Abstract
Body condition scoring (BCS) is a subjective semiquantitative method of assessing body fat and muscle by palpation of key anatomic features. A previously published BCS system for rhesus macaques (Macaca mulatta) uses a scale comprising both whole and half units, in which the midrange represents optimal body condition (3.0), lower values represent emaciated to lean conditions (1.0 to 2.0), and higher values (4.0 to 5.0) indicate excessive body fat. A valid BCS system is well described, relevant to the species, has agreement within and between raters, and is consistent with objective measures. Here we correlate the subjective BCS assigned during physical exam with percentage body fat as determined by dual-energy X-ray absorptiometry (DEXA). Adult rhesus monkeys from an indoor-housed breeding colony were evaluated by the veterinary staff and assigned to 1 of 9 BCS score groups to give a minimum of 6 animals in each group. DEXA was used to obtain objective body composition measurements for macaques in each BCS group. Animals in the ‘optimal’ BCS group (3.0) had 25% body fat on average. Each full unit change in BCS was associated with an approximate 10% change in body fat percentage for macaques in the 2.0-to-5.0 BCS range. Absolute body fat in animals with BCS of 1.0 or 1.5 may be too low for accurate assessment by DEXA.
Abbreviation: BCS, body condition score; DEXA, dual-energy X-ray absorptiometry
Body condition scoring (BCS) is a subjective means of assessing an animal's lean body mass and body fat. Although a scoring system has been described for visual assessment of rhesus macaques (Macaca mulatta),3 most systems, including that which we use in the current study, involve a hands-on approach. BCS systems have been developed for several mammalian species, including dogs, cats, sheep, mice, rats, horses, and cattle.2,12,16,24,25,37,40 To be validated, a BCS system must be assessed in comparison with an objective means of measuring body composition.8 Several methods for determining body composition are used in both human physiology and animal research. Methods that have the highest degree of precision tend to be highly invasive, whereas less invasive methods compromise accuracy. Some methods used in human physiology are impractical for use in animals.13,17,34 For the current study, we used dual-energy X-ray absorptiometry (DEXA) to determine percentage body fat and compared this objective measurement with subjective assessment of body condition through BCS. We then used statistical analysis to determine whether increasing BCS correlated well with increasing percentage body fat and whether the BCS was a better indicator of percentage body fat than was weight alone.
A large amount of morphometric data exists for rhesus monkeys.20,42 One study evaluated the correlation between morphometric measurements and body composition assessed by using DEXA in rhesus monkeys.10 Although morphometric measurements are useful, both biologic and technical variation are introduced with this methodology.27 We chose DEXA as an objective measure of body composition owing to the substantial available literature on this technique and its relative noninvasiveness for reproductively active animals. DEXA analysis is frequently performed at our facility, and technicians at this institution have considerable experience and training in working proficiently with this modality.
We hypothesize that the subjective BCS of adult rhesus monkeys will be an accurate predictor of average percentage body fat as determined by DEXA analysis. Furthermore, we hypothesize that BCS is a better predictor of average percentage body fat than is weight alone.
Materials and Methods
All animals used in the current study were indoor-housed adult rhesus monkeys from the breeding colony of the California National Primate Research Center (Davis, CA), which is an AAALAC-accredited animal research facility. All animals were housed and fed in accordance with guidelines in the Guide for Care and Use of Laboratory Animals,18 and the current study was assigned to an IACUC-approved protocol at the University of California at Davis. All macaques were negative for simian retrovirus, SIV, and simian T-lymphotropic virus. A total of 71 animals (age: mean, 12 y 3 mo; range, 6 y 11 mo to 17 y 10 mo; weight: mean, 9.2 kg; range, 4.25 to 20. 72 kg) were included in our study set; 45% (n = 32) of these were male, and 55% (n = 39) were female. Macaques were fasted for 12 h prior to BCS or DEXA, which required sedation with either ketamine (10 mg/kg IM; Fort Dodge Animal Health, Fort Dodge, IA) as a sole agent or in conjunction with medetomidine hydrochloride (30 µg/kg IM; Pfizer Animal Health, Espoo, Finland). Eight staff and resident veterinarians contributed to this study and had reviewed the BCS system (Figure 1) .8 Two veterinarians had to independently agree on the same BCS for an animal to be assigned to a score group. Where possible, the same numbers of male and female macaques were assigned to each score group. To provide sufficient data for statistical analysis, at least 6 macaques were assigned to each score group, except score group 5 (n = 5). Initial evaluation of data from 6 macaques revealed no significant difference between the determined percentage body fat between score groups 1.5, 2.0, and 2.5. For this reason, 6 additional animals were added to each of these score groups.
