Abstract
Recognition of pain and stress is a common challenge when working with laboratory mice. The aim of the current study was to identify noninvasive parameters to assess the severity and duration of possible pain and stress after vasectomy in BALB/c mice. Mice underwent isoflurane anesthesia with or without vasectomy. Body weight, food and water intake, and fecal corticosterone metabolites (FCM) were measured 3 d before and 3 d after the procedure. Behavior was recorded 1, 2, 4, and 8 h after the procedure. Food and water consumption and defecation were reduced postoperatively in the vasectomized group compared with mice given anesthesia only. FCM were elevated the first day after anesthesia in the control mice but not in the vasectomized group. Vasectomy resulted in behavioral changes that were not seen in the group that was anesthetized only. In conclusion, food and water consumption and pain-related behaviors, but not FCM, may be useful as noninvasive parameters to assess postoperative pain and stress in vasectomized mice.
Abbreviation: FCM, fecal corticosterone metabolites
Recognition of pain and stress due to invasive experimental procedures is important for evaluation of wellbeing in laboratory animals. Pain and stress are undesirable conditions because they may affect the outcome of an experiment and increase variation between and within experimental groups. Furthermore, validated methods for assessing pain are required for evaluation of analgesic efficacy.14,23,37,59 Analgesia used in suboptimal concentrations may be ineffective in alleviating pain, and the study outcome may be biased by, for example, high levels of circulating corticosteroids. In addition, excessive doses of analgesia, regardless of the pharmacologic analgesic class, may cause serious side effects. The most common side effects of most classes of NSAID are gastric and intestinal ulceration, and in combination with anesthesia, renal toxicosis may develop.38 Opioids, which are used frequently in laboratory animal experimentation, may suppress cardiovascular, respiratory, and gastrointestinal functions, and high doses may inhibit postsurgical recovery.1 This information illustrates why analgesic drugs should be used with caution and in correct doses and why establishing reliable pain assessment methods is important.20
Analgesiometric studies have been used frequently to evaluate the clinical effects of analgesia. However, knowledge about pain and analgesia obtained from analgesiometric studies may have little relevance in the clinical evaluation of postsurgical pain, because the pathology of the pain stimulated in these tests is not comparable to postoperative pain.15,53 Clinical studies evaluating pain of individual surgical procedures are therefore an important step to refine analgesic treatment and to ensure optimal postsurgical welfare.1
The effects of the anesthesia may bias pain assessment when evaluating postoperative pain in newly operated animals. Handling, loss of consciousness, and recovery from anesthesia have been shown in many species, including mice, to be significant stressors.5,11,16,22,66 Changes in clinical biochemical (for example, chloride, potassium, glucose, and alkaline phosphatase), hematologic (for example, erythrocyte indices, hematocrit, and white blood cells), and hormonal parameters (for example, prolactin and thyroxine) occur after various inhalation anesthetic regimens in rats.16 In mice, anesthesia with ketamine–xylazine (100 mg/kg, 10 mg/kg) or avertin (240 mg/kg) caused sustained hyperglycermia,11 whereas methoxyflurane and diethylether increased heart rate and locomotor activity and decreased body temperature.5 In addition, methoxyflurane decreased body weight by the second day after the anesthesia.5 Serum corticosterone was increased several hours after isoflurane anesthesia in rats and for more than 24 h in rats that underwent CO2 anesthesia.2 Serum glucocorticoids in rabbits anesthetized with either halothane or isoflurane anesthesia remained elevated for 24 h.21 Despite differences between species, strains, and sex, all animals that undergo anesthesia, regardless of the drug, seem to be affected by the procedure. Therefore, the effects of anesthesia must be differentiated from postsurgical pain when evaluating the severity of a procedure and optimizing analgesic treatment. Isoflurane is widely used in experimental procedures due to its relatively minor effects on cardiac and respiratory function, rapid induction of and recovery from anesthesia, and minimal toxic effects.19 However, isoflurane provides no analgesia, and the reported effects of isoflurane anesthesia on the stress response differ depending on the stress assessment method used.2,21,48
Behavioral observation is one of only a few noninvasive approaches to assessing pain and stress and does not involve retrospective assessment but gives immediate information on the animals’ physiologic and psychologic state. However, behavioral monitoring of mice for adverse signs has limitations. Mice are prey animals and may show minimal signs of pain. Even a person entering the room may evoke a mouse's natural defense behavior to hide any signs of weakness or pain.52 This defensive response makes monitoring of pain-associated behavior difficult and has resulted in behavioral monitoring of mice being perceived as a difficult and unreliable method for assessing pain.4 However, behavioral measures are effective in many species,25,41,43,52,57 and recent data suggest that behavioral analysis can be used to assess postoperative pain in mice.17,54,67
Because mice are crepuscular, they have different activity levels during night and daytime. In particular, mice have a diurnal variation in their pain reactions, with a higher pain score when tested during the dark phase (1900 to 2200) than when tested during the light phase (0700 to 1000) of the day.45,46 Therefore, behavioral observations performed only during daytime could underestimate pain in mice. In addition, behavior can be influenced by social factors, stress, and mouse strain.8,34,63 By combining behavioral observations with quantitative physiologic measures such as body weight, food and water consumption, and glucocorticoid levels, reliable information about the physiologic and psychologic state of the animal can be obtained.25,28,33 Food and water consumption, body weight, and corticosterone levels can provide reliable information regarding animal wellbeing during evaluation of postoperative pain and stress in several species.15,37,53,65 Levels of fecal corticosterone and corticosterone metabolites accurately represent the integrated amounts of these steroids over time, whereas blood levels of corticosterone provide only a ‘snapshot’ of the stress level. Furthermore, quantification of corticosterone metabolites in feces is unlikely to be biased by the sampling technique, because it is noninvasive and does not involve interaction with the animal during the period of interest.
