Abstract
Most mice used in research are purchased devoid of specific pathogens. Experimental studies required us to evaluate the profile of infective agents harbored in mice sold as pets or food for captive reptiles. Anecdotal reports regarding disease in these mice abound, but there are few published reports on disease prevalence. Purchasers are unaware of the potential zoonotic or adventitious infections carried by these mice. This survey investigated the prevalence of ectoparasites, endoparasites, and viral, bacterial, and fungal agents carried by apparently healthy mice (n = 18) obtained from 6 pet stores in New York City, with an emphasis on those pathogens with zoonotic potential. Serology revealed the presence of antibodies to numerous murine specific viral agents in most mice tested. Ectoparasites were present on most mice. Examination of intestinal contents revealed nematode and cestode parasites, including a potential cause of human cestodiasis, Rodentolepis nana. A multidrug-resistant β-hemolytic Enterococcus faecium was isolated from the skin of mice from a single pet store; this organism causes community-acquired infections in humans. This study confirms that pet-store mice are exposed to or carry numerous pathogens that are excluded from laboratory rodent colonies. The potential for laboratory animal personnel to serve as mechanical vectors of unwanted infective agents likely is increased when these persons handle pet-store mice at home.
House mice, Mus musculus, are a recognized carrier of various infective agents. These agents may have zoonotic potential or be a threat to laboratory animal facilities, which often house SPF research mice. Infective agent contamination of laboratory mouse colonies may be associated with wild mice infesting personnel's homes or various facility locations including loading docks. In addition, escaped laboratory (feral) mice that establish colonies in interstitial and other building spaces may pose a risk to SPF colonies. Recent work demonstrates that urban wild mice are not a likely source for many agents found in laboratory mice.17 Wild mice pose an increased risk for zoonotic disease, such as from lymphocytic choriomeningitis virus.1,25
Another population to consider is mice purchased from pet stores as pets or as food for captive reptiles. Although presumed not to pose a threat to human health, reports exist of pet store mice carrying zoonotic agents such as lymphocytic choriomeningitis virus or Streptobacillus moniliformis.1,9 In addition, pet-store mice might carry pathogens of concern to laboratory mouse colonies. Personnel can act as mechanical vectors for disease transmission to laboratory animals.24 A research project required us to use pet-store mice. The current comprehensive survey investigated the prevalence of ectoparasites, endoparasites, and viral, bacterial, and fungal organisms of apparently healthy mice obtained from pet stores in the New York City area, with an emphasis on those pathogens with zoonotic potential.
Materials and Methods
Animal use was approved by Memorial Sloan Kettering Cancer Center's Institutional Animal Care and Use Committee. Mice (13 female; 5 male) of unknown genetic background, age, and health status were purchased from 6 regional or national pet-store chains in New York City, NY; 3 mice were purchased from each location. Several pet stores cohoused male and female mice, and verification of sex was not feasible prior to purchase. Mice were transported in sealed, ventilated (passive) containers from the place of purchase to the Laboratory of Comparative Pathology at our institution. Mice were euthanized immediately by CO2 asphyxiation, and blood was collected aseptically by cardiocentesis.
Serologic and PCR analysis.
Serologic evaluation of samples was performed by ELISA (Laboratory of Comparative Pathology, NY) using commercially available reagents (Charles River Laboratories, Wilmington, MA) for the following agents: mouse hepatitis virus, Sendai virus, Theiler murine encephalomyelitis virus, pneumonia virus of mice, mouse parvovirus 1 and 2, minute virus of mice, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, rotavirus, ectromelia, reovirus 3, K virus, adenovirus, polyoma virus, Encephalitozoon cuniculi, cilia-associated respiratory bacillus, murine cytomegalovirus, Hantaan virus, and Clostridium piliforme. An immunofluorescent assay was used to test for mouse thymic virus. Positive results were confirmed through multiplexed fluorometric immunoassay or immunofluorescent assay (Charles River Research Animal Diagnostic Services). PCR testing for lymphocytic choriomeningitis virus was done on frozen kidney sections pooled by pet store of origin (Research Animal Diagnostic Laboratory, Columbia, MO). Nasal aspirates and swabs were submitted for PCR testing for S. moniliformis (Research Animal Diagnostic Laboratory).