Figure 1.
Body condition scoring chart for rhesus monkeys. Image reproduced from reference 8 with permission.
Within 2 wk of assignment to a score group, macaques underwent body composition analysis by DEXA (software version 3.9.4; Norland VR Bone Densitometer, Siemens, Berkley, CA). The scanner was calibrated and maintained according to the manufacturer's guidelines. Resolution was set at 6.5 × 13.0 mm, 130 mm/s. After sedation, macaques were placed on the scanner bed in dorsal recumbency; limbs were secured to the scanner table with porous tape. Each macaque was scanned 3 to 5 times sequentially over a period of 8 to 10 min. Averages for bone mass, lean body mass, and body fat were determined for each animal. Percentage body fat was used as an objective measurement and evaluated in relation to the BCS assigned to each animal.
Pearson linear correlation coefficients were calculated to determine the capability of using the assigned body condition score to predict percentage body fat.9,21 In addition, Spearman correlation coefficients were calculated to reflect the sensitivity of the coefficients to nonnormal distributions of variables.28 Because Spearman and Pearson analyses yielded similar results, only the Pearson results are presented here. In addition, data were evaluated statistically to determine whether BCS is a better predictor of percentage body fat than is weight alone within the full data set as well as within subgroups defined by sex, age, and weight. All statistical analyses were performed by using R Project software (www.gnu.org/software/r/).
Results
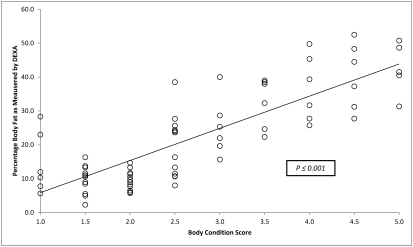
Average weight, weight range, average percentage body fat, and range for percentage body fat for each BCS are presented in Table 1. A scatter plot of average percentage body fat for individual macaques is presented in Figure 2. A summary of the average percentages for bone mass, lean body mass, and fat mass as determined by DEXA are presented in Figure 3. Adult animals with BCS in the ‘optimum’ range have approximately 25% body fat, with approximately a 10% change in body fat with each full unit of change in assigned BCS for macaques with BCS of 2.5 to 5.0. Using linear regression analysis, we determined that animals in the 1.0, 1.5, and 2.0 BCS groups had approximately 5%, 10%, and 15% body fat, respectively. The data on BCS and percentage body fat were analyzed by using ANOVA, which indicated a strong predictive value of the BCS system to assess average percentage body fat in adult rhesus monkeys (F8,58 = 23.7, P < 0.001).
Table 1.
Summary of results from DEXA analysis of all macaques
| Body weight (kg) |
Body fat (%) |
||||
| BCS | No. of macaques scanned | Range | Average | Range | Average |
| 1.0 | 6 | 4.25–10.02 | 6.57 | 0.1–41.0 | 14.9 |
| 1.5 | 12 | 5.47–10.64 | 7.02 | 0.3–24.0 | 10 |
| 2.0 | 12 | 5.10–11.36 | 7.96 | 3.0–17.0 | 9.4 |
| 2.5 | 12 | 5.75–12.75 | 9.18 | 6.0–40.0 | 19.7 |
| 3.0 | 6 | 7.10–12.20 | 8.71 | 15.0–41.0 | 25.2 |
| 3.5 | 6 | 9.52–16.03 | 13.01 | 20.0–42.0 | 32.8 |
| 4.0 | 6 | 10.81–17.22 | 13.91 | 24.0–52.0 | 36.3 |
| 4.5 | 6 | 13.51–19.62 | 16.35 | 26.0–55.0 | 39.9 |
| 5.0 | 5 | 12.21–20.72 | 15.04 | 31.0–50.8 | 42.7 |
Figure 2.
Average percentage of body fat for each body condition score as determined by DEXA analysis.
Figure 3.
Averages for lean body mass, fat mass, and bone mass as determined by DEXA analysis for each body condition score group.
