Abstract
CO2 administration is a common euthanasia method for research mice, yet questions remain regarding whether CO2 euthanasia is associated with pain and stress. Here we assessed whether premedication with acepromazine, midazolam, or anesthetic induction with isoflurane altered behavioral and physiologic parameters that may reflect pain or stress during CO2 euthanasia. Mice were assigned to 1 of 6 euthanasia groups: CO2 only at a flow rate of 1.2 L/min which displaces 20% of the cage volume per minute (V/min; control group); premedication with acepromazine (5 mg/kg), midazolam (5 mg/kg), or saline followed by 20% V/min CO2; induction with 5% isoflurane followed by greater than 100% V/min CO2 (>6L/min); and 100% V/min CO2 only (6 L/min). Measures included ultrasonic sound recordings, behavioral analysis of video recordings, plasma ACTH and corticosterone levels immediately after euthanasia, and quantification of c-fos from brain tissue. Compared with 20% V/min CO2 alone, premedication with acepromazine or midazolam did not significantly alter behavior but did induce significantly higher c-fos expression in the brain. Furthermore, the use of isoflurane induction prior to CO2 euthanasia significantly increased both behavioral and neuromolecular signs of stress. The data indicate that compared with other modalities, 20% V/min CO2 alone resulted in the least evidence of stress in mice and therefore was the most humane euthanasia method identified in the current study.
Abbreviation: CCAC, Canadian Council on Animal Care in Science; GABA, γ-aminobutyric acid
CO2 administration is a frequently used form of euthanasia in laboratory mice due to its ease of use, availability, low expense, and high level of personnel safety.3,7 Furthermore, because this method does not require handling or manipulation of mice prior to euthanasia, it avoids stress associated with these activities. However, questions remain regarding the pain and distress that mice may experience during CO2 euthanasia.7,13,23
CO2 stimulates receptors in the nasal mucosa.31 Pain associated with this stimulation is concentration-dependent, and studies in humans indicate that a single full breath of CO2 at concentrations ranging from 50% to 100% can be painful.9 Human subjects reported that exposure to 50% CO2 induced tingling sensations and was associated with an unpleasant odor or taste; high CO2 concentrations (100%) induced pain that was described as piercing or stabbing.9 Rodents, like humans, possess these receptors in the nasal mucosa.31 Rodent studies assessing the use of CO2 as a euthanizing agent are unclear, and some suggest that CO2 induces pain and distress, whereas others indicate there are no signs of pain or distress.7,9,13
Current euthanasia guidelines from both the American Veterinary Medical Association and the American College of Laboratory Animal Medicine recommend that CO2 be administered in a gradual fill method so that the influx of CO2 fills at least 20% of the euthanasia chamber volume per minute.1,3 Concerns over the use of CO2 have led the Canadian Council for Animal Care (CCAC) to recommend the use of an inhalant anesthetic followed by CO2 for euthanasia of rodents.6 In addition, the Morris Animal Foundation does not consider the use of CO2 alone to be an appropriate method of euthanasia and will not fund projects that propose to use CO2 for euthanasia without premedication.29 In some larger species, premedication prior to euthanasia is standard practice; however, the potential benefit of this practice has not been analyzed prospectively in mice. Therefore, we tested the hypothesis that premedication with a sedative or inhalant anesthesia alleviates pain and stress associated with CO2 euthanasia in mice. Specifically, we evaluated 3 types of premedication to determine whether their use before euthanasia affected behavioral, physiologic, or neuromolecular indicators of pain and stress during CO2 euthanasia. In addition, we evaluated mouse euthanasia due to CO2 alone at a flow rate of 20% chamber volume displacement per minute (V/min) and at the higher rate of 100% V/min. Behavioral measures analyzed in the current study included ultrasonic sound recordings to assess any change in vocalization which might be associated with distress calls14,40 and posthoc evaluation of videorecordings by a blinded observer trained in assessing pain and stress in rodents. Physiologic parameters measured in this study included plasma ACTH and corticosterone levels. We also used quantitative PCR to measure the c-fos transcript in the brain. The c-fos gene is an immediate early gene that is rapidly and transiently expressed in neurons in response to stimulation and has been used in numerous studies as an indicator of pain and stress in rodents.15,36
Materials and Methods
Animals.
Female CD1 mice (n = 10 per group; age, 8 to 11 wk) were purchased from Charles River Laboratories (Wilmington, MA) and acclimated for at least 1 wk prior to experimental procedures. All mice were screened by the vendor and were deemed to be SPF for all commonly tested bacterial and viral pathogens and parasites. All animal procedures were approved by Cornell's IACUC.
Housing.
Mice were housed in an AAALAC-accredited facility in groups of 2 to 3 in individually ventilated polycarbonate cages (29.2 cm × 16.5 cm × 12.7 cm) with autoclaved corncob bedding (7097A; Harlan Teklad, Frederick, MD). Cages were maintained on a rack system (Micro-FLO/Micro-VENT Environmental Rack System, Allentown Caging Equipment Company, Allentown, NJ). The mice were housed in a room with controlled temperature (20 to 22 °C), humidity (30% to 70%), and photoperiod (14:10-h light:dark cycle). All mice had free access to food (irradiated maintenance mouse diet 7912, Harlan Teklad) and acidified reverse-osmosis water through an automated watering system (Edstrom, Waterford, WI). All cages were provided with sterile nesting pads for enrichment.
Experimental design.