Parasitology.
A no. 10 scalpel blade was used to scrape a 1-cm2 area of skin in 3 locations: scalp between and immediately caudal to the ears, midline dorsal thoracolumbar junction, and midline ventrum at the level of the inguinal canal.23 Hair samples were transferred to a 2 × 2 cm piece of cellophane tape and affixed to a microscope slide for evaluation. An anal tape test was performed by firmly placing and removing a 2 × 2 cm piece of cellophane tape over the perineum. This tape was affixed to a microscope slide for evaluation. All microscopic evaluations were performed under 40× magnification. The pelt was removed en bloc, placed into a culture dish (100 × 15 mm, Fisher Scientific, Waltham, MA), and examined for ectoparasites under a dissecting microscope at 20× magnification. Pelt hair was examined for ectoparasites by using 2 wooden applicator sticks to separate the hair in a continuous grid pattern progressing cranial to caudal.
Pooled fecal pellets (6 to 10) were evaluated by flotation for each store group. Intestinal contents were flushed gently with sterile saline and the contents collected and evaluated at 20× magnification by one of the authors (GSR). All other parasitology examinations were performed by a single experienced medical technologist except for fecal flotations, which were examined by a different experienced medical technologist, and skin scrapes, which were examined by an experienced medical technologist and veterinarian.
Microbiology.
Skin and fecal swabs (BactiSwab NPG, Remel, Lenexa, KS) were taken from each mouse and evaluated and cultured for aerobic bacteria and fungi (skin only). Skin was swabbed along the head, dorsum, and ventrum. A 1-cm midline, ventral incision was made along the cervical neck to visualize the trachea. Bronchoalveolar lavage fluids were collected by flushing 0.1 mL tryptic soy broth (Becton Dickinson, Franklin Lakes, NJ) caudad from the cervical trachea into the lungs and aspirating. Nasal aspirates were obtained by flushing 0.1 mL tryptic soy broth through a 25-gauge needle craniad into the cervical trachea and aspirating when broth was noted at the nares. Oropharyngeal swabs were obtained by passing a swab through the mouth and oral cavity into the oropharynx. Samples from each site were pooled by pet store of origin and submitted for aerobic culture and sensitivity. Tympanic bullae were visualized after removal of the pelt, opened ventrally, and swabbed. Swabs were submitted for aerobic culture and sensitivity testing. After pelt removal, sterile instruments were used to open the peritoneal cavity and small sections of spleen and liver were collected aseptically. These samples were pooled together by pet store of origin and submitted for aerobic and anaerobic culture and sensitivity testing.
Aerobic cultures were inoculated on trypticase soy agar with 5% sheep blood, chocolate II agar (GC II Agar with hemoglobin and IsoVitaleX, Becton Dickinson), Columbia CNA agar with 5% sheep blood, and MacConkey II agar (Becton Dickinson). In addition, skin, liver, and spleen samples were inoculated on enriched media (BBL Enriched Thioglycollate Medium with Vitamin K and Hemin, Becton Dickinson). Fecal cultures were inoculated on trypticase soy agar with 5% sheep blood, MacConkey II agar, and Hektoen enteric agar (Becton Dickinson). Fungal cultures were inoculated on Sabouraud dextrose agar (Becton Dickinson). Antibiotic sensitivity was performed for all bacterial isolates (BBL Sensi-Disc Susceptibility Test Discs, Becton Dickinson).
Clinical and anatomic pathology.
Blood smears were prepared and stained (Accustain Wright Stain, Modified, Sigma-Aldrich, St Louis, MO). A manual differential count was performed by an experienced medical technologist. All external and internal organs were examined grossly. After aseptic removal of spleen and liver for microbiologic culture, the gastrointestinal tract was removed and divided into 3 sections (colon, distal jejunum–ileum, and duodenum–proximal jejunum). Small intestine and colon were ‘Swiss rolled’ for evaluation.16 Tissues were collected from all major organ systems, fixed in neutral buffered 10% formalin (Leica Microsystems, IL), processed by standard methods, sectioned at 5 µm, stained with hematoxylin and eosin or Warthin–Starry silver stain, and examined by light microscopy by an experienced veterinary pathologist.