Because data from animals in BCS groups 1.0 and 1.5 did not follow an expected linear scale for percentage body fat, we assessed the data by using animals from all BCS groups (full data set, Table 2) as well as those with BCS of 2.0 to 5.0 (partial data set; Table 3). With the full data set, the Pearson correlation coefficient for BCS and average percentage body fat was 0.83, whereas the Pearson correlation coefficient for weight and percentage body fat was only 0.65. A t test for comparing dependent correlation coefficients9 revealed that 0.83 was significantly higher than 0.65 (P < 0.001). When data from animals with BCS less than 2.0 were excluded from analysis, the Pearson coefficient for BCS and percentage body fat was 0.86 compared with 0.61 between weight and percentage body fat (t test, P < 0.001). Therefore, we concluded that the correlation between BCS and percentage body fat is higher than that between weight and percentage body fat, especially for macaques with a BCS of 2.0 or greater.
Table 2.
Comparison of BCS and weight as predictors of percentage body fat as influenced by sex, weight, and age among macaques with BCS ≥ 2.0
| Pearson correlation coefficient |
||
| BCS and % body fat | Weight and % body fat | |
| Male | 0.91 | 0.84 |
| Female | 0.88 | 0.82 |
| ≤12 ya | 0.86 | 0.59 |
| >12 ya | 0.81 | 0.47 |
| ≤9.2 kga | 0.81 | 0.47 |
| >9.2 kga | 0.86 | 0.59 |
t test comparing the 2 Pearson coefficients yielded P < 0.05.
Table 3.
Comparison of BCS and weight as predictors of percentage body fat as influenced by sex, weight, and age among all macaques
| Pearson correlation coefficient |
||
| BCS and % body fat | Weight and %body fat | |
| Male | 0.83 | 0.79 |
| Female | 0.88 | 0.85 |
| ≤12 ya | 0.82 | 0.52 |
| >12 ya | 0.81 | 0.67 |
| ≤9.2 kg | 0.39 | 0.11 |
| >9.2 kga | 0.83 | 0.46 |
t test comparing the 2 Pearson coefficients yielded P < 0.05.
Furthermore, using the subset of data for animals with a BCS ≥ 2.0, we found that the combination of BCS and body weight provided a significantly better predictor of percentage body fat than did body weight alone (likelihood ratio test, P < 0.001) but not significantly better than BCS alone (likelihood ratio test, P = 0.098). This result again indicates that BCS itself adequately predicts percentage body fat, and that adding body weight does not substantially improve the predictability of BCS for average percentage body fat.
Next, we evaluated the data to determine the influences of sex (male compared with female), age (12 y or younger compared with older than 12 y), and weight (9.2 kg or less compared with more than 9.2 kg) on the predictive values of BCS assigned to adult macaques. Visual comparison of Pearson correlation coefficients (Tables 2 and 3) revealed that all of the coefficients were higher for the correlation between BCS and percentage body fat than that between weight and percentage body fat. Within each age- or sex-specific subgroup of animals with a BCS score of 2.0 or greater (Table 2), the correlation coefficient between BCS and percentage body fat was significantly (t test, P < 0.05) higher than that between weight and percentage body fat.9 When data for all BCS groups were considered (Table 3), similar and significant (t test, P < 0.05) results were obtained after comparing the correlation coefficients, except for animals weighing 9.2 kg or less (P = 0.38). Furthermore, there was no significant difference between the use of BCS or weight as a predictor of percentage body fat within the sex-specific subgroups among all BCS groups or those of 2.0 or greater.
Discussion
In the context of communication in a veterinary medical record, it is helpful to have a standardized method of ‘scoring’ an animal's body condition. A previously described BCS system for rhesus monkeys uses a 1-to-5 scale, with midrange values representing optimal body condition, lower values representing emaciated or lean conditions, and higher values representing excessive body fat.8 The primary goal of the current study was to evaluate how well data from an objective means of assessing body fat correlated with a score assigned by using the described BCS system.
In human physiology research, several noninvasive means of measuring body composition have been well described.13,34 Of these, underwater weighing (hydrodensitometry) has long been considered the ‘gold standard’ for evaluating body composition in humans.13 This methodology is impractical for use in research animals, because subjects must be completely submerged under water while breath-holding. Air displacement methods are influenced by the amount of body hair, again making this methodology impractical for nonhuman primate species.17 Total body water assessment, total body potassium calculations, and CT all require exposure to radioactivity, which we elected to avoid because all of the macaques we used here are part of the institution's breeding colony. MRI is a reasonable method for determining body fat, but our review of the literature did not yield sufficient information to justify the use of MRI over DEXA for determination of body fat.1,31
Traditionally, carcass analysis has been the ‘gold standard’ for assessing body composition in research and food animals. Although this method has the advantage of precision, it necessitates euthanasia of subjects. For the current study, we elected to use only minimally invasive means of assessing body composition in the interest of preserving animal resources. The use of DEXA technology to determine body composition by using carcass analysis has been validated several species including, mice,32 rats,4 pigs,6,7,22,30 rhesus monkeys,4 dogs, and cats.37 These studies have shown good correlation between DEXA and carcass analysis in the assessment of body fat and lean body mass.