Cages of mice were allocated randomly into 6 euthanasia groups; mice were weighed the day prior to euthanasia to ensure correct dosing. Group 1 (control) mice were euthanized by CO2 only; the mice in group 2 received acepromazine (5 mg/kg IP, 0.20 mL in sterile saline) 10 min prior to euthanasia. Mice in group 3 received midazolam (5 mg/kg IP, 0.20 mL in sterile saline) 10 min prior to euthanasia. Group 4 mice received 0.20 mL saline IP 10 min prior to euthanasia (negative control group for handling and intraperitoneal injection). Mice in these groups were all euthanized with CO2 at a flow rate of 20% chamber air displacement per minute (20% V/min; 1.2 L/min). Mice in the group 5 were anesthetized with 5% isoflurane at a flow rate of 1.2 L/min O2 until all mice in the cage were unconscious and then immediately euthanized with CO2 at a flow rate exceeding 100% V/min (more than 6 L/min; consistent with the CCAC recommendation6). Mice in group 6 were euthanized by using CO2 at flow rate of 100% V/min (6 L/min). Flowmeter settings were calculated by using the following formula: (% air displacement per min) × (volume of cage in L; without filter top).
The cages were moved one at a time from the housing room to a procedure room across the hall. The mice were maintained in their social groups of 2 or 3 mice per cage. Mice that received intraperitoneal injections were injected in the procedure room and left undisturbed for 10 min prior to CO2 exposure. CO2 was administered at a set flow rate from a compressed tank through a tube that was inserted into the home cage of the mice through the lixit port (Figure 1).
Figure 1.
The euthanasia set-up. Mice remained in the home cage, CO2 was administered through the lixit port (arrow), and an ultrasonic microphone was placed over the cage.
Behavioral measures.
Digital videorecordings of each cage were collected for approximately 2 min immediately after intraperitoneal injection, if applicable (induction), 2 min immediately prior to starting either isoflurane or CO2 (preeuthanasia), and for the duration of isoflurane and CO2 exposure (euthanasia). Videorecordings were collected by using a digital videocamera (Sony, San Jose, CA) directed at the long axis of the cage. Each recording was assigned a random number and blindly scored for increased respiratory effort (dyspnea); abnormal activity such as flipping and spinning or an abnormal alteration in activity level (a marker of agitation); and presence of any behaviors indicative of pain (such as pawing at the face) on a scale of 0 to 3 (0, none; 1, mild; 2, moderate; 3, severe) by a laboratory animal veterinarian trained in assessing rodent behavior but who was unfamiliar with the study design and group identity. Times to unconsciousness and death were recorded for each mouse, starting from the onset of gas exposure (isoflurane or CO2). Behavioral scores were assessed only until unconsciousness was reached (regardless of whether unconsciousness was due to anesthesia or CO2). Unconsciousness was defined as cessation of voluntary movement, and death was defined as complete cessation of breathing.
Ultrasonic sound recordings.
Sound recordings in the range of 0 to 120 kHz were collected for 2 min prior to (preeuthanasia) and during (euthanasia) gas administration by using an ultrasonic microphone (USG 116-200, UltraSoundGate Kit, Avisoft, Berlin, Germany) to capture vocal emissions made by the mice.14,40 Sounds were recorded until mice lost consciousness (regardless of whether unconsciousness was due to anesthesia or CO2). The microphone was directed into the cage through a hole in a platform that was placed on top of the cage after removing the bonnet and wire rack (Figure 1). An averaged power spectrogram of each cage recording was created by using software provided by the manufacturer (SASLab Pro, version 4.3, Avisoft). Sonograms from 2 cages of each group were averaged and plotted on a graph comparing preeuthanasia and euthanasia values.
Physiologic measures.
Immediately after euthanasia, mice were exsanguinated by cardiocentesis, and blood was collected into EDTA tubes. Euthanasia of all groups was coordinated to occur at the same time (0900 to 1100) in a random order to ensure no fluctuations in circadian rhythm and that order of euthanasia was not a factor in the parameters analyzed. Plasma was isolated and stored at −20 °C until assayed for ACTH and corticosterone levels. A chemiluminescent ELISA (Calbiotech, Spring Valley, CA) was used to measure plasma ACTH levels, and a competitive ELISA (VWR, Radnor, PA) was used to measure plasma corticosterone levels.
Quantification of c-fos mRNA in brain.
A straight razor blade was used to cut a 2-mm coronal section of brain tissue at the level of the hypothalamus, which was processed for c-fos mRNA quantification. This section was chosen because differences due to distress in mice typically occur in this region.27 Total RNA was isolated from the samples by using the EZNA Tissue RNA kit (Omega Bio-Tek, Norcross, GA), and 1 μg of RNA was converted to cDNA (Quanta Biosciences, Gaithersburg, MD). Quantitative PCR was performed for c-fos and GAPDH mRNA expression (SYBR/Lo Rox, Quanta Biosciences) and run on an ABI Fast 7500 machine. Expression of c-fos was normalized to that of GAPDH and analyzed by using the ∆∆Ct method as described previously.28
Statistics.
All statistical analyses were conducted by using GraphPad Prism (version 5.04, GraphPad Software, San Diego, CA). All parametric data sets, including time measurements, ACTH, corticosterone, and c-fos mRNA expression were analyzed by using one-way ANOVA and Tukey posttest. Behavior data were analyzed by using a nonparametric Kruskal–Wallis test followed by Dunn posttest. Differences were considered significant at a P value of less than 0.05.
Results
Time measurements.