Results
Serologic and PCR analysis.
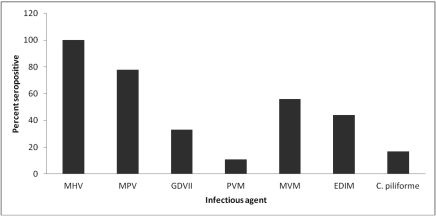
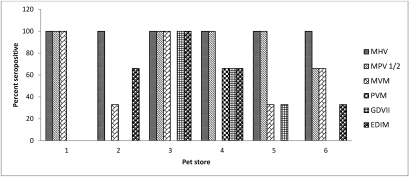
Serology revealed the presence of antibodies to mouse hepatitis virus (all 18 mice evaluated), mouse parvovirus types 1 and 2 (14 of 18 mice), mouse rotavirus (6 mice), pneumonia virus of mice (2 mice), minute virus of mice (10 mice), and epizootic diarrhea of infant mice virus (8 mice; Figure 1). Antibodies against C. piliforme (3 mice) were identified by ELISA in mice from a single store (Figure 2). No antibodies against the remaining infectious agents were detected. All PCR results for lymphocytic choriomeningitis virus and S. moniliformis were negative.
Figure 1.
Distribution of seropositive mice among those purchased (n = 18) from 6 pet stores in New York City. C. piliforme was detected in only one store. MHV, mouse hepatitis virus; MPV, mouse parvovirus; GDVII, mouse rotavirus; PVM, pneumonia virus of mice; MVM, minute virus of mice; EDIM, epizootic diarrhea of infant mice virus.
Figure 2.
Distribution of mice seropositive to various murine viruses (n=18). MHV, mouse hepatitis virus; MPV 1/2, mouse parvovirus types 1 and 2; GDVII, mouse rotavirus; PVM, pneumonia virus of mice; MVM, minute virus of mice; EDIM, epizootic diarrhea of infant mice virus.
Parasitology.
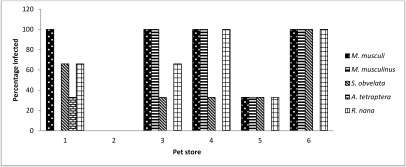
Postmortem microscopic examination of the pelt detected both Myocoptes musculinus (11 of 18 mice) and Myobia musculi (13 of 18). Dual infestations were detected on 50% of the mice with ectoparasites. Skin scrapes identified mite infestations in only 40% of the cases identified on postmortem pelt exam. Direct examination of intestinal contents revealed Syphacia obvelata (7 mice), Aspiculuris tetraptera (1 mouse), and Rodentolepis nana (9 mice). No other ecto- or endoparasites were identified. Fecal flotation identified R. nana in the pooled samples from pet store 3 (Figure 3). Pooled fecal exams were negative for animals from 3 stores, although R. nana was identified in at least one animal from each store by direct exam of intestinal contents or histology. No pinworm ova were identified on fecal flotation or anal tapes.
Figure 3.
The distribution of endo- and ectoparasites found in mice (n = 18) from 6 pet stores. Data shown are an accumulation of findings confirmed by either microscopic or histopathologic observation.
Microbiology.
Multidrug-resistant β-hemolytic E. faecium was cultured from the skin of 3 mice, all of which were from the same pet store. This strain of E. faecium was resistant to 9 antibiotics tested: penicillin, oxacillin, tetracycline, trimethoprin–sulfa, erythromycin, ceftriaxone, ampicillin, cephalothin, and gentamicin. In addition, Pasteurella pneumotropica was found in 5 of 18 mice, 3 in nasal aspirates and 2 in the tympanic bullae. No other clinically significant bacteria were isolated. There was no growth of fungal organisms.
Clinical and anatomic pathology.
No gross lesions associated with disease were noted at necropsy in any mouse. A relative eosinophilia was present in 6 of the 18 mice. No other significant findings were noted on blood smear examination.