Limitations of DEXA for assessment of body composition are well documented. Sources of variation include instrument manufacturer, mode of operation, patient position, type of platform on which the subject is placed, and subject age (infant, adult). 15,23,39 In addition, DEXA has been shown to under-represent fat content compared with that from chemical analysis,30 and the software of DEXA machines may fail to recognize fat percentages of less than approximately 4%.30 Furthermore, because DEXA software assumes a fixed constant for fat-free mass, the hydration status of the patient affects the imaging results.36,41 Despite these limitations, the ability of DEXA to assess body composition accurately generally is well accepted.29
A complete review of physical concepts of DEXA technology is provided elsewhere.33 Briefly, the components of a DEXA scanner include an X-ray tube and k-edge filter, which generate X-rays at 2 main energy peaks. DEXA scanners have a detector, which receives the X-rays, and an integrated computer system. Different body compartments will attenuate the 2 X-ray energies to different degrees, depending on the patient's mass and composition, thereby allowing differentiation between bone mineral and soft tissues. Soft tissues can then be divided into fat mass and fat-free mass.11
Body composition changes over the lifetime of each individual animal. Our described BCS for rhesus macaques focuses on fully mature, nongeriatric adults. Although the scale can be used for animals outside of this age range, the BCS assigned must be interpreted in light of the animal's age. For example, a healthy infant or juvenile rhesus macaque may frequently be assigned a BCS of 1.5 to 2.0 or 2.0 to 2.5, respectively, which the interpreter must acknowledge as appropriate for young, growing animals. Conversely, geriatric animals may have excessive fat deposits in the abdominal region, with muscle loss over the hips, back, and extremities. In this situation, it may be appropriate to assign 2 scores, one for fat and one for muscle, to each animal. In the current study, we are attempting to validate the scoring system as written for adult rhesus macaques in their prime, so we included animals from this age group only.
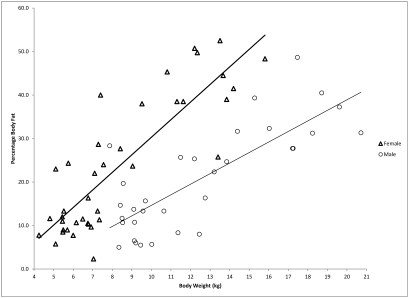
In our study, weight was a good predictor of percentage body fat within subgroups divided by sex. However, BCS has the advantage of being independent of sex in predicting percentage body fat. As seen in Figure 4, body weight is a strong predictor of percentage body fat in both female and male macaques, but its predictability is quite different between the 2 sexes. As illustrated, a 1-kg change in body weight is associated with a 2.69% increase in percentage body fat in male macaques but a 3.96% increase in female macaques. A more consistent relationship is observed between BCS and its predictability for percentage body fat in both female and male subgroups, in which each full-unit increase in BCS correlates with a 10% increase in percentage body fat. Therefore, we claim that BCS is a convenient and accurate tool for predicting percentage body fat in practice.
Figure 4.
Average percentage of body fat compared with body weight in male and female rhesus monkeys.
Overall, BCS was a better predictor of average percentage body fat than was weight alone, especially for animals in higher weight and BCS ranges. We speculate that DEXA technology likely cannot accurately assess the absolute body fat in macaques with little appreciable body fat and correspondingly low BCS.30
The current study shows that the previously described BCS system for rhesus monkeys is valid for adult animals, independent of sex and age and over a wide weight range. Direct carcass analysis likely would provide additional valuable data, especially for very lean or emaciated animals, and might be used in studies with a defined endpoint.15,19,26,33-35
Acknowledgments
This publication was made possible by grant 5P51 RR000169 from the National Center for Research Resources (NCRR), a component of the NIH, and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Additional funding for this research was provided by the Association for Primate Veterinarians.
We thank Vanessa Bakula, Jennifer VandeVegte, and Ross Allen from the CNPRC for their technical assistance and tireless patience with this project.