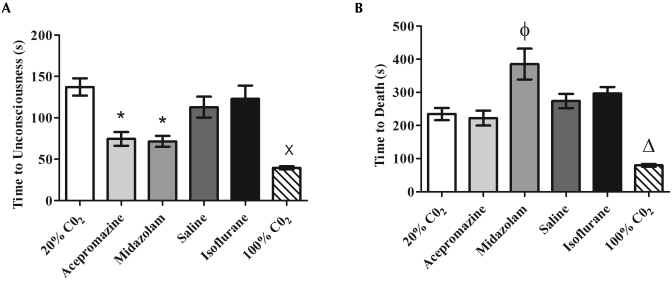
Mice euthanized with 100% V/min CO2 had the most rapid loss of consciousness (39.6 ± 1.9 s), which was significantly (P < 0.05) faster than that of the 20% V/min CO2 (137.2 ± 10.5 s), saline (112.9 ± 12.7 s), and isoflurane (122.9 ± 16.0 s) groups (Figure 2 A). Mice that received 100% V/min CO2 also reached death (79.9 ± 4.2 s) significantly (P < 0.05) faster than did all other groups (range, 222.3 ± 22.5 s to 385.2 ± 46.7 s; Figure 2 B).
Figure 2.
Times (mean ± SEM) to (A) unconsciousness and (B) death were analyzed for each euthanasia group. Mice given either acepromazine or midazolam or euthanized with 100% V/min CO2 experienced the most rapid time to unconsciousness, but only mice euthanized with 100% V/min CO2 experienced a significantly more rapid death compared with other groups. Despite a shorter time to unconsciousness, mice euthanized with midazolam experienced a significantly increased time to death compared with that of mice that received 20% V/min CO2. (A) *, Significant (P < 0.05) decrease in time compared with values for 20% V/min CO2 and isoflurane groups; ×, significant (P < 0.05) decrease in time compared with 20% V/min CO2, saline, and isoflurane groups; (B) Φ, significant (P < 0.05) increase in time compared with values for 20% V/min CO2, acepromazine, and saline groups; Δ, significant (P < 0.05) decrease in time compared with values for all other groups.
Premedication of mice with acepromazine or midazolam significantly (P < 0.05) decreased the time to unconsciousness (74.5 ± 8.3 s and 71.6 ± 6.6 s, respectively) compared with 20% V/min CO2 alone (137.2 ± 10.5 s; Figure 2 A). However, premedication with midazolam significantly (P < 0.05) lengthened the time to death (385.2 ± 46.7 s) compared with 20% V/min CO2 alone (234.6 ± 18.4 s; Figure 2 B). Induction with isoflurane did not reduce time to unconsciousness or death but instead produced the unwanted side effect of 5 of the 10 mice recovering consciousness while the cage was being treated with greater than 100% V/min CO2.
Behavior analysis.
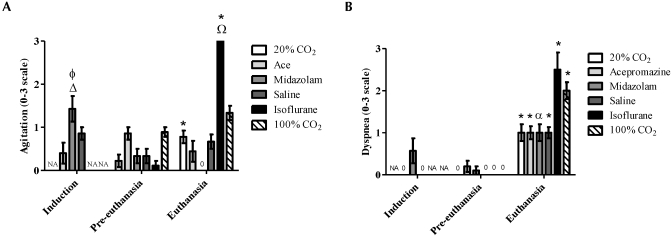
Blinded evaluations of behavior were conducted for the induction, preeuthanasia, and euthanasia periods. Groups of mice were compared according to both treatment and time point (induction, preeuthanasia, euthanasia). Behavior scores at induction were compared among the acepromazine, midazolam, and saline groups. Induction with midazolam resulted in significantly (P < 0.05) higher agitation scores compared with induction with acepromazine (1.4 ± 0.3 and 0.4 ± 0.2, respectively) but did not differ significantly from the saline control (0.9 ± 0.1; Figure 3 A). Midazolam induction resulted in significantly (P < 0.05) higher agitation scores compared with preeuthanasia and euthanasia time points (0.3 ± 0.2 and 0 ± 0, respectively). Two mice received a pain score of 1 (mild): one midazolam-treated mouse during induction and one acepromazine-treated mouse during preeuthanasia, both due to pawing at the face (data not shown).
Figure 3.
A blinded observer examined videotapes taken at induction, preeuthanasia, and euthanasia and scored levels of (A) agitation (increased and altered activity) and (B) dyspnea (increased respiratory effort) on a scale of 0 to 3 (0, none; 1, mild; 2, moderate; 3, severe). For both agitation and dyspnea, mice anesthetized with isoflurane displayed the highest mean scores at euthanasia. Regardless of treatment, all groups displayed significant (P < 0.05) dyspnea at the time of euthanasia compared with either preeuthanasia or induction levels. NA, no applicable data. (A) Δ, Significant (P < 0.05) increase in agitation compared with values at preeuthanasia and euthanasia time points; Φ, significant (P < 0.05) increase in agitation compared with that of with mice induced with acepromazine; *, significant (P < 0.05) increase in agitation compared with that of respective preeuthanasia score; Ω, significant (P < 0.05) increase in agitation at euthanasia compared with that of mice treated with 20% V/min CO2, acepromazine, midazolam, or saline. (B) *, Significant (P < 0.05) increase in dyspnea compared with that of respective preeuthanasia and induction scores (if applicable); α, indicates a significant increase in dyspnea compared with that for the respective preeuthanasia score.
During euthanasia, the isoflurane group had agitation scores (3.0 ± 0) that were significantly (P < 0.05) higher than those of the 20% V/min CO2 (0.8 ± 0.1), acepromazine (0.4 ± 0.2), midazolam (0 ± 0), and saline (0.7 ± 0.2) groups (Figure 3 A). Because the observer stopped scoring at unconsciousness, this effect is entirely due to isoflurane and not to CO2 administration after isoflurane. Euthanasia caused a significant (P < 0.05) increase in dyspnea in all groups of mice compared with their respective preeuthanasia scores, but dyspnea scores did not differ significantly between any of the groups (Figure 3 B).