The aim of the current study was to identify noninvasive parameters to assess the severity and duration of possible pain and stress after vasectomy in BALB/c mice. We hypothesized that isoflurane anesthesia in the absence of surgery would induce a stress response but that it would be of short duration and not affect basic behaviors, such as eating and drinking. In contrast, anesthetized mice that underwent abdominal surgery were expected to show changes in feeding and drinking pattern and possible changes in behavioral profile. We also hypothesized that both anesthesia and surgery would stimulate the hypothalamic–pituitary–adrenal axis as indicated through corticosterone metabolites (FCM) levels.
Materials and Methods
The experiments performed in the current study were approved by the Animal Experiments Inspectorate under the Danish Ministry of Justice (license number 2005/561-1059). All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals31 in a fully AAALAC-accredited facility.
Animals and housing conditions.
Male BALB/c mice (n = 16; age, 8 to 10 wk; weight, 21 to 32 g) were obtained from Taconic (Ry, Denmark). Mice were vendor-designated as SPF for mouse hepatitis virus, minute virus of mice, mouse parvovirus, mouse rotavirus, encephalomyelitis virus, pneumonia virus of mice, Sendai virus, lymphocytic choriomeningitis virus, murine norovirus, ectromelia virus, Hantaan virus, mouse adenovirus 1 and 2, mouse cytomegalovirus, respiratory enteric virus III, K virus, lactic dehydrogenase elevating virus, polyoma virus, thymic virus, β-hemolytic Streptococcus, Bordetella bronchoseptica, Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutscheri, Klebsiella oxytoca, K. pneumonia, Mycoplasma spp., Pasteurella pneumotropica, other Pasteurella spp., Pseudomonas aeruginosa, Salmonella spp., Staphylococcus aureus, Streptococcus pneumonia, cilia-associated respiratory bacillus, Heliobacter hepaticus, H. bilis, Pneumocystis carinii, and endo- and ectoparasites.
The mice were housed individually before and after surgery to avoid social stress from affecting the results of the behavioral analysis50 and to ensure a calm postsurgical recovery period. Short-term isolation of mice generally is considered to be not stressful,9,24 and for habituation, the mice were housed individually 1 wk prior the study. The mice were housed in Macrolon cages (Techniplast, Varese, Italy) with food pellets (Altromin 1319, Brogaarden, Gentofte, Denmark) and acidified tap water provided ad libitum. Woodchips (Tapvei Oy, Kortteinen, Finland) were used as bedding, and plastic houses (Brogaarden, Gentofte, Denmark) were provided as environmental enrichment. Room temperature was maintained at 20 ± 2 °C, relative humidity was 30% to 60%, and the light regimen was a 12:12-h dark:light cycle (lights on, 0630). The day after anesthesia with or without surgery, the lighting period was expanded 1 h in the evening (from 0630 to 1930) to allow all behavioral observations to be made during the light period.
Study design.
The mice were divided randomly into 2 groups of 8 animals each to undergo anesthesia only or anesthesia plus vasectomy. Due to practical reasons, the study was conducted in 2 experimental periods with an interval of 1 mo. Both groups were equally distributed during both experimental periods.
Experimental procedure.
Experimental manipulations were performed between 0730 and 1130. The same person anesthetized and performed surgery on all mice in a randomized order. The mice were placed in an induction chamber, and anesthesia was induced with 5% isoflurane (Forene, Abbot Scandinavia, Stockholm, Sweden) delivered in pure oxygen. Once the paw withdrawal reflex was absent, the mice were shaved at the incision sites and attached to an anesthetic face mask for spontaneous respiration. Isoflurane was maintained at approximately 2.5% to ensure adequate anesthesia. All mice were placed on a heating pad (HB101, Panlab, Cornella, Spain), and rectal body temperature was maintained at 36 to 38 °C. The skin was disinfected with 80% ethanol. Surgery consisted of a 1-cm transverse incision through the skin and linea alba. The vas deferens was identified and after ligation with 6-0 resorbable Vicryl suture (Ethicon, St Stevens Woluwe, Belgium), a piece of 6 to 8 mm was removed by cutting. The abdominal wall and skin were closed separately with 6-0 Vicryl. About 0.5 mL 37 °C isotonic saline was given subcutaneously to counteract dehydration and to ensure adequate body temperature during recovery. The time of the procedure, from induction of anesthesia to regaining righting reflex, was 15 to 20 min. The mice were allowed to recover in a quiet room, in which filming was performed. The control mice were anesthetized by using 5% isoflurane delivered in pure oxygen for induction and maintained for 15 to 20 min at 2.5% isoflurane in oxygen; each mouse received about 0.5 mL 37 °C isotonic saline subcutaneously, as for the vasectomized mice.
Data collection.