In 3 mice from 3 different stores, there were numerous cross sections of intestinal nematodes in the large intestinal lumen. Histologic findings including a thin eosinophilic cuticle, platymyarian musculature, and the presence of gravid females containing embryonated ova with a characteristic pointed ovular shape and flattened side identified the organisms as S. obvelata. In addition, grossly identifiable S. obvelata infections were noted in 2 of these 3 mice, and 7 other mice from 4 stores contained large intestinal luminal parasites most consistent with adult or juvenile pinworms. Features necessary to confirm genus were lacking in the histologic sections examined. In 4 of these 7 mice, intestinal parasites were identified grossly as S. obvelata. Histology revealed nonidentifiable nematode larvae in 3 mice, 1 of which mice had histologic evidence of undefined juvenile or adult nematodes and gross confirmation of S. obvelata. The remaining 2 mice had neither gross nor histologic evidence of more-developed nematode life stages (Table 1).
Table 1.
Distribution of endoparasites by detection methods among 18 mice from 6 pet stores
| Identified by |
||||||
| No. of mice positive for endoparisites | Gross exam only | Histopathology only | Both methods | |||
| Nematodes | ||||||
| S. obvelata | 8 | 5 | 1 | 2 | ||
| A. tetrapteraa | 1 | 1 | 0 | 0 | ||
| Unconfirmedb | 5 | 0 | 5 | 0 | ||
| Cestodes | ||||||
| R. nanac | 11 | 6 | 2 | 3 | ||
A. tetraptera was found along with S. obvelata in one mouse.
Mice with unidentified nematodes on histopathology and no nematodes identified on gross exam.
S. obvelata and R. nana were found together in 4 mice.
In 5 mice, multiple segments of small intestine contained longitudinal and oblique sections of a luminal cestode. This cestode was identified as R. nana histologically; in some sections, an armed rostellum was present. Furthermore, 3 of these 5 mice had R. nana identified grossly (Table 1). Trichomonads were noted histologically in 2 mice, although these parasites were not seen on microscopic exam of gross intestinal contents.
Eight mice had mild to moderate lymphoplasmacytic colitis or enteritis; eosinophilic infiltration accompanied these lesions in 5 of these mice, although a relative eosinophilia was present in only 2 of these 5 cases. Argyrophilic bacterial rods consistent with C. piliforme were not observed in Warthin–Starry silver-stained hepatic or intestinal sections of any mouse. This stain did not reveal the presence of spirochete bacteria consistent with Leptospira spp. in the kidneys.
Discussion
This survey confirms that apparently healthy pet store mice carry or have seroconverted to a number of infective agents which may pose a health risk to either humans or laboratory mice. Agents with zoonotic potential identified during this survey include the multidrug-resistant bacterium E. faecium and the cestode R. nana.
E. faecium generally is considered to be a harmless commensal of the gastrointestinal tract of healthy animals and humans. Recently, enterococcal organisms, especially multidrug-resistant E. faecalis and multidrug-resistant E. faecium, have been recognized as important causes of community acquired or nosocomial infections in humans.10 Enterococci harbor antimicrobial resistance genes, which may transfer between animals and humans due to handling or ingestion of animals or animal products.10 The strain of E. faecium isolated in the current survey was resistant to gentamicin as well as penicillin. To our knowledge, this report is the first to describe multidrug-resistant E. faecium isolated from pet mice. The isolation of the bacterium from the skin is unusual, given that most isolates are from the feces. Perhaps fecal material contaminated the fur, but the bacterium was not isolated on fecal culture from these animals. Further investigation is necessary to characterize the genomic relationship of this isolate to known human isolates.