References
- 1.Abate N, Burns D, Peshock RM, Garg A, Grundy SM. 1994. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res 35:1490–1496 [PubMed] [Google Scholar]
- 2.Baldwin K, Bartges J, Buffington T, Freeman LM, Grabow M, Legred J, Ostwald D., Jr 2010. AAHA nutritional assessment guidelines for dogs and cats. J Am Anim Hosp Assoc 46:285–296 [DOI] [PubMed] [Google Scholar]
- 3.Berman CM, Schwartz S. 1988. A nonintrusive method for determining relative body fat in free-ranging monkeys. Am J Primatol 14:53–64 [DOI] [PubMed] [Google Scholar]
- 4.Bertin E, Ruiz JC, Mourot J, Peiniau P, Portha B. 1998. Evaluation of dual-energy X-ray absorptiometry for body composition assessment in rats. J Nutr 128:1550–1554 [DOI] [PubMed] [Google Scholar]
- 5.Black A, Tilmont EM, Baer DJ, Rumpler WV, Ingram DK, Roth GS, Lane MA. 2001. Accuracy and precision of dual-energy X-ray absorptiometry for body composition measurements in rhesus monkeys. J Med Primatol 30:94–99 [DOI] [PubMed] [Google Scholar]
- 6.Brunton JA, Bayley HS, Atkinson SA. 1993. Validation and application of dual-energy X-ray absorptiometry to measure bone mass and body composition in small infants. Am J Clin Nutr 58:839–845 [DOI] [PubMed] [Google Scholar]
- 7.Brunton JA, Weiler HA, Atkinson SA. 1997. Improvement in the accuracy of dual energy X-ray absorptiometry for whole body and regional analysis of body composition: validation using piglets and methodologic considerations in infants. Pediatr Res 41:590–596 [DOI] [PubMed] [Google Scholar]
- 8.Clingerman KJ, Summers L. 2005. Development of a body condition scoring system for nonhuman primates using Macaca mulatta as a model. Lab Anim (NY) 34:31–36 [DOI] [PubMed] [Google Scholar]
- 9.Cohen J, Cohen P. 1975. Applied multiple regression–correlation analysis for the behavioral sciences. Hillsdale (NJ): Lawrence Erlbaum Associates. [Google Scholar]
- 10.Colman RJ, Hudson JC, Barden HS, Kemnitz JW. 1999. A comparison of dual-energy X-ray absorptiometry and somatometrics for determining body fat in rhesus macaques. Obes Res 7:90–96 [DOI] [PubMed] [Google Scholar]
- 11.DeVita MV, Stall SH. 1999. Dual-energy X-ray absorptiometry: a review. J Ren Nutr 9:178–181 [DOI] [PubMed] [Google Scholar]
- 12.Edmonson AJ, Lean IJ, Weaver LD, Farver T, Webster G. 1989. A body condition scoring chart for Holstein dairy cows. J Dairy Sci 72:68–78 [Google Scholar]
- 13.Ellis KJ. 2000. Human body composition: in vivo methods. Physiol Rev 80:649–680 [DOI] [PubMed] [Google Scholar]
- 14.Grier SJ, Turner AS, Alvis MR. 1996. The use of dual-energy x-ray absorptiometry in animals. Invest Radiol 31:50–62 [DOI] [PubMed] [Google Scholar]
- 15.Haarbo J, Gotfredsen A, Hassager C, Christiansen C. 1991. Validation of body composition by dual energy X-ray absorptiometry (DEXA). Clin Physiol 11:331–341 [DOI] [PubMed] [Google Scholar]
- 16.Henneke DR, Potter GD, Kreider JL, Yeates BF. 1983. Relationship between condition score, physical measurements, and body fat percentage in mares. Equine Vet J 15:371–372 [DOI] [PubMed] [Google Scholar]
- 17.Higgins PB, Fields DA, Hunter GR, Gower BA. 2001. Effect of scalp and facial hair on air displacement plethysmography estimates of percentage of body fat. Obes Res 9:326–330 [DOI] [PubMed] [Google Scholar]
- 18. Institute for Laboratory Animal Research. 2010. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press. [Google Scholar]
- 19.Jensen MD, Kanaley JA, Roust LR, O'Brien PC, Braun JS, Dunn WL, Wahner HW. 1993. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc 68:867–873 [DOI] [PubMed] [Google Scholar]