Data from all animals were obtained daily between 0800 and 0830. Body weight, daily food and water intake, mass of feces produced, and FCM levels of each mouse were recorded 3 d before the procedure to provide preprocedural values and 3 d after surgery or anesthesia. The daily food and water consumption were calculated by subtracting the measured weight of food or water from the amount measured the previous day. At the time of weighing, all bedding was removed from each cage and frozen at −21 °C until feces were separated from bedding. The nesting material, mouse hut, and cage were reused for each mouse throughout the experiment to maintain individual olfactory signals within the mouse's environment and thereby minimizing stress in relation to bedding change. The fecal pellets were weighed and are presented as grams of feces per day. FCM were quantified as described previously.32,58 Corticosterone metabolites were extracted by incubating feces in 96% ethanol (5 mL per gram feces) overnight. Corticosterone levels were analyzed in duplicate (EIA4164, DRG Instruments, Marburg, Germany) in accordance with the manufacturer's instructions. Standards included in the kit were replaced with a custom 9-point standard curve prepared in 96% ethanol from analytical grade corticosterone (catalog no. 46148, Sigma-Aldrich, St Louis, MO) in concentrations from 50 to 0.19 ng/mL. The kit has been verified to have a crossreactivity equivalent to 7.4% with progesterone, 3.4% with deoxycorticosterone, 1.6% with 11-dehydrocorticosterone, 0.3% with cortisol and pregnenolone, and less than 0.1% with other steroids. The absorbencies were recorded at 450 nm (reference wavelength, 650 nm; Thermo Fisher Scientific, Waltham, MA). Results are presented as total nanograms of FCM and nanograms of FCM per gram of feces daily.
Behavioral observations.
Behavioral observations were obtained by videorecording with digital cameras (GZ MG6800, JVC, Yokohama, Japan). The camera was fixed on a tripod (JVC) and placed so that the entire cage could be seen at all times; 6-min intervals were recorded at 1, 2, 4, and 8 h after mice regained consciousness. For recording, one person carefully entered the room, turned on all cameras (one camera per cage), and then left the room.
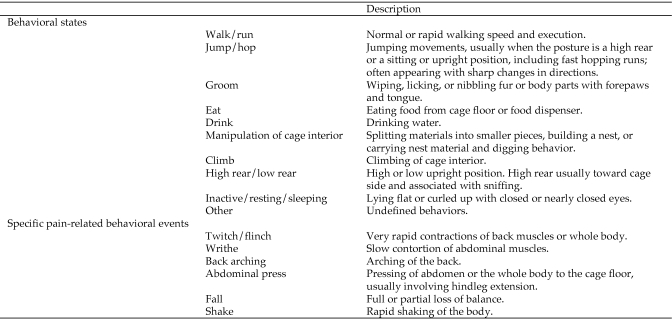
Behaviors were identified by using an ethogram (Figure 1) consisting of 16 behavioral categories comprising 10 states and 6 events. The ethogram was based on those used in previous studies on mice and was adapted to the present study.54,67 States are defined as behaviors of prolonged duration, and brief behaviors are regarded as events. States were sampled by using an instantaneous-sampling technique3 with a 15-s intersample interval. No behavior was recorded at the start time of filming (time 0), thus resulting in 23 state scorings per 6 min. Events were sampled by a modified one–zero sampling technique,3 and all occurrences of events within the sampling interval were recorded.
Figure 1.
Behavioral states and specific pain-related behavioral events. Behavioral states were defined as prolonged behaviors, whereas specific pain-related events were of momentary duration.
Statistical analysis.
Q-Q plots were constructed to test for normal distribution. Body weight, food and water consumption, and the amount of feces produced were normally distributed. FCM values were found to conform to a log-normal distribution. Body weight was analyzed as mean gain or loss. Water consumption values on day −3 were excluded due to incorrect handling of the water bottles and therefore incorrect measurements. A multivariate general linear model with Tukey multiple comparisons test, with treatment group and day as fixed factors, was applied on parameters measured days −3, −2, and −1 to test for differences in preprocedural levels. Body weight, food and water consumption, amount of feces produced, and FCM levels on days 1, 2, and 3 after the procedure were analyzed by using a multivariate general linear model with Tukey multiple comparisons test, with treatment group and day as fixed factors. When significant interaction (a nonadditive effect of multiple factors) between treatment group and day occurred, 2-tailed unpaired t tests with Bonferroni correction were performed to determine on which days the groups differed.
Behavioral states and events were analyzed separately by using the nonparametric Kruskal–Wallis test. P values less than 0.05 were considered significant. All statistical tests were performed by using PASW Statistics v.18 (SPSS, Chicago, IL). Graphs were made in GraphPad Prism version 5.00 (Graph Software, San Diego, CA).
Results
Preprocedural values.
No differences were seen between the 2 groups or between the 3 d during the preprocedural period in any of the categories (data not shown). Any difference observed during the days after anesthesia with or without surgery therefore is concluded to represent effects due to the procedure.
Body weight.
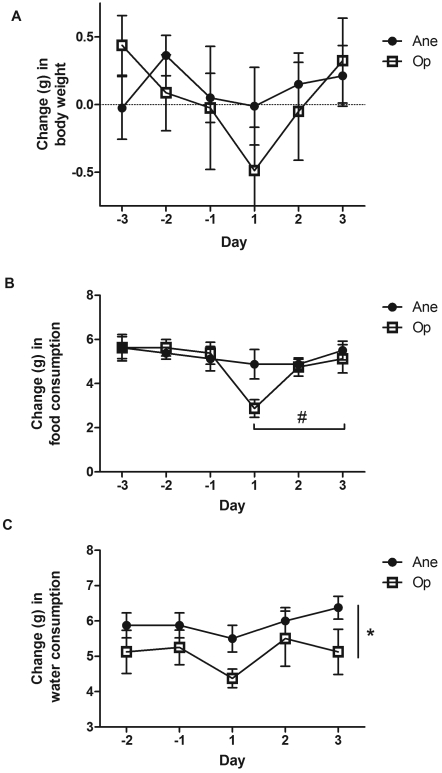
No difference in body weight was seen between treatment groups (F[1;47] = 0.611, P = 0.439) or across days (F[2;47] = 1.573, P = 0.219; Figure 2 A).
Figure 2.