Ultrasonic sound recordings.
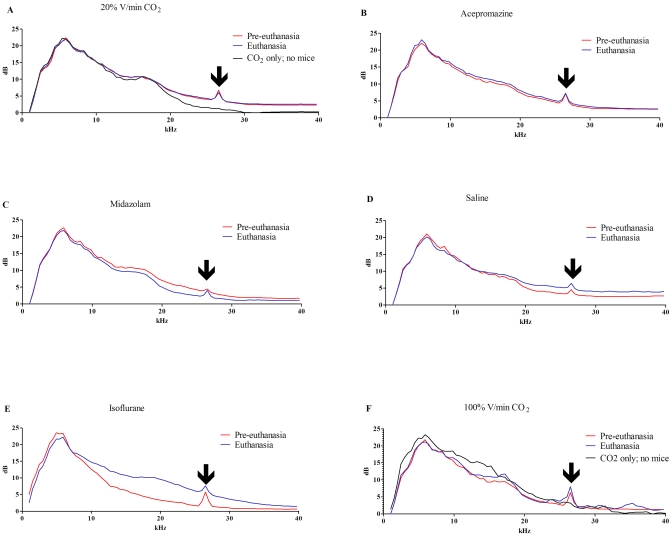
Ultrasonic sound recordings were taken to capture any altered vocalizations made during euthanasia. All preeuthanasia and euthanasia sound spectrograms displayed sound peaks at 26.5 kHz that were not present in background noise control recordings for which no mice were present (black line, Figure 4 A); therefore, this peak is presumed to be mouse vocalization (arrows, Figure 4). Mice euthanized with 20% V/min CO2 or premedicated with acepromazine had euthanasia spectrograms that were identical to their respective preeuthanasia spectrograms (Figure 4 A and B). Mice premedicated with midazolam had an overall lower-amplitude spectrogram during euthanasia than during preeuthanasia for all data points greater than 7 kHz, except at the 26.5-kHz vocalization peak (Figure 4 C). This finding is consistent with the marked decreased activity noted in this group but demonstrates that vocalization was unaltered. The saline-premedicated group had a higher amplitude spectrogram during euthanasia compared with preeuthanasia for all data points greater than 18 kHz, consistent with increased activity and increased vocalization (Figure 4 D). Mice induced with isoflurane had a large increase in amplitude during euthanasia, which differed from the preeuthanasia values for all data points greater than 8 kHz, including the 26.5-kHz vocalization peak (Figure 4 E), consistent with the noted agitation in this group and increased vocalization. Mice euthanized with 100% V/min CO2 had some fluctuations of higher amplitude during euthanasia (9 to 11 kHz, 13 to 18.6 kHz, 23 to 27 kHz, 28 to 32 kHz, and 33 to 37 kHz), some of which were similar to fluctuations seen in background noise control recordings of 100% V/min CO2 in which no mice were present (Figure 4 F); however, there was an increase at the 26.5-kHz vocalization peak similar to that noted in isoflurane-treated mice.
Figure 4.
Spectrograms of ultrasonic sound recordings for each euthanasia group were graphed. Preeuthanasia recordings were generated for 2 min prior to administration of gas. Euthanasia recordings were initiated at the beginning of gas administration until all mice in the cage were unconscious. Each figure represents the averaged values from 2 cages of mice from each group. All groups displayed a peak at 26.5 kHz (arrow), during both recordings, which was absent when mice were not in the cage. This peak represents the only peak readily attributable to vocalization. Mice (A) euthanized with 20% V/min CO2 or (B) administered acepromazine had identical baseline preeuthanasia and euthanasia recordings. In contrast, (C) mice treated with midazolam had lower dB recordings in the 10- to 25-kHz range during euthanasia, consistent with heavy sedation and decreased movement, but not a decrease at the 26.5-kHz vocalization peak. Mice given (D) saline, (E) isoflurane, or (F) 100% V/min CO2 had higher amplitude recordings at the vocalization peak, consistent with increased vocalization at euthanasia. (E) Isoflurane euthanasia also produced a large degree of background noise (10- to 26.5-kHz range), consistent with increased movement and agitation noted in this group.
Physiologic measures.
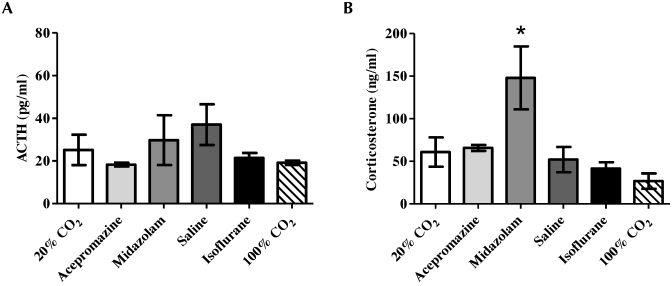
Plasma ACTH and corticosterone concentrations are used often to quantitatively assess stress. No significant differences in ACTH concentration were noted between any treatment groups (Figure 5 A). In contrast, midazolam-treated mice had significantly (P < 0.05) higher corticosterone concentrations (147.9 ± 37.0 ng/mL) than did all other groups (range, 26.7 ± 9.0 ng/mL to 65.8 ± 3.5 ng/mL; Figure 5 B).
Figure 5.