- 20.Kemnitz JW, Francken GA. 1986. Characteristics of spontaneous obesity in male rhesus monkeys. Physiol Behav 38:477–483 [DOI] [PubMed] [Google Scholar]
- 21.Kendall MG, Stuart A. 1977. The advanced theory of statistics. London (UK): C Griffin. [Google Scholar]
- 22.Koo WW, Hammami M, Hockman EM. 2004. Validation of bone mass and body composition measurements in small subjects with pencil beam dual energy X-ray absorptiometry. J Am Coll Nutr 23:79–84 [DOI] [PubMed] [Google Scholar]
- 23.Koo WW, Hockman EM, Hammami M. 2004. Dual energy X-ray absorptiometry measurements in small subjects: conditions affecting clinical measurements. J Am Coll Nutr 23:212–219 [DOI] [PubMed] [Google Scholar]
- 24.Laflamme D. 1997. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract 25:13–18 [Google Scholar]
- 25.Laflamme D. 1997. Development and validation of a body condition score system for dogs. Canine Pract 22:10–15 [Google Scholar]
- 26.Lohman TG. 1971. Biological variation in body composition. J Anim Sci 32:647–653 [DOI] [PubMed] [Google Scholar]
- 27.Lohman TG. 1981. Skinfolds and body density and their relation to body fatness: a review. Hum Biol 53:181–225 [PubMed] [Google Scholar]
- 28.Maritz JS. 1981. Distribution-free statistical methods. London (UK): Chapman and Hall. [Google Scholar]
- 29.Mazess RB, Barden HS, Bisek JP, Hanson J. 1990. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 51:1106–1112 [DOI] [PubMed] [Google Scholar]
- 30.Mitchell AD, Scholz AM, Conway JM. 1998. Body composition analysis of small pigs by dual-energy X-ray absorptiometry. J Anim Sci 76:2392–2398 [DOI] [PubMed] [Google Scholar]
- 31.Mitchell AD, Scholz AM, Wang PC, Song H. 2001. Body composition analysis of the pig by magnetic resonance imaging. J Anim Sci 79:1800–1813 [DOI] [PubMed] [Google Scholar]
- 32.Nagy TR, Clair AL. 2000. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res 8:392–398 [DOI] [PubMed] [Google Scholar]
- 33.Pietrobelli A, Formica C, Wang Z, Heymsfield SB. 1996. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol 271:E941–E951 [DOI] [PubMed] [Google Scholar]
- 34.Pietrobelli A, Wang Z, Heymsfield SB. 1998. Techniques used in measuring human body composition. Curr Opin Clin Nutr Metab Care 1:439–448 [DOI] [PubMed] [Google Scholar]
- 35.Plank LD. 2005. Dual-energy X-ray absorptiometry and body composition. Curr Opin Clin Nutr Metab Care 8:305–309 [DOI] [PubMed] [Google Scholar]
- 36.Roubenoff R, Kehayias JJ, Dawsonhughes B, Heymsfield SB. 1993. Use of dual-energy X-ray absorptiometry in body-composition studies: not yet a gold standard. Am J Clin Nutr 58:589–591 [DOI] [PubMed] [Google Scholar]
- 37.Russel A. 1984. Body condition scoring of sheep. In Pract 6:91–93 [DOI] [PubMed] [Google Scholar]
- 38.Speakman JR, Booles D, Butterwick R. 2001. Validation of dual energy X-ray absorptiometry (DXA) by comparison with chemical analysis of dogs and cats. Int J Obes Relat Metab Disord 25:439–447 [DOI] [PubMed] [Google Scholar]
- 39.Tothill P, Avenell A, Love J, Reid DM. 1994. Comparisons between Hologic, Lunar and Norland dual-energy X-ray absorptiometers and other techniques used for whole-body soft-tissue measurements. Eur J Clin Nutr 48:781–794 [PubMed] [Google Scholar]
- 40.Ullman-Cullere MH, Foltz CJ. 1999. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 49:319–323 [PubMed] [Google Scholar]
- 41.Van Loan MD. 1998. Is dual-energy X-ray absorptiometry ready for prime time in the clinical evaluation of body composition? Am J Clin Nutr 68:1155–1156 [DOI] [PubMed] [Google Scholar]
- 42.Walker ML, Schwartz SM, Wilson ME, Musey PI. 1984. Estimation of body fat in female rhesus monkeys. Am J Phys Anthropol 63:323–329 [DOI] [PubMed] [Google Scholar]