Mean change (bar, SEM) in (A) body weight, (B) daily food consumption, and (C) daily water consumption in mice that underwent isoflurane anesthesia alone (Ane) or with vasectomy (Op). Days –3,–2, and –1 are before surgery; days 1, 2, and 3 are after surgery. Values that differed significantly between days (#, P < 0.05) or groups (*, P < 0.05) are indicated.
Food consumption.
No significant difference in food consumption was seen between the 2 groups (Figure 2 B); however, a trend was seen in which vasectomized mice ate less than did control mice (F[1;47] = 3.818, P = 0.057). Across days, a significant change in food consumption was seen (F[2;47] = 3.217, P = 0.05), and Tukey post hoc tests found a significant difference between days 1 and 3 (P = 0.04).
Water consumption.
Water intake differed between the 2 groups (F[1;47] = 5.602, P = 0.023; Figure 2 C), with the vasectomized mice drinking significantly less than did the anesthetized mice. No difference in water intake was seen across days (F[2;47] = 1.790, P = 0.18).
Fecal production.
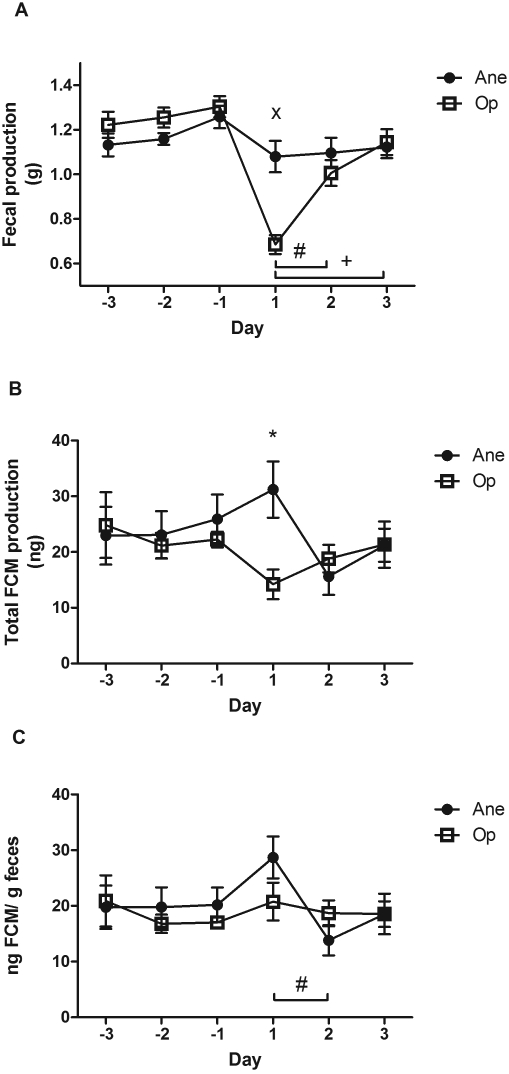
All mice excreted feces each day, with vasectomized mice excreting significantly less than did anesthetized mice (F[1;47] = 10.376, P = 0.002; Figure 3 A). The amount of feces produced differed significantly across days (F[2;47] = 9.577, P < 10−4), with day 1 differing from days 2 and 3 as determined by Tukey post hoc test (P = 0.018 and P < 10−4, respectively). A significant interaction between day and treatment group was found (F[2;47] = 6.733, P = 0.003), with a significant difference between groups on day 1 (t = 4.832, P < 10−4).
Figure 3.
Mean (bar, SEM) (A) fecal production (g), (B) total fecal corticosterone metabolites (FCM; ng), and (C) nanograms FCM per gram of feces in mice that underwent isoflurane anesthesia alone (Ane) or with vasectomy (Op). Days –3,–2, and –1 are before surgery; days 1, 2, and 3 are after surgery. Values that differed significantly between days (#, P < 0.05; +, P < 0.001) or groups (*, P < 0.05; ×, P < 0.001) are indicated.
FCM.
Total FCM produced daily did not differ between groups (F[1;47] = 1.486, P = 0.230) or across days (F(2;47) = 1.061, P = 0.355; Figure 3 B). However, a significant interaction between day and group was found (F[2;47] = 4.701, P = 0.014), with a significant difference between groups on day 1 (t = 2.968, P = 0.01). When adjusted for the amount of feces produced, FCM levels showed no difference between groups (F[1;47] = 0.018, P = 0.894; Figure 3 C) but a significant difference across days (F[2;47] = 3.375, P = 0.044), specifically between days 1 and 2 (P = 0.037).
Behavioral observations.
When compared between individual time points (that is, 1, 2, 4, and 8 h after the procedures), behavioral states and events did not differ within groups (as analyzed by a Kruskal–Wallis test with time as a grouping variable). The data obtained at the individual time points therefore were pooled for statistical comparison. No difference between groups was observed in any of the behavioral states after the procedure (data not shown). However, a Kruskal–Wallis test demonstrated significantly higher levels of twitch–flinch behavior (P = 0.006), writhing behavior (P < 10−4), and abdominal press (P = 0.005) in the operated group.
Discussion
To permit assessment of the effect of vasectomy on parameters related to pain and stress, none of our mice received any preemptive or postoperative analgesia. This decision raises important animal welfare concerns. However, if any mice had shown clear signs of substantial pain, they would have been withdrawn from the study for humane reasons. Regardless, no mouse demonstrated overt signs of discomfort.