Plasma levels (mean ± SEM) of (A) ACTH and (B) corticosterone for each euthanasia group were evaluated. The mice that received midazolam displayed a significant (*, P < 0.05) increase in corticosterone but not ACTH; this result was suggestive of a hypoxic ACTH-independent activation of adrenal steroidogenesis. This finding was consistent with the increased time to death following unconsciousness in this group.
Measurement of c-fos expression.
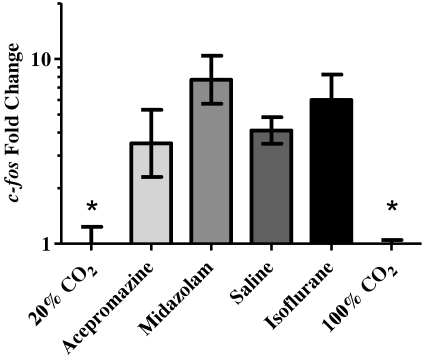
All premedication groups and the isoflurane anesthetized group displayed significantly (P < 0.05; range, 3- to 7-fold) higher c-fos expression as compared with the 20% V/min CO2 control group (Figure 6). There was no significant difference in c-fos expression between the 2 CO2 flow rates.
Figure 6.
Relative mean expression (mean ± SEM) of c-fos in the brains of mice compared with the 20% V/min CO2 control group calculated by using the ΔΔct method. Both the 20%- and 100%-CO2–treated groups expressed significantly (*, P < 0.05) less c-fos than did all other groups.
Discussion
The hypothesis of the current study was that premedication with acepromazine, midazolam, or anesthetic induction with isoflurane prior to CO2 euthanasia would alleviate pain and stress compared with CO2 administration alone in mice. The data described agree with a similar study that assessed the use of sedation or anesthesia prior to CO2 euthanasia of rats.13 Specifically, premedication or anesthetic induction of mice prior to CO2 euthanasia did not provide any advantage over the use of CO2 alone, in that premedication or anesthesia did not decrease behavioral, physiologic, or neuromolecular markers of pain and stress. Furthermore, all measured parameters were minimal in mice euthanized with CO2 at a flow rate of 20% chamber air displacement per minute. In addition, to our knowledge, the current study is the first that provides direct evidence that, within the flow rates examined, isoflurane induction results in higher stress measurements than does euthanasia with CO2 alone.
Numerous studies, primarily using rats, have attempted to determine whether CO2 is a humane and acceptable method of euthanasia; however, there has been great variation in methodology. The major questions that remain over the use of CO2 for euthanasia stem from the ability of CO2 to induce pain in human2,9,18,21 and rodent studies.22,31,38 Unfortunately, the manner in which those studies were conducted differs greatly from the way that rodents typically are euthanized with CO2, in that specific concentrations of CO2 were applied directly on mucosal surfaces.2,9,18,21,22,31,38 In a human study, the mean concentration of CO2 that was reported to induce pain was 47.1%;2 similarly in a study involving rats, nociceptors in the nasal mucosa responded to concentrations of 37% to 50% CO2, although no assessment of pain was made.31 Human subjects reported that increasing concentrations of CO2 were progressively more noxious, with 50% V/min CO2 being considered “highly unpleasant” bordering on “uncomfortable” and 100% V/min CO2 being rated as “painful.”9 In contrast to the conduct of those cited studies, our mice experienced a chamber filled with 100% CO2 mixed with ambient air at a specified flow rate (20% chamber volume air displacement per minute). Mice generally lost consciousness approximately 2 min after initiation of CO2, indicating that they lost consciousness prior to experiencing concentrations (approximately 50%) that are reported to be painful in humans. Specifically, concentrations of CO2 in the cage after 2 min of exposure would be approximately 40%. Therefore, gradually filling the cage with CO2 likely achieves unconsciousness before reaching the exposure level that is reported to be painful in humans.
In CO2 euthanasia studies, despite observing the same behaviors, some observers describe the animals as experiencing no pain or stress, whereas others conclude that the animals experienced considerable pain or stress.7,9 To avoid basing recommendations on subjective data alone, authors also have evaluated objective measures, including heart rate and blood pressure monitoring, plasma ACTH, corticosterone, and glucose level quantifications, and ultrasonic vocalizations; the resulting data supported the notion that CO2 is a humane method of euthanasia in rodents.5,13,35
We included acepromazine in the current study because it is a frequently used premedication and tranquilizer in veterinary medicine. There are species-specific differences in the reported effectiveness of acepromazine prior to euthanasia. Specifically, acepromazine reduces agitation in dogs8 but provides no beneficial effect in rats.13 In the present study, the use of acepromazine reduced time to unconsciousness, but it also increased c-fos expression.
We evaluated midazolam because it is a fast-acting anxiolytic with strong sedative properties. Like acepromazine, midazolam significantly reduced the time to unconsciousness but significantly prolonged the time to death compared with all other euthanasia groups except isoflurane. This prolonged time to death after unconsciousness may be explained by the mechanism of action of midazolam, which increases the efficiency of γ-aminobutyric acid (GABA), an inhibitory neurotransmitter in the brain. GABA is neuroprotective against ischemia during hypoxia,33,41 and the protective effects of GABAergic drugs, such as midazolam, against neural hypoxic damage may have prolonged the time to death in these mice. Induction with midazolam was associated with significantly more agitation than was induction with acepromazine. This greater agitation at induction is likely due to paradoxical excitement, which occasionally occurs with sedative doses of benzodiazepines, such as midazolam. This benzodiazepine-induced excitation has been reported in numerous species including humans and may include restlessness, agitation, violent behavior, and self-mutilation.12,19,26 The mechanism of action for this reaction is unknown. Mice pretreated with midazolam also had similar ACTH levels to those of the other treatment groups; however, their corticosterone levels were significantly higher. This finding suggests a hypoxic ACTH-independent activation of adrenal steroidogenesis.32 The elevated corticosterone in the midazolam-treated group was consistent with the extended time period of hypoxia that the group experienced during the prolonged time to death, although direct activation by midazolam cannot be completely discounted. The c-fos levels in the midazolam treated group were the highest of all the groups, 7.7-fold higher than the control. Therefore, midazolam does not appear to provide any benefit as a premedication for euthanasia. These data are similar to studies in rats, in which sedation with oral acepromazine or anesthesia with pentobarbital intraperitoneally did not appreciably alter behavioral or biochemical parameters of pain in rats euthanized with CO2.13
Like midazolam and acepromazine treatment, saline-treated mice showed greater c-fos expression. Handling and intraperitoneal injection may be more potent stimuli in this respect than is CO2 euthanasia itself. These findings imply fundamental differences between mice and standard companion animals in that standard preeuthanasia regimes used in companion species appear deleterious in animals for which human contact and handling is itself a stressful event.