Two additional bacterial species with zoonotic potential are C. piliforme and P. pneumotropica. These organisms have been reported to cause rare, opportunistic infections in immunocompromised persons.6,26 Human disease associated with P. pneumotropica as the primary pathogen was reported after exposure of patients to dogs and cats.6,8 Our pet-store mice have a high prevalence of P. pneumotropica (28%) compared with that of laboratory mice (4.8%).20 Although no statistical significance can be ascribed after comparing the results of the current study to previous studies, the data indicate relevant trends. There is a single report of C. piliforme causing disease in an AIDS patient after exposure to rodents.26 In the current survey, C. piliforme antibody was detected in 3 of the 18 mice. None of the infected mice showed clinical signs, nor were any bacteria or suggestive lesions noted in stained tissues, indicating that the serologic results were either false-positives or that the mice had eliminated the infection after seroconversion, which is believed to occur.21 False-positive results can occur due to nonspecific cross-reaction with closely related commensal clostridial species.5
A majority (5 of 6) of the surveyed pet stores sold mice positive for a potentially zoonotic parasite, R. nana. This outcome agrees with a previous finding, in which mice from 75% of surveyed Connecticut pet stores were positive for R. nana.4 The cited study found R. nana in several species, including mice, rats, and hamsters. R. nana (previously Hymenolepis nana) is the most common cestode infecting humans worldwide, with infection rates estimated at 50 to 75 million people.2 Infection occurs after ingestion of contaminated food or infected arthropod intermediates. Most cases of infection are asymptomatic in both humans and mice, but large worm burdens might lead to clinical disease. Whether humans and mice have their own strains of R. nana which do not cross infect is under debate.13 Regardless, the Centers for Disease Control and Prevention recommends taking precautions when handling infected rodents, because of the possibility of human infection.3
Although human clinical disease is rare, all of the zoonotic pathogens identified present a risk to immunocompromised adults as well as children, who are less likely than are adults to stringently wash their hands after animal handling. For this study, we purchased mice from multiple stores throughout the borough of Manhattan. The source of these mice is undefined, although discussions with store staff indicated that they were purchased from breeders. Whether the stores surveyed purchased from the same or multiple breeders is unknown. According to conversations with store employees, no prophylactic treatment was provided. The other pathogens we detected in the pet-store mice pose a risk to facilities housing SPF mice through inadvertent introduction by personnel owning or handling pet-store mice. Although the mice appeared healthy, and few, if any, significant clinical, gross, or histopathologic indicators of bacterial or viral disease were seen, the results clearly indicate that multiple parasitic, bacterial, and viral agents can be harbored.
Interestingly, adventitious disease transmission from pet store mice may pose a greater risk to lab animal facilities than that from populations of wild mice. The viral milieu of pet-store mice appears more similar to that of laboratory mice than wild mice caught in an urban environment. Serology detected mouse hepatitis virus in only 2% of urban wild mice as compared with 100% of our pet-store mice.18 In addition, pet-store mice had greater serologic prevalence of epizootic diarrhea of infant mice virus, mouse parvovirus, minute virus of mice, and mouse rotavirus than that reported in urban wild mice.18 These data, combined with negative results for murine cytomegalovirus, indicate that contact between the pet-store mice evaluated and wild mice was unlikely at either the breeder or store, given that murine cytomegalovirus is found frequently in wild mice in both urban and rural environments.18,25
A previous study examined ectoparasites infesting pet-store mice in South Carolina22 and revealed a greater variety of ectoparasites, including Ornithonyssus bacoti, than that in the current survey. However, the prevalence of M. musculi in mice (66.7% in South Carolina compared with 72.2% here) was similar, but that of M. musculinus was lower in the current study (93.3% compared with 55.6%). However, when M. musculinus was present, it tended to infest all 3 mice sampled from a store. This pattern is expected in light of the rapidity and ease by which M. musculinus spreads by contact.27
Pet-store mice had a higher prevalence of ectoparasites, cestodes, and nematodes than do urban wild mice and laboratory mice.18,20 Trichomonads, nonpathogenic protozoa, were noted at low prevalence in our pet store mice, as previously has been reported for both wild and laboratory mice.18,20 The high prevalence for the majority of infections is predictable due to the high-density housing and continuous influx of naïve animals typical of pet stores. We are unable to state whether transmission occurs at the breeder, the pet store, or both.
Several mice with lymphoplasmacytic colitis or enteritis had endo- or ectoparasite infestations (7 of 8 mice). In addition, 5 of these mice had an associated eosinophilic intestinal infiltrate, although only 2 had a relative eosinophilia of the peripheral blood. An association between infesting parasite species and eosinophilia cannot be drawn from these data. The most likely cause for the intestinal eosinophilia is R. nana, because experimental R. nana infections in mice resulted in intestinal eosinophilia.7,17 R. nana was found in 4 of the 5 mice with eosinophilic enteritis. The 2 mice purchased with the remaining mouse were positive for R. nana, making an undetected infection in this mouse likely. S. obvelata infections do not appear to lead to eosinophilia in mice.12,15 Peripheral blood eosinophilia has been reported in association with mite-infested mice,19 but no report yet has correlated mite infestation with gastroenteritis.