Even seemingly innocuous laboratory routines such as handling and cage change can affect physiologic parameters in mice (for example, serum corticosterone, heart rate, and blood pressure).6,49 Therefore, minimization of unwanted sources of stress is important in pain and stress assessment studies. The potential stress of daily handling and bedding removal in the present study did not affect any of the measured parameters during the 3 d prior to the experimental procedures. Perhaps the parameters we used were not sufficiently sensitive to detect small changes in the stress response. Furthermore, the mice retained the same mouse hut and nesting material throughout the experimental period. The 1-h change in lighting regimen on the day of surgery is a potential source of error, because such changes can be stressful to animals.29,62 However, because the change in lighting regime was of short duration and affected both groups equally, any differences between the groups on the day after anesthesia with or without vasectomy are assumed to be related to the different experimental procedures.
The knowledge obtained in the current study is important in the process of evaluating optimal analgesic regimens and ensuring high animal welfare in laboratory mice. Only a few studies54,67 have investigated pain in relation to vasectomy in laboratory mice, and none of these, to our knowledge, evaluated BALB/c mice, which are used frequently both as animal models and in breeding strategies. One study reported marked differences in the behavioral phenotype of 6 inbred mouse strains,51 and strain-associated differences in the behavioral profiles of inbreed mice are well known.64 As demonstrated previously, 17,67 interstrain differences also exist in regard to behavioral and corticosterone responses to postsurgical pain, and these differences imply that different dose rates of analgesia may be required to control postoperative pain in various strains of mice.
Vasectomy is a common procedure in many laboratory animal facilities due to the use of embryo transfer and genetically modified mice. This surgery is often classified as mildly to moderately invasive because it involves abdominal manipulation, tissue injury, and stimulates the hypothalamic–pituitary–adrenal axis.33 Therefore, this operative procedure be a good model for investigating whether different noninvasive parameters can be relied on for assessing postoperative pain. In the present study, mice in the vasectomized group had lower levels of food and water consumption than did the control group. The same was seen in the amount of feces voided, for which the vasectomized mice had significantly lower levels the day after surgery. These results suggest that these parameters are potential indicators of postsurgical pain in vasectomized BALB/c mice. Two days after surgery, no differences could be seen between the 2 groups. This finding correlates well with previous studies, demonstrating that pain related to mild and moderate surgical procedures often is of short duration.1,10,15,30
Quantification of glucocorticoid levels in blood and feces has been used extensively as biomarkers of stress in several species, including mice,26 even though the correlation is not always straightforward.18,55 Measuring FCM levels seems to provide reliable information regarding pain due to invasive procedures in rats.32,35,56 However, the FCM measurements we obtained may be biased by reduced fecal output, which might have been due to the reduced food intake in the vasectomized group. In addition, postoperative ileus is a common complication of abdominal surgery.7,39,40 The reduced fecal output seen in the vasectomized group could explain the low levels of total corticosterone.27 However, even when expressed as FCM produced per gram of feces,36,60 the vasectomized group did not differ significantly from the anesthetized group. This result contrasts with a previous study evaluating FCM obtained at different time points by stroking the tail of the mouse and applying light pressure to the back to encourage the mouse to defecate.67 Analyzing only a few fecal pellets may be misleading, owing to high variability in glucocorticoid content between fecal pellets voided at the same time in several species.13,42,44,47 Furthermore, only increases in serum glucocorticoids of sustained duration may have clinical significance. Peaks of FCM at narrow sampling windows may reflect only a brief stress response. In the present study, we expected that isoflurane anesthesia and vasectomy combined would markedly activate the hypothalamic–pituitary–adrenal axis, such that increases in FCM would have been measurable over 24-h intervals. Our broad sampling window might have missed small transient increases in FCM.
A previous study did not detect increased FCM at 12 or 24 h after surgical removal of the mammary fat pad in female mice.1 Although the surgery was regarded as minor and did not induce pain or distress sufficient to increase FCM, food consumption and wheel running activity indicated the presence of pain during the first 24 h after surgery.1 Despite many differences between our current work and this previous experiment1 (for example, invasiveness of the procedure, anesthesia used, and FCM sampling interval), the 2 studies demonstrate the difficulties in using and interpreting FCM levels as indices of pain in mice. Even serum levels of corticosterone may not precisely reflect postsurgical pain in mice. A recent work showed no difference in serum corticosterone between mice that experienced partial hepatectomy but different analgesic strategies, even though other parameters indicated that not all treatments provided effective pain relief.61 Perhaps corticosterone output over time is affected by physiologic changes in the hypothalamic–pituitary–adrenal axis immediately after surgery, given that high levels of circulating corticosterone may inhibit further release of ACTH through negative feedback inhibition.12 Further studies are needed to validate the use of serum corticosterone and FCM as indications of pain and to explain the absence of a peak of fecal corticosterone in the vasectomized group in the present study.
The study showed no difference in behavioral states of vasectomized and anesthetized mice. Previous studies of postsurgical behavior in mice have demonstrated that vasectomy reduced the duration of digging, high-rearing,67 walking, and rearing behaviors54 and caused changes in a range of behavioral states such as walk, sleep, hanging vertically, and rearing compared with those in control mice when analyzed with an automated behavioral scoring method.17 Continuous scoring of behavioral states by using automated systems has been demonstrated to be as reliable as manual sampling and thus encourages prolonged behavioral scoring.17,54 However, the use of computer software is not always feasible or practical, and simple methods of pain assessment in mice therefore are needed. The behavioral states and sampling method used in the present study may have lacked the sensitivity necessary to differentiate the 2 groups.
Behavioral events have been shown to be reliable behavioral parameters when assessing pain in laboratory mice.17,52 In the present investigation, the behavioral events twitch/flinch, writhing, and abdominal press were useful in differentiating between the 2 groups, because the vasectomized mice had significantly more occurrences of these behaviors. Therefore, behavioral events may be more useful than are behavioral states when performing short-term behavioral pain assessment. This difference may reflect that behavioral states are complex and sustained activities, whereas pain-related behavioral events are simple actions that are associated with focally painful stimuli due to the surgically traumatized tissue.