We included anesthetic induction with isoflurane prior to CO2 euthanasia in the current study in acknowledgement of current recommendations in the CCAC guidelines regarding the euthanasia of animals used in science. According to the CCAC, where practical, animals should be anesthetized, preferably with an inhalant anesthetic, prior to the use of CO2.6 The 2 studies that were cited in support of this recommendation used approach–avoidance tests to assess rodent aversion to gas stimuli.23,25 However, one study assessed rat and mouse aversion to prefilled chambers of CO2, CO2–argon mixture, or argon without the presence of a food reward in the test chamber,23 whereas the other study compared rat aversion to gradual-fill halothane or isoflurane with a food reward in the test chamber.25 In the CO2–argon study, the authors concluded that induction with CO2 either alone or in combination with argon is likely to cause considerable distress in rodents before they lose consciousness.23 This finding was based on significantly shorter initial withdrawal times and total dwelling times in the test chamber that was prefilled with CO2 at concentrations that ranged from 25.5% to 50.8% compared with the control of room air. The halothane–isoflurane study concluded that both inhalant anesthetics were aversive to rats.25 However, because some of the rats remained in the test cage long enough to become ataxic, the authors concluded that the rats likely were sedated at the time they chose to leave the test cage; therefore, continued forced exposure from the onset of aversion to unconsciousness may be more humane than forced exposure to CO225 The 2 studies were not comparable in their design (prefill compared with gradual fill; no food reward compared with food reward), and neither study directly compared aversion of CO2 with isoflurane. In addition, neither of these studies23,25 involved euthanizing the rodents nor assessed any direct or indirect indicators of pain or stress.
The present study tested the hypothesis that isoflurane anesthesia prior to CO2 euthanasia would decrease the level of pain or stress experienced with CO2 alone. However, isoflurane induction required longer CO2 exposure at greater than 100% V/min than did the use of 100% V/min CO2 alone. This prolonged time to death may be due to the hypothermic effect of general anesthesia, which can be neuroprotective during hypoxia.11,33 In addition to prolonged euthanasia times, isoflurane generated the highest scores for both dyspnea and agitation, which were significantly higher than pre-gas exposure scores, and produced significantly higher agitation scores than did 20% V/min CO2 during euthanasia. In addition, the ultrasonic spectrogram indicated increased vocalization at the 26.5-kHz peak, potentially indicative of stress.14 Furthermore, c-fos expression was 6-fold higher in the isoflurane-treated group compared with the 20% V/min CO2 group. An additional negative effect of isoflurane is that some mice awoke after isoflurane induction and during the euthanasia phase. This finding highlights a weakness in the CCAC recommendation, in that, due to the rapid recovery associated with isoflurane, mice may recover once the CO2 displaces the isoflurane from the chamber. Indeed, with newer gas anesthetics (for example, sevoflurane), this problem may become even greater. Previous studies that recommended the use of isoflurane prior to CO2 euthanasia23,25 do not account for the rapid recovery from isoflurane that occurs while the chamber is filling with CO2. Furthermore, avoidance tests do not assess the possibility of pain or stress associated with anesthetic induction. The use of an air-tight induction chamber might retard recovery, but this modification would require handling of the mice and removal from the home cage. Regardless, based on behavioral and neuromolecular markers, isoflurane does not provide any benefit. In short, in the current study, prior anesthesia with isoflurane was the worst option as an adjunct to CO2 euthanasia.
A group of mice euthanized with CO2 at a flow rate of 100% air displacement per minute was included in this study because the American Veterinary Medical Association currently recommends concentrations of 20% V/min CO2 or greater. Times to unconsciousness and death were significantly faster for 100% V/min than the 20% V/min CO2 control. However, ACTH, corticosterone, and c-fos levels were comparable between the 100% and 20% V/min CO2 groups. Furthermore, because mice were still conscious in the cage during the timeframe when the CO2 concentration exceeded 50%, they might have experienced mucosal pain from CO2 at this flow rate. These data support evaluation of higher CO2 flow rates in future studies to determine the recommended upper flow rate limit for euthanasia.
