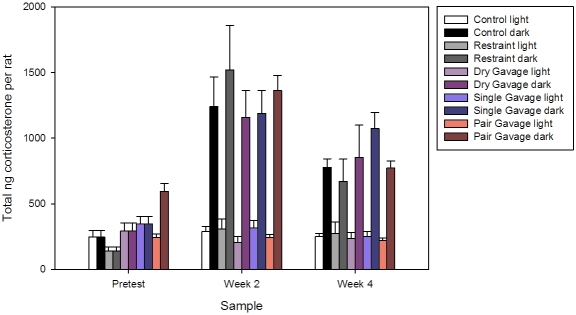
Total corticosterone results for the biweekly evaluation periods occurring over the course of the 4-wk gavage trial are shown in Figure 4. Normal circadian variation in corticosterone levels was seen between dark and light periods at each of the time points for weeks 2 and 4 but not at pretest. There were no significant differences in fecal corticosterone levels within or between any groups at any time point during the light phases. The total fecal corticosterone dark-phase value obtained at pretest from paired rats was significantly (P < 0.05) elevated compared with those for other groups. Total corticosterone levels were significantly (P < 0.05) increased during the dark phase for animals in all groups at week 2, compared with similar phase values obtained at pretest and at week 4; however, there were no between group differences (F[4, 80] = 0.55, P = 0.70). Results were almost identical when fecal corticoid levels were calculated per gram of feces (data not shown).
Figure 4.
Total fecal corticosterone values (mean ± SE) for male Sprague–Dawley rats after 4 wk of oral gavage. *, Value significantly (P < 0.05) increased compared with other pretest dark-phase values.
Organ:body weight ratios.
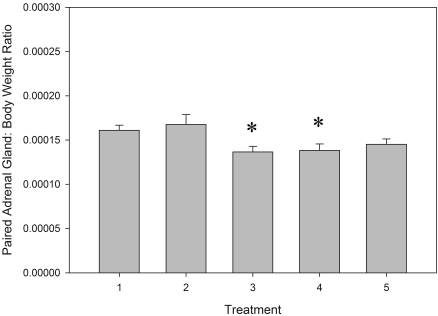
The dry gavage and single-housed gavage rats had mild but significant (F[4, 51] = 3.13, P < 0.02) decreases in paired adrenal gland:body weight ratios, compared with control animals (Figure 5). No significant difference was found in brain:body weight ratios between groups (data not shown; F[4,52] = 1.44, P = 0.233).
Figure 5.
Paired adrenal gland:body weight ratios (mean ± SE) for male Sprague–Dawley rats after 4 wk of oral gavage, where 1 = control group, 2 = restraint alone group, 3 = dry gavage, 4 = gavage-singly housed, and 5 = gavage-pair housed. *, Value significantly (P < 0.02) decreased compared with other paired adrenal gland:body weight group values.
Discussion
The results of the current study suggest that chronic orogastric gavage of rats with aqueous solutions at 5 mL/kg is readily tolerated and does not negatively affect their welfare. These findings are based on similarities in growth rates over the 4 wk of the study, fecal corticosteroid metabolite output, use of in-cage enrichment, and a lack of motivational influence of the anxiolytic drug, chlordiazepoxide, to induce a difference in conditioned place preference between groups. Socially housed rats did not respond differently to gavage compared with single housed rats. Although minor but significant differences were found between paired adrenal gland:body weight ratios for the dry gavage and singly housed gavaged rats, the overall range of relative adrenal gland weights obtained in this study are within the normal range seen for Charles River Sprague–Dawley rats of this age16 and were interpreted to be biologically insignificant.
Furthermore, the current study found that provision of a nylon chew toy was an accepted enrichment item for male Sprague–Dawley rats, given that 70% to 89% of the bones were used during the 5 wk that rats had access to them, and on average, 3.8 g of the bone was gnawed. Paired rats did not gnaw proportionately more of the bones than did singly housed animals. Because chronically stressed rats show less interest in their environments,14,15 these findings indirectly support that singly and pair-housed animals were not chronically stressed and adapted equally well to the routines of this study.
A recent study evaluating the effect of gavaging different volumes of barium sulfate to rats demonstrated that heart rate and blood pressure were elevated above baseline for 30 min after gavage whereas body temperature was elevated for as long as 1 h afterward, regardless of whether rats received dry gavage treatment or 4, 10, or 40 mL/kg barium sulphate.3 In that study, rats were not handled except for cage changing, there were no restraint-alone controls, and the duration that the catheter needle was inserted was indicated to last between 15 to 50 s. These times seem quite lengthy, given that the gavage and restraint procedures in the current study, when performed by technically skilled personnel, lasted approximately 2 s. Other research groups have demonstrated that restraint alone can be as stressful for rodents, as indicated by heart rate and body temperature,8 as concurrently performed procedures such as tail snipping and ear notching, suggesting that restraint should be minimized when performing procedures. The difference in restraint and procedural time may account for conclusion differences with the barium sulfate study and the present one. The present study did not measure acute physiologic responses to gavage but instead evaluated chronic responses to daily oral gavage. Habituation of rats to handling during the week prior to study initiation did subjectively improve ease of animal handling in the current study.