In conclusion, food and water consumption were useful parameters for detecting possible pain and stress after vasectomy of mice. FCM levels revealed that handling and isoflurane anesthesia induced a stress response but FCM were not useful for pain assessment in the present experimental setup, perhaps because of the decreased amount of feces produced after surgery. Behavioral events such as twitch/flinch, abdominal press, and writhing seem to be reliable indices of pain and may be useful in assessing pain in male vasectomized mice.
Acknowledgments
The present investigation was supported by a generous grant from the Danish Council for Strategic Research. We thank Trine Marie Nielsen and Shama Khalid for technical assistance.
References
- 1.Adamson TW, Kendall LV, Goss S, Grayson K, Touma C, Palme R, Chen JQ, Borowsky AD. 2010. Assessment of carprofen and buprenorphine on recovery of mice after surgical removal of the mammary fat pad. J Am Assoc Lab Anim Sci 49:610–616 [PMC free article] [PubMed] [Google Scholar]
- 2.Altholtz LY, Fowler KA, Badura LL, Kovacs MS. 2006. Comparison of the stress response in rats to repeated isoflurane or CO2: O2 anesthesia used for restraint during serial blood collection via the jugular vein. J Am Assoc Lab Anim Sci 45:17–22 [PubMed] [Google Scholar]
- 3.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49:227–267 [DOI] [PubMed] [Google Scholar]
- 4.Arras M, Rettich A, Cinelli P, Kasermann H, Burki K. 2007. Assessment of post-laparotomy pain in laboratory mice by telemetric recording of heart rate and heart rate variability. BMC Vet Res 3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arras M, Rettich A, Seifert B, Kasermann HP, Rulicke T. 2007. Should laboratory mice be anaesthetized for tail biopsy? Lab Anim 41:30–45 [DOI] [PubMed] [Google Scholar]
- 6.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51 [PubMed] [Google Scholar]
- 7.Bauer AJ, Boeckxstaens GE. 2004. Mechanisms of postoperative ileus. Neurogastroenterol Motil 16:54–60 [DOI] [PubMed] [Google Scholar]
- 8.Belknap JK, Lame M, Danielson PW. 1990. Inbred strain differences in morphine-induced analgesia with the hot plate assay: a reassessment. Behav Genet 20:333–338 [DOI] [PubMed] [Google Scholar]
- 9.Benton D, Brain PF. 1981. Behavioral and adrenocortical reactivity in female mice following individual or group housing. Dev Psychobiol 14:101–107 [DOI] [PubMed] [Google Scholar]
- 10.Blaha MD, Leon LR. 2008. Effects of indomethacin and buprenorphine analgesia on the postoperative recovery of mice. J Am Assoc Lab Anim Sci 47:8–19 [PMC free article] [PubMed] [Google Scholar]
- 11.Brown ET, Umino Y, Solessio E, Barlow R. 2005. Anesthesia can cause sustained hyperglycemia in C57/BL6J mice. Vis Neurosci 22:615–618 [DOI] [PubMed] [Google Scholar]
- 12.Bugajski J, Gadek-Michalska A, Bugajski AJ. 2001. A single corticosterone pretreatment inhibits the hypothalamic–pituitary–adrenal responses to adrenergic and cholinergic stimulation. J Physiol Pharmacol 52:313–324 [PubMed] [Google Scholar]
- 13.Carlsson HE, Lyberg K, Royo F, Hau J. 2007. Quantification of stress sensitive markers in single fecal samples do not accurately predict excretion of these in the pig. Res Vet Sci 82:423–428 [DOI] [PubMed] [Google Scholar]
- 14.Carr DB, Goudas LC. 1999. Acute pain. Lancet 353:2051–2058 [DOI] [PubMed] [Google Scholar]
- 15.Cooper DM, Hoffman W, Wheat N, Lee HY. 2005. Duration of effects on clinical parameters and referred hyperalgesia in rats after abdominal surgery and multiple doses of analgesic. Comp Med 55:344–353 [PubMed] [Google Scholar]
- 16.Deckardt K, Weber I, Kaspers U, Hellwig J, Tennekes H, van Ravenzwaay B. 2007. The effects of inhalation anaesthetics on common clinical pathology parameters in laboratory rats. Food Chem Toxicol 45:1709–1718 [DOI] [PubMed] [Google Scholar]
- 17.Dickinson AL, Leach MC, Flecknell PA. 2009. The analgesic effects of oral paracetamol in 2 strains of mice undergoing vasectomy. Lab Anim 43:357–361 [DOI] [PubMed] [Google Scholar]
- 18.Duncan IJH. 2005. Science-based assessment of animal welfare: farm animals. Rev Sci Tech 24:483–492 [PubMed] [Google Scholar]
- 19.Flecknell P. 2009. Laboratory animal anaesthesia, 3rd ed. New York (NY): Academic Press [Google Scholar]
- 20.Flecknell PA. 1994. Refinement of animal use—assessment and alleviation of pain and distress. Lab Anim 28:222–231 [DOI] [PubMed] [Google Scholar]
- 21.Gil AG, Silvan G, Illera JC. 2007. Pituitary–adrenocortical axis, serum serotonin, and biochemical response after halothane or isoflurane anaesthesia in rabbits. Lab Anim 41:411–419 [DOI] [PubMed] [Google Scholar]