Data that call into question the humane nature of rodent CO2 euthanasia generally fall into 2 categories: those that rely on human studies indicating that CO2 is painful at high concentrations and those that rely on approach–avoidance tests. With regard to the former, our data indicate that with 20% V/min flow rates, mice reach unconsciousness prior to the build-up of potentially painful concentrations of CO2. With regard to the latter, we suggest that approach-avoidance tests are not ideal tests to determine a humane euthanasia method. Specifically, aversion does not necessarily indicate pain or distress. Several examples support this idea. Specifically, a brief puff of air induces aversion in mice,39 and urine collected from male mice infected with Polyplax serrata induces aversion in female mice.20 In these examples, nonconditioned avoidance–aversion responses are not necessarily indicators of pain or distress, but rather evolutionary survival cues (puff of air = predator, infected mouse urine = poor mate). Indeed, nonconditioned CO2 avoidance is conserved evolutionarily (presumably to avoid hypoxic death) and can be demonstrated in organisms as diverse as nematodes (Caenorhabditis elegans), Drosophila, fish, and mice, which can actually sense CO2 concentrations at near atmospheric levels (that is, far below those which would ever be reported as painful or unpleasant in humans).4,16,17,24,37 Further, approach–avoidance tests fail to adequately distinguish between aversion and distress, which is the inability to appropriately respond (either behaviorally or physiologically) to a stressor.30,34 That is, just because an animal encounters an external stressor (for example, puff of air, mouse urine, CO2) and chooses to avoid it does not mean that distress is induced. Our data indicate that behavioral, physiologic, and neuromolecular signs of pain or distress do not occur with CO2 euthanasia at the 20% V/min flow rate tested. The avoidance studies provide evidence that indeed CO2 is likely aversive, but all currently available euthanasia agents probably fall into this category.
In summary, the present study demonstrates that premedication with acepromazine or midazolam did not improve CO2 euthanasia. Furthermore, the use of isoflurane induction prior to CO2 euthanasia significantly increased c-fos expression and adverse behavioral signs. A limitation of the current study is that none of the methodologies used can indicate definitively whether the mice encountered pain or stress–distress. However, taken together our results indicate that, compared with the other readily accepted treatments (isoflurane, premedication) analyzed in this study, 20% V/min CO2 alone is the most humane method of euthanasia for mice.
Acknowledgments
This research was funded by Wyeth Pharmaceutical through Helen Valentine's residency funding. We thank Drew Kirby for technical support and Keith Jarosinski for manuscript review.
References
- 1. American Veterinary Medical Association. [Internet]. 2007. AVMA guidelines on euthanasia, 2007 update. [Cited 16 June 2011]. Available at: http://www.avma.org/issues/animal_welfare/euthanasia.pdf.
- 2.Anton F, Euchner I, Handwerker HO. 1992. Psychophysical examination of pain induced by defined CO2 pulses applied to the nasal mucosa. Pain 49:53–60 [DOI] [PubMed] [Google Scholar]
- 3.Artwohl J, Brown P, Corning B, Stein S. 2006. Report of the ACLAM Task Force on Rodent Euthanasia. J Am Assoc Lab Anim Sci 45:98–105 [PubMed] [Google Scholar]
- 4.Bretscher AJ, Busch KE, de Bono M. 2008. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci USA 105:8044–8049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkholder TH, Niel L, Weed JL, Brinster LR, Bacher JD, Foltz CJ. 2010. Comparison of carbon dioxide and argon euthanasia: effects on behavior, heart rate, and respiratory lesions in rats. J Am Assoc Lab Anim Sci 49:448–453 [PMC free article] [PubMed] [Google Scholar]
- 6.Charbonneau R, Niel L, Olfert E, von Keyserlingk M, Griffin G.2010. [Internet] CCAC guidelines on: euthanasia of animals used in science. Secondary CCAC guidelines on euthanasia of animals used in science. [Cited 16 June 2011]. Available at: http://www.ccac.ca/Documents/Standards/Guidelines/Euthanasia.pdf.
- 7.Conlee KM, Stephens ML, Rowan AN, King LA. 2005. Carbon dioxide for euthanasia: concerns regarding pain and distress, with special reference to mice and rats. Lab Anim 39:137–161 [DOI] [PubMed] [Google Scholar]
- 8.Dallaire A, Chalifoux A. 1985. Premedication of dogs with acepromazine or pentazocine before euthanasia with carbon monoxide. Can J Comp Med 49:171–178 [PMC free article] [PubMed] [Google Scholar]
- 9.Danneman PJ, Stein S, Walshaw SO. 1997. Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci 47:376–385 [PubMed] [Google Scholar]
- 10.Elliott KJ, Brodsky M, Hynansky AD, Foley KM, Inturrisi CE. 1995. Dextromethorphan suppresses both formalin-induced nociceptive behavior and the formalin-induced increase in spinal cord c-fos mRNA. Pain 61:401–409 [DOI] [PubMed] [Google Scholar]
- 11.Fish RE, Brown MJ, Danneman PJ, Karas AZ. 2008. Anesthesia and analgesia in laboratory animals. New York (NY): Elsevier. [Google Scholar]
- 12.Golparvar M, Saghaei M, Sajedi P, Razavi SS. 2004. Paradoxical reaction following intravenous midazolam premedication in pediatric patients—a randomized placebo-controlled trial of ketamine for rapid tranquilization. Paediatr Anaesth 14:924–930 [DOI] [PubMed] [Google Scholar]