Although the number of mice and pet stores we evaluated was limited, the survey reveals the potential for zoonotic disease transmission, especially to immunocompromised persons. In addition, this survey reinforces the importance of preventing laboratory animal personnel from purchasing and handling pet store mice, even if kept temporarily for live feeding of captive reptiles. The potential for contact by laboratory animal personnel with rodents outside of the facility reinforces the need for the use of appropriate personal protective equipment within a facility. Correct compliance with facility guidelines regarding the use of personal protective equipment and personal hygiene practices, such as hand washing and showering, will reduce the risk of personnel transferring adventitious agents into a facility.14
Finally and importantly, we experienced several common difficulties in the detection of parasitic infections in live animals. Fecal flotation detected R. nana infections in only 20% of the pooled samples and did not detect S. obvelata or A. tetraptera eggs in any samples; in addition, S. obvelata infection was never noted on anal tape analysis. We suspect that this outcome is due to the periodic shedding of the organism.11 Furthermore, in a single mouse, intestinal content examination failed to reveal S. obvelata found on histologic sections. Five other animals had histologic evidence of nematode infection without similar gross findings. In addition, skin scrapes identified mite infestations in only 40% of the cases identified on postmortem pelt exam. These findings have considerable implications for rodent quarantine programs in which only live animals are evaluated.
Acknowledgments
We thank the staff of the Laboratory of Comparative Pathology, especially Aziz Toma, Jacqueline Candelier, Desiree Powell, and Carmela Bacani, for their diagnostic support. We also thank Chris Gardiner for his assistance with histopathologic identification of endoparasites.
References
- 1.Ceianu C, Tatulescu D, Muntean M, Molnar GB, Emmerich P, Gunther S, Schmidt-Chanasit J. 2008. Lymphocytic choriomeningitis in a pet-store worker in Romania. Clin Vaccine Immunol 15:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho SC, Lee HL, Lee OY, Yoon BC, Choi HS, Hahm JS, Ryu JS, Ahn MH. 2009. Hymenolepis nana infection of the colon in an adult male. Gastrointest Endosc 70:784–785 [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention and National Institutes of Health. 2009. Biosafety in microbiological and biomedical laboratories, 5th ed. Bethesda (MD): Department of Health and Human Services. [Google Scholar]
- 4.Duclos LM, Richardson DJ. 2000. Hymenolepis nana in pet store rodents. Comp Parasitol 67:197–201 [Google Scholar]
- 5.Feldman SH, Kiavand A, Seidelin M, Reiske HR. 2006. Ribosomal RNA sequences of Clostridium piliforme isolated from rodent and rabbit: reexamining the phylogeny of the Tyzzer disease agent and development of a diagnostic PCR assay. J Am Assoc Lab Anim Sci 45:65–73 [PubMed] [Google Scholar]
- 6.Frebourg NB, Berthelot G, Hocq R, Chibani A, Lemeland JF. 2002. Septicemia due to Pasteurella pneumotropica: 16S rRNA sequencing for diagnosis confirmation. J Clin Microbiol 40:687–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedberg W, Neas BR, Faulkner DN, Congdon CC. 1979. Hymenolepis nana: intestinal tissue phase in actively immunized mice. J Parasitol 65:61–64 [PubMed] [Google Scholar]
- 8.Gadberry JL, Zipper R, Taylor JA, Wink C. 1984. Pasteurella pneumotropica isolated from bone and joint infections. J Clin Microbiol 19:926–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glastonbury JR, Morton JG, Matthews LM. 1996. Streptobacillus moniliformis infection in Swiss white mice. J Vet Diagn Invest 8:202–209 [DOI] [PubMed] [Google Scholar]