The typical 2- to 3-fold variation in nocturnal–diurnal levels of fecal corticiosterone excretion27,28 was noted only during the second and third fecal corticosterone sampling periods but was absent in the first sampling period after the initial week of acclimation. In paired rats, there was a mild increase in the dark-phase fecal corticosterone levels at pretest, but the levels were still lower than anticipated. This finding suggests that rats may not have been fully acclimated to the reversed light cycle prior to study initiation and that paired animals may acclimate faster to light cycle changes than do singly housed rats. Therefore rats housed under conditions with a reversed light cycle may require more than 7 d of acclimation to fully acclimate to these conditions prior to experimental manipulation.
A potential reason for the marked increase in fecal corticosterone values obtained after the second sampling period in all groups was the loud and persistent noise of the fire alarm during the early part of the dark phase. Other studies have demonstrated that loud, persistent noises in the vivarium, such as white noise at 90 dB or rock music, elicit short term changes in adrenal gland ultrastructure, immune system function, gastrointestinal permeability, heart rate, and blood pressure in rats.1,10,22,26 Fecal corticosterone values reflect changes in systemic levels occurring in the previous 6 to 12 h,23 and the alterations noted in the current study occurred during this time frame for the second collection period. Other studies similarly have demonstrated that fecal corticosterone assay can be used to monitor acute procedures or incidents in laboratory rats.7
Efforts to refine procedures with laboratory animals within the research and welfare communities require biologically meaningful measurement and evaluation of systemic effects, rather than subjective or anthropomorphic assessment.17 Daily oral gavage of rodents is an important dosing technique that is used to precisely administer a fixed volume of material to research subjects. Voluntary consumption of dosing substances is always preferred; however, this option may not be possible if all animals must receive a complete dose, large numbers of animals must be accurately and precisely dosed within a fixed period of time, or a substance cannot be administered into the mouth because of palatability issues. In these cases, research personnel can be confident that, when performed correctly, oral gavage does not induce chronic stress in rats.
In conclusion, the findings of this study suggest that daily orogastric gavage of male, Sprague–Dawley rats with 5 mL/kg of aqueous material is not chronically stressful when performed by skilled personnel in rats that have been acclimated to handling. Furthermore, small nylon bones can be used successfully as enrichment chew toys for rats.
Acknowledgments
We thank Jutta Hammermueller and Andrew Junkin for technical support throughout this study. Elizabeth Vaughn was supported by an Ontario Veterinary College Summer Leadership Scholarship.
References
- 1.Baldwin AL. 2005. Effect of noise on rodent physiology. Int J Comp Psychol 20:134–144 [Google Scholar]
- 2.Bardo MT, Rowlett JK, Harris MJ. 1995. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev 19:39–51 [DOI] [PubMed] [Google Scholar]
- 3.Bonnichsen M, Dragsted N, Hansen AK. 2005. The welfare impact of gavaging laboratory rats. Anim Welf 14:223–227 [Google Scholar]
- 4.Brown AP, Dinger N, Levine BS. 2000. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci 39:17–21 [PubMed] [Google Scholar]
- 5.Canadian Council on Animal Care. [Internet]. Standards and Guidance. [Cited 12 December 2012]. Available at: http://ccac.ca/en_/standards.