- 13.Hackbarth H, Kuppers N, Bohnet W. 2000. Euthanasia of rats with carbon dioxide—animal welfare aspects. Lab Anim 34:91–96 [DOI] [PubMed] [Google Scholar]
- 14.Han JS, Bird GC, Li W, Jones J, Neugebauer V. 2005. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J Neurosci Methods 141:261–269 [DOI] [PubMed] [Google Scholar]
- 15.Harris JA. 1998. Using c-Fos as a neural marker of pain. Brain Res Bull 45:1–8 [DOI] [PubMed] [Google Scholar]
- 16.Herbert NA, Skjaeraasen JE, Nilsen T, Salvanes AGV, Steffensen JF. 2011. The hypoxia avoidance behaviour of juvenile Atlantic cod (Gadus morhua L.) depends on the provision and pressure level of an O2 refuge. Mar Biol 158:737–746 [Google Scholar]
- 17.Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. 2007. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science 317:953–957 [DOI] [PubMed] [Google Scholar]
- 18.Hummel T, Roscher S, Jaumann MP, Kobal G. 1996. Intranasal chemoreception in patients with multiple chemical sensitivities: a double-blind investigation. Regul Toxicol Pharmacol 24:S79–S86 [DOI] [PubMed] [Google Scholar]
- 19.Kahn CM, Line S. 2005. The Merck veterinary manual. Whitehouse Station (NJ): Merck. [Google Scholar]
- 20.Kavaliers M, Fudge MA, Colwell DD, Choleris E. 2003. Aversive and avoidance responses of female mice to the odors of males infected with an ectoparasite and the effects of prior familiarity. Behav Ecol Sociobiol 54:423–430 [Google Scholar]
- 21.Kobal G. 1985. Pain-related electrical potentials of the human nasal mucosa elicited by chemical stimulation. Pain 22:151–163 [DOI] [PubMed] [Google Scholar]
- 22.Komai M, Bryant BP. 1993. Acetazolamide specifically inhibits lingual trigeminal nerve responses to carbon dioxide. Brain Res 612:122–129 [DOI] [PubMed] [Google Scholar]
- 23.Leach MC, Bowell VA, Allan TF, Morton DB. 2002. Aversion to gaseous euthanasia agents in rats and mice. Comp Med 52:249–257 [PubMed] [Google Scholar]
- 24.Ludsin SA, Zhang XS, Brandt SB, Roman MR, Boicourt WC, Mason DM, Costantini M. 2009. Hypoxia avoidance by planktivorous fish in Chesapeake Bay: implications for food web interactions and fish recruitment. J Exp Mar Biol Ecol 381:S121–S131 [Google Scholar]
- 25.Makowska IJ, Weary DM. 2009. Rat aversion to induction with inhalant anaesthetics. Appl Anim Behav Sci 119:229–235 [Google Scholar]
- 26.Mancuso CE, Tanzi MG, Gabay M. 2004. Paradoxical reactions to benzodiazepines: literature review and treatment options. Pharmacotherapy 24:1177–1185 [DOI] [PubMed] [Google Scholar]
- 27.Martinez M, Calvo-Torrent A, Herbert J. 2002. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress 5:3–13 [DOI] [PubMed] [Google Scholar]
- 28.Maurer KJ, Rao VP, Ge Z, Rogers AB, Oura TJ, Carey MC, Fox JG. 2007. T-cell function is critical for murine cholesterol gallstone formation. Gastroenterology 133:1304–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morris Animal Foundation. [Internet]. 2010. Animal involvement justification. Secondary animal involvement justification. [Cited 16 June 2011. Available at: http://www.morrisanimalfoundation.org/for-grant-seekers/
- 30. National Research Council. 2008. Recognition and alleviation of distress in laboratory animals. Washington (DC): National Academies Press. [PubMed]
- 31.Peppel P, Anton F. 1993. Responses of rat medullary dorsal horn neurons following intranasal noxious chemical stimulation: effects of stimulus intensity, duration, and interstimulus interval. J Neurophysiol 70:2260–2275 [DOI] [PubMed] [Google Scholar]
- 32.Raff H, Jacobson L, Cullinan WE. 2003. Elevated corticosterone and inhibition of ACTH responses to CRH and ether in the neonatal rat: effect of hypoxia from birth. Am J Physiol Regul Integr Comp Physiol 285:R1224–R1230 [DOI] [PubMed] [Google Scholar]
- 33.Schwartz-Bloom RD, Sah R. 2001. γ-Aminobutyric acid A neurotransmission and cerebral ischemia. J Neurochem 77:353–371 [DOI] [PubMed] [Google Scholar]
- 34.Selye H. 1975. Confusion and controversy in the stress field. J Human Stress 1:37–44 [DOI] [PubMed] [Google Scholar]
- 35.Smith W, Harrap SB. 1997. Behavioural and cardiovascular responses of rats to euthanasia using carbon dioxide gas. Lab Anim 31:337–346 [DOI] [PubMed] [Google Scholar]
- 36.Stamp JA, Herbert J. 1999. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience 94:1313–1322 [DOI] [PubMed] [Google Scholar]
- 37.Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. 2004. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431:854–859 [DOI] [PubMed] [Google Scholar]
- 38.Thurauf N, Friedel I, Hummel C, Kobal G. 1991. The mucosal potential elicited by noxious chemical stimuli with CO2 in rats: is it a peripheral nociceptive event? Neurosci Lett 128:297–300 [DOI] [PubMed] [Google Scholar]
- 39.Voikar V, Colacicco G, Gruber O, Vannoni E, Lipp HP, Wolfer DP. 2010. Conditioned response suppression in the IntelliCage: assessment of mouse strain differences and effects of hippocampal and striatal lesions on acquisition and retention of memory. Behav Brain Res 213:304–312 [DOI] [PubMed] [Google Scholar]
- 40.Williams WO, Riskin DK, Mott AK. 2008. Ultrasonic sound as an indicator of acute pain in laboratory mice. J Am Assoc Lab Anim Sci 47:8–10 [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao P, Qian H, Xia Y. 2005. GABA and glycine are protective to mature but toxic to immature rat cortical neurons under hypoxia. Eur J Neurosci 22:289–300 [DOI] [PubMed] [Google Scholar]