- 22.Gil AG, Silvan G, Illera M, Illera JC. 2004. The effects of anesthesia on the clinical chemistry of New Zealand white rabbits. Contemp Top Lab Anim Sci 43:25–29 [PubMed] [Google Scholar]
- 23.Goecke JC, Awad H, Lawson JC, Boivin GP. 2005. Evaluating postoperative analgesics in mice using telemetry. Comp Med 55:37–44 [PubMed] [Google Scholar]
- 24.Goldsmith JF, Brain PF, Benton D. 1978. Effects of the duration of individual or group housing on behavioural and adrenocortical reactivity in male mice. Physiol Behav 21:757–760 [DOI] [PubMed] [Google Scholar]
- 25.Graham MJ, Kent JE, Molony V. 1997. Effects of 4 analgetic treatments on the behavioural and cortisol responses of 3-week-old lambs to tail docking. Vet J 153:87–97 [DOI] [PubMed] [Google Scholar]
- 26.Harper JM, Austad SN. 2000. Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol Biochem Zool 73:12–22 [DOI] [PubMed] [Google Scholar]
- 27.Hau J, Kalliokoski O, Jacobsen KR, Abelson KSP. 2011. Interpretations of faecal concentrations of corticosteroids. Lab Anim 45:129–130 [DOI] [PubMed] [Google Scholar]
- 28.Hawkins P. 2002. Recognizing and assessing pain, suffering, and distress in laboratory animals: a survey of current practice in the UK with recommendations. Lab Anim 36:378–395 [DOI] [PubMed] [Google Scholar]
- 29.Hayashi O, Aoki N, Kikuchi M. 1982. Influence of phase shift in light–dark cycle on humoral immune responses of mice against sheep red blood cells and polyvinylpyrrolidone. In: Abstracts of the 20th annual meeting of the Japanese Society of Biometeorology, Ube, 26–27 November 1981. Int J Biometeorol 26:347 [Google Scholar]
- 30.Hayes KE, Raucci JA, Jr, Gades NM, Toth LA. 2000. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Top Lab Anim Sci 39:18–23 [PubMed] [Google Scholar]
- 31.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 32.Kalliokoski O, Abelson KSP, Koch J, Boschian A, Thormose SF, Fauerby N, Rasmussen RS, Johansen FF, Hau J. 2010. The effect of voluntarily ingested buprenorphine on rats subjected to surgically induced global cerebral ischaemia. In Vivo 24:641–646 [PubMed] [Google Scholar]
- 33.Kohn DF, Martin TE, Foley PL, Morris TH, Swindle MM, Vogler GA, Wixson SK. 2007. Guidelines for the assessment and management of pain in rodents and rabbits. J Am Assoc Lab Anim Sci 46:97–108 [PubMed] [Google Scholar]
- 34.LaBuda CJ, Sora I, Uhl GR, Fuchs PN. 2000. Stress-induced analgesia in µ-opioid receptor knockout mice reveals normal function of the δ-opioid receptor system. Brain Res 869:1–5 [DOI] [PubMed] [Google Scholar]
- 35.Lepschy M, Touma C, Hruby R, Palme R. 2007. Noninvasive measurement of adrenocortical activity in male and female rats. Lab Anim 41:372–387 [DOI] [PubMed] [Google Scholar]
- 36.Lepschy M, Touma C, Palme R. 2010. Faecal glucocorticoid metabolites: how to express yourself. Comparison of absolute amounts versus concentrations in samples from a study in laboratory rats. Lab Anim 44:192–198 [DOI] [PubMed] [Google Scholar]
- 37.Liles JH, Flecknell PA. 1992. The effects of buprenorphine, nalbuphine, and butorphanol alone or following halothane anaesthesia on food and water consumption and locomotor movement in rats. Lab Anim 26:180–189 [DOI] [PubMed] [Google Scholar]
- 38.Liles JH, Flecknell PA. 1992. The use of nonsteroidal antiinflammatory drugs for the relief of pain in laboratory rodents and rabbits. Lab Anim 26:241–255 [DOI] [PubMed] [Google Scholar]
- 39.Luckey A, Livingston E, Tache Y. 2003. Mechanisms and treatment of postoperative ileus. Arch Surg 138:206–214 [DOI] [PubMed] [Google Scholar]
- 40.Luckey A, Wang LX, Jamieson PM, Basa NR, Million M, Czimmer J, Vale W, Tache Y. 2003. Corticotropin-releasing factor receptor 1-deficient mice do not develop postoperative gastric ileus. Gastroenterology 125:654–659 [DOI] [PubMed] [Google Scholar]
- 41.Malavasi LM, Nyman G, Augustsson H, Jacobson M, Jensen-Waern M. 2006. Effects of epidural morphine and transdermal fentanyl analgesia on physiology and behaviour after abdominal surgery in pigs. Lab Anim 40:16–27 [DOI] [PubMed] [Google Scholar]
- 42.Millspaugh JJ, Washburn BE. 2003. Within-sample variation of fecal glucocorticoid measurements. Gen Comp Endocrinol 132:21–26 [DOI] [PubMed] [Google Scholar]
- 43.Molony V, Kent JE, Hosie BD, Graham MJ. 1997. Reduction in pain suffered by lambs at castration. Vet J 153:205–213 [DOI] [PubMed] [Google Scholar]
- 44.Paramastri Y, Royo F, Eberova J, Carlsson HE, Sajuthi D, Fernstrom AL, Pamungkas J, Hau J. 2007. Urinary and fecal immunoglobulin A, cortisol, and 11-17–dioxoandrostanes, and serum cortisol in metabolic cage housed female cynomolgus monkeys (Macaca fascicularis). J Med Primatol 36:355–364 [DOI] [PubMed] [Google Scholar]