- 10.Hammerum AM, Lester CH, Heuer OE. 2010. Antimicrobial-resistant enterococci in animals and meat: a human health hazard? Foodborne Pathog Dis 7:1137–1146 [DOI] [PubMed] [Google Scholar]
- 11.Hill WA, Randolph MM, Mandrell TD. 2009. Sensitivity of perianal tape impressions to diagnose pinworm (Syphacia spp.) infecions in rats (Rattus norvegicus) and mice (Mus musculus). J Am Assoc Lab Anim Sci 48:378–380 [PMC free article] [PubMed] [Google Scholar]
- 12.Liu LX, Chi J, Upton MP, Ash LR. 1995. Eosinophilic colitis associated with larvae of the pinworm Enterobius vermicularis. Lancet 346:410–412 [DOI] [PubMed] [Google Scholar]
- 13.Macnish MG, Morgan UM, Behnke JM, Thompson RC. 2002. Failure to infect laboratory rodent hosts with human isolates of Rodentolepis (= Hymenolepis) nana. J Helminthol 76:37–43 [DOI] [PubMed] [Google Scholar]
- 14.McGarry MP, Martin TA. 2003. Dressing for success: choosing garbing standards for the laboratory animal facility. Lab Anim (NY) 32:32–36 [DOI] [PubMed] [Google Scholar]
- 15.Michels C, Goyal P, Nieuwenhuizen N, Brombacher F. 2006. Infection with Syphacia obvelata (pinworm) induces protective Th2 immune responses and influences ovalbumin-induced allergic reactions. Infect Immun 74:5926–5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moolenbeek C, Ruitenberg EJ. 1981. The ‘Swiss roll’: a simple technique for histological studies of the rodent intestine. Lab Anim 15:57–59 [DOI] [PubMed] [Google Scholar]
- 17.Niwa A, Miyazato T. 1996. Enhancement of intestinal eosinophilia during Hymenolepis nana infection in mice. J Helminthol 70:33–41 [DOI] [PubMed] [Google Scholar]
- 18.Parker SE, Malone S, Bunte RM, Smith AL. 2009. Infectious diseases in wild mice (Mus musculus) collected on and around the University of Pennsylvania (Philadelphia) Campus. Comp Med 59:424–430 [PMC free article] [PubMed] [Google Scholar]
- 19.Pochanke V, Hatak S, Hengartner H, Zinkernagel RM, McCoy KD. 2006. Induction of IgE and allergic-type responses in fur-mite–infested mice. Eur J Immunol 36:2434–2445 [DOI] [PubMed] [Google Scholar]
- 20.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173 [DOI] [PubMed] [Google Scholar]
- 21.Pritt S, Henderson KS, Shek WR. 2010. Evaluation of available diagnostic methods for Clostridium piliforme in laboratory rabbits (Oryctolagus cuniculus). Lab Anim 44:14–19 [DOI] [PubMed] [Google Scholar]
- 22.Reeves WK, Cobb KD. 2005. Ectoparasites of house mice (Mus musculus) from pet stores in South Carolina, USA. Comp Parasitol 72:193–195 [Google Scholar]
- 23.Ricart Arbona RJ, Lipman NS, Wolf FR. 2010. Treatment and eradication of murine fur mites: III. Treatment of a large mouse colony with ivermectin-compounded feed. J Am Assoc Lab Anim Sci 49:633–637 [PMC free article] [PubMed] [Google Scholar]
- 24.Shek WR, Gaertner DJ. 2002. Microbiological quality control for laboratory rodents and lagomorphs, p 365–393. In: Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed. New York (NY): Elsevier. [Google Scholar]
- 25.Smith AL, Singleton GR, Hansen GM, Shellam G. 1993. A serologic survey for viruses and Mycoplasma pulmonis among wild house mice (Mus domesticus) in southeastern Australia. J Wildl Dis 29:219–229 [DOI] [PubMed] [Google Scholar]
- 26.Smith KJ, Skelton HG, Hilyard EJ, Hadfield T, Moeller RS, Tuur S, Decker C, Wagner KF, Angritt P. 1996. Bacillus piliformis infection (Tyzzer disease) in a patient infected with HIV1: confirmation with 16S ribosomal RNA sequence analysis. J Am Acad Dermatol 34:343–348 [DOI] [PubMed] [Google Scholar]
- 27.Watson DP. 1961. The effect of the mite Myocoptes musculinus (C. L. Koch 1840) on the skin of the white laboratory mouse and its control. Parasitology 51:373–378 [DOI] [PubMed] [Google Scholar]