- 6.Calcagnetti DJ, Schechter MD. 1993. Extinction of cocaine-induced place approach in rats: a validation of the ‘biased’ conditioning procedure. Brain Res Bull 30:695–700 [DOI] [PubMed] [Google Scholar]
- 7.Cavigelli SA, Guhad FA, Ceballos RM, Whetzel CA, Nevalainen T, Lang CM, Klein LC. 2006. Fecal corticoid metabolites in aged male and female rats after husbandry-related disturbances in the colony room. J Am Assoc Lab Anim Sci 45:17–21 [PubMed] [Google Scholar]
- 8.Cinelli P, Rettich A, Seifert B, Bürki K, Arras M. 2007. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim 41:174–184 [DOI] [PubMed] [Google Scholar]
- 9.Eriksson E, Royo F, Lyberg K, Carlsson H-E, Hau J. 2004. Effect of metabolic cage housing on immunoglobulin A and corticosterone excretion in faeces and urine of young male rats. Exp Physiol 89:427–433 [DOI] [PubMed] [Google Scholar]
- 10. Fagan KD, Shinsako J, Dallman MF. 1983. Effects of housing and chronic cannulation on plasma ACTH and corticosterone in the rat. Am J Physiol 245: E515–E520. [DOI] [PubMed]
- 11.File SE. 1986. Aversive and appetitive properties of anxiogenic and anxiolytic agents. Behav Brain Res 21:189–194 [DOI] [PubMed] [Google Scholar]
- 12.Foltz C, Carbone L, DeLong D, Rollin BE, Van Loo P, Whitaker J, Wolff A. 2007. Considerations for determining optimal mouse caging density. Lab Anim (NY) 36:40–49 [DOI] [PubMed] [Google Scholar]
- 13.Grella SL, Levy AM, Campbell A, Djazayer Si, Allen C, Goddard B, Leri F. 2011. Oxycodone dose-dependently imparts conditioned reinforcing properties to discrete sensory stimuli in rats. Pharmacol Res. 64:364–70 [DOI] [PubMed] [Google Scholar]
- 14.Katz RJ, Roth KA, Carroll BJ. 1981. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev 5:247–251 [DOI] [PubMed] [Google Scholar]
- 15.Korte SM. 2001. Corticoids in relation to fear, anxiety, and psychopathology. Neurosci Biobehav Rev 25:117–142 [DOI] [PubMed] [Google Scholar]
- 16.Lang PL, White WJ. 1994. Growth, development, and survival of the Crl:CD(SD)BR stock and CDF(F344)/CrlBR strain, p 588–608. In: Dungworth DL, Mohr U, Capen CC. Pathobiology of the aging rat, vol 1. Washington (DC): International Life Sciences Institute. [Google Scholar]
- 17.Langkilde T, Shine R. 2006. How much stress do researchers inflict on their study animals? A case study using a Scincid lizard, Eulampus heat wolei. J Exp Biol 209:1035–1043 [DOI] [PubMed] [Google Scholar]
- 18.Leri F, Franklin KB. 2000. Effects of diazepam on conditioned place preference induced by morphine or amphetamine in the rat. Psychopharmacology (Berl) 150:351–360 [DOI] [PubMed] [Google Scholar]
- 19.Õkva K, Tamoševiciute E, Cižiute A, Pokk P, Rukšenas O, Nevalainen T. 2006. Refinements for intragastric gavage in rats. Scand J Lab Anim Sci 33:243–252 [Google Scholar]
- 20.Ontario Ministry of Agriculture and Food.[Internet]. Animals for Research Act. R.R.O. 1990, Regulation 24 research facilities and supply facilities. [Cited 12 December 2011]. Available at: http://www.e-laws.gov.on.ca/html/regs/english/elaws_regs_900024_e.htm
- 21.Parker LA, Limebeer CL, Simpson GR. 1998. Chlordiazepoxide-induced conditioned place and taste aversion learning in rats. Pharmacol Biochem Behav 59:33–37 [DOI] [PubMed] [Google Scholar]
- 22.Pellegrini A, Soldani P, Gesi M, Lenzi P, Natale G, Paparelli A. 1997. Effect of varying noise stress duration on rat adrenal gland: an ultrastructural study. Tissue Cell 29:597–602 [DOI] [PubMed] [Google Scholar]
- 23.Pihl L, Hau J. 2003. Faecal corticosterone and immunoglobulin A in young adult rats. Lab Anim 37:166–171 [DOI] [PubMed] [Google Scholar]
- 24.Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F. 2005. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res 1040:55–63 [DOI] [PubMed] [Google Scholar]
- 25.Smith RA. 1971. The effect of unequal group sizes on Tukey's HSD procedure. Psychometrika 36:31–34 [Google Scholar]
- 26.Sobrian SK, Vaughn VT, Ashe WK, Markovic B, Djuric V, Jankovic BD. 1997. Gestational exposure to loud noise alters the developmental and postnatal responsiveness of humoral and cellular components of the immune system in offspring. Environ Res 73:227–241 [DOI] [PubMed] [Google Scholar]
- 27.Thanos PK, Cavigelli SA, Michaelides M, Olvet DM, Patel U, Diep MN, Volkow ND. 2009. A noninvasive method for detecting the metabolic stress response in rodents: characterization and disruption of the circadian corticosterone rhythm. Physiol Res 58:219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmermann E, Critchlow V. 1967. Effects of diurnal variation in plasma corticosterone levels on adrenocortical response to stress. Proc Soc Exp Biol Med 125:658–663 [DOI] [PubMed] [Google Scholar]