- 45.Perissin L, Boccalon S, Scaggiante B, Petrelli L, Ortolani F, Porro CA. 2004. Diurnal changes of tonic nociceptive responses in mice: evidence for a proalgesic role of melatonin. Pain 110:250–258 [DOI] [PubMed] [Google Scholar]
- 46.Perissin L, Facchin P, Porro CA. 2000. Diurnal variations in tonic pain reactions in mice. Life Sci 67:1477–1488 [DOI] [PubMed] [Google Scholar]
- 47.Pihl L, Hau J. 2003. Faecal corticosterone and immunoglobin A in young adult rats. Lab Anim 37:166–171 [DOI] [PubMed] [Google Scholar]
- 48.Pomplun D, Mohlig M, Spranger J, Pfeiffer AFH, Ristow M. 2004. Elevation of blood glucose following anaesthetic treatment in C57Bl/6 mice. Horm Metab Res 36:67–69 [DOI] [PubMed] [Google Scholar]
- 49.Rasmussen S, Miller MM, Filipski SB, Tolwani R. 2011. Cage change influences serum corticosterone and anxiety-like behaviors in the mouse. J Am Assoc Lab Anim Sci 50:479–483 [PMC free article] [PubMed] [Google Scholar]
- 50.Rodgers RJ, Hendrie CA. 1983. Social conflict activates status-dependent endogenous analgesic or hyperalgesic mechanisms in male mice: effects of naloxone on nociception and behaviour. Physiol Behav 30:775–780 [DOI] [PubMed] [Google Scholar]
- 51.Rogers DC, Fisher EMC, Brown SDM, Peters J, Hunter AJ, Martin JE. 1997. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 8:711–713 [DOI] [PubMed] [Google Scholar]
- 52.Roughan JV, Flecknell PA. 2001. Behavioural effects of laparotomy and analgesic effects of ketoprofen and caprofen in rats. Pain 90:65–74 [DOI] [PubMed] [Google Scholar]
- 53.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating postoperative pain in animals. Lab Anim 36:322–343 [DOI] [PubMed] [Google Scholar]
- 54.Roughan JV, Wright-Williams SL, Flecknell PA. 2009. Automated analysis of postoperative behaviour: assessment of HomeCageScan as a novel method to rapidly identify pain and analgesic effects in mice. Lab Anim 43:17–26 [DOI] [PubMed] [Google Scholar]
- 55.Rushen J. 1991. Problems associated with the interpretation of physiological data in the assessment of animal welfare. Appl Anim Behav Sci 28:381–386 [Google Scholar]
- 56.Siswanto H, Hau J, Carlsson HE, Goldkuhl R, Abelson KSP. 2008. Corticosterone concentrations in blood and excretion in faeces after ACTH administration in male Sprague–Dawley rats. In Vivo 22:435–440 [PubMed] [Google Scholar]
- 57.Stafford KJ, Mellor DJ, Todd SE, Bruce RA, Ward RN. 2002. Effects of local anaesthesia or local anaesthesia plus a nonsteroidal antiinflammatory drug on the acute cortisol response of calves to 5 different methods of castration. Res Vet Sci 73:61–70 [DOI] [PubMed] [Google Scholar]
- 58.Sundbom R, Jacobsen KR, Kalliokoski O, Hau J, Abelson KSP. 2011. Postoperative corticosterone levels in plasma and feces of mice subjected to permanent catheterization and automated blood sampling. In Vivo 25:335–342 [PubMed] [Google Scholar]
- 59.Thompson AC, Kristal MB, Sallaj A, Acheson A, Martin LB, Martin T. 2004. Analgesic efficacy of orally administered buprenorphine in rats: methodologic considerations. Comp Med 54:293–300 [PubMed] [Google Scholar]
- 60.Touma C, Palme R, Sachser N. 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22 [DOI] [PubMed] [Google Scholar]
- 61.Tubbs JT, Kissling GE, Travlos GS, Goulding DR, Clark JA, King-Herbert AP, Blankenship-Paris TL. 2011. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J Am Assoc Lab Anim Sci 50:185–191 [PMC free article] [PubMed] [Google Scholar]
- 62.Van der Meer E, Van Loo PLP, Baumans V. 2004. Short-term effects of a disturbed light–dark cycle and environmental enrichment on aggression and stress-related parameters in male mice. Lab Anim 38:376–383 [DOI] [PubMed] [Google Scholar]
- 63.Van Loo PLP, Kuin N, Sommer R, Avsaroglu H, Pham T, Baumans V. 2007. Impact of ‘living apart together’ on postoperative recovery of mice compared with social and individual housing. Lab Anim 41:441–455 [DOI] [PubMed] [Google Scholar]
- 64.Van Oortmerssen GA. 1971. Biological significance, genetics, and evolutionary origin of variability in behaviour within and between inbred strains of mice (Mus musculus): a behaviour genetic study. Behaviour 38:1–92 [DOI] [PubMed] [Google Scholar]
- 65.Wheat NJ, Cooper DM. 2009. A simple method for assessing analgesic requirements and efficacy in rodents. Lab Anim (NY) 38:246–247 [DOI] [PubMed] [Google Scholar]
- 66.Whitten PL, Stavisky RC, Aureli F, Russel E. 1998. Response of fecal cortisol to stress in captive chimpanzees (Pan troglodytes). Am J Primatol 44:57–69 [DOI] [PubMed] [Google Scholar]
- 67.Wright-Williams SL, Courade JP, Richardson CA, Roughan JV, Flecknell PA. 2007. Effects of vasectomy surgery and meloxicam treatment on faecal corticosterone levels and behaviour in 2 strains of laboratory mouse. Pain 130:108–118 [DOI] [PubMed] [Google Scholar]