Abstract
BACKGROUND:
CYP3A5 was observed to be an important genetic contributor to inter individual differences in CYP3A-dependent drug metabolism in acute leukemic patients. Loss of CYP3A5 expression was mainly conferred by a single nucleotide polymorphism at 6986A>G (CYP3A5*3). We investigated the association between CYP3A5*3 polymorphism and acute leukemia.
MATERIALS AND METHODS:
Two hundred and eighty nine acute leukemia cases comprising of 145 acute lymphocytic leukemia (ALL), 144 acute myeloid leukemia and 241 control samples were analyzed for CYP3A5*3 polymorphism using PCR-RFLP method. Statistical analysis was performed with SPSS version (15.0) to detect the association between CYP3A5*3 polymorphism and acute leukemia.
RESULTS:
The CYP3A5*3 polymorphism 3/3 genotype was significantly associated with acute leukemia development (χ2- 133.53; df-2, P 0.000). When the data was analyzed with respect to clinical variables, mean WBC, blast % and LDH levels were increased in both ALL and AML cases with 3/3 genotype. The epidemiological variables did not contribute to the genotype risk to develop either AML or ALL.
CONCLUSION:
The results suggest that the CYP3A5*3 polymorphism might confer the risk to develop ALL or AML emphasizing the significance of effective phase I detoxification in carcinogenesis. Association of the polymorphism with clinical variables indicate that the 3/3 genotype might also contribute to poorer survival of the patients.
Keywords: Acute leukemia, CYP3A5, drug metabolism, polymorphism
Introduction
Cytochrome P450 enzymes are involved in initial oxidation, reduction or alkylation of xenobiotics that convert inactive carcinogens into active electrophiles which have the capacity to interact with biological molecules. CYP genes convert several anti-neoplastic agents into intermediate reactive metabolites, some of which can damage DNA.[1] Polymorphisms in the CYP genes have been associated with increased or decreased risk of certain cancers, because of extensive or poor metabolism of carcinogens or drugs.[2] The genetic variations which result in altering the activity of these enzymes had been found to be associated with the risk of developing secondary cancers and t –AML/t-MDS.[3] CYP3A5 family plays a significant role in the metabolism of more than 100 different drugs, carcinogens and toxic chemicals.[4]
The CYP3A5 gene is located on chromosome 7q21.1. CYP3A5 was observed to be an important genetic contributor to inter individual differences in CYP3A-dependent drug metabolism. Loss of CYP3A5 expression was mainly conferred by a single nucleotide polymorphism at 6986A>G (CYP3A5*3). This SNP produces a cryptic splice site resulting in the inclusion of exon 3B followed by splicing which consequently introduces a stop codon. As a result, CYP3A5*3 protein is truncated with greatly reduced enzyme activity in homozygous CYP3A5*3 individuals.
In the present study, attempt has been made to identify the association of CYP3A5*3 polymorphism with the development of acute leukemia.
Materials and Methods
Study subjects
The study includes 289 acute leukemia cases comprising of 145 acute lymphocytic leukemia (ALL), 144 acute myeloid leukemia (AML) and 241 control samples. Blood samples were collected from patients being treated at NIMS (Nizams Institute of Medical Sciences), Hyderabad. The age and sex matched control samples were randomly selected from different areas from the local population. Patient's clinical data like WBC count, blast%, platelet count, Hb, LDH, complete remission rate (CR) and disease free survival (DFS) was noted from the tumor registry file with the help of a medical oncologist. Genomic DNA was isolated by using salting-out method.[5]
Restriction fragment length polymorphism
CYP3A5*3 polymorphism was analyzed through PCR-RFLP (restriction fragment length polymorphism) method. 121 bp fragment was amplified using specific primer sequences: Forward: 5’- gtt gta cgc cac aca gca cc0 -3’; Reverse: 5’- CTC TTT AAA GAG CTC TTT TGT CTC TCA - 3’. The 25 μl PCR reaction mixture consists of approximately 100-150 ng of genomic DNA, 15 pmol/l of each primer, 200 μmol/l of dNTPs, 20 mmol/l of tris HCl, 50 mM of KCl, 2.5 mmol/l of MgCl2, 0.5 U of Taq DNA polymerase and deionized water (varied).
The PCR cycling conditions include initial denaturation at 95°C for 12 min followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 59°C for 20 s, extension at 72°C for 40 s and final extension at 72°C for 6 min. After amplification, PCR products were subjected for restriction digestion using Dde 1 enzyme (New England Biolabs, USA). The samples were genotyped on 3% agarose gel. CYP3A5*3 wild type allele has no restriction site and produces fragment of size 121bp whereas mutant allele with restriction site produces two fragments of size 97 and 24 base pairs.
Statistical analysis
All the statistical analyses were performed with SPSS version 15. 0. (Statistical Package for the Social Science). Chi square values and Odds ratios were calculated to test the significance of genotype association with the occurrence of acute leukemia. t test was done to test the significance of association of clinical variables with the risk of acute leukemia. All the P values were two sided and the level of significance was taken as P <0.05.
Results
CYP3A5*3 homozygous genotype (3/3) and 3 allele frequency were significantly elevated in both ALL and AML groups as compared to control group. The genotype distribution of CYP3A5*3 polymorphism in AML group did not deviate from Hardy-Weinberg equilibrium, whereas the control group showed significant deviation from Hardy-Weinberg equilibrium (χ2-28.1) [Table 1]. Odds ratios were significant for 1/1 vs 1/3 and 1/3 vs 3/3 groups among AML cases and for 1/1 vs 1/3 and 1/1 vs 3/3 groups among ALL cases when compared with controls [Table 2].
Table 1.
Genotype distribution of CYP3A5*3 polymorphism in acute leukemia and controls
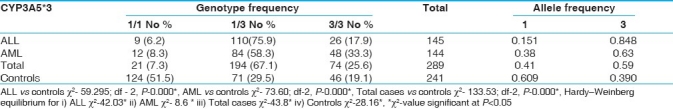
Table 2.
Odds ratios (OR) in disease versus controls

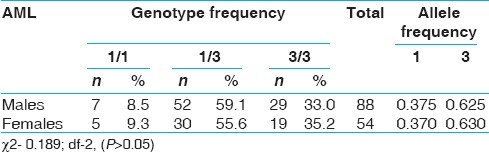
When the genotype data was analyzed with respect to epidemiological variables like sex, age at onset, none of the variables showed significant association with CYP3A5*3 [Tables 3–6]. However, 3/3 genotype frequency was found to be elevated among ALL patients with age at onset >20 years (28.2%) as compared to those with age at onset <10 years (14.3%) [Table 5]. In AML, 3/3 genotype frequency was elevated considerably in early age at onset group (41.7%) as compared to later age at onset group (29.7%) [Table 6].
Table 3.
CYP3A5*3 polymorphism and sex in all groups
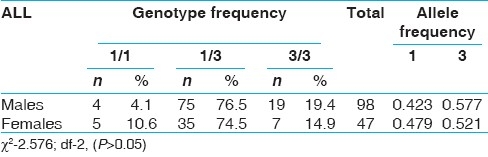
Table 6.
CYP3A5*3 polymorphism and age at onset in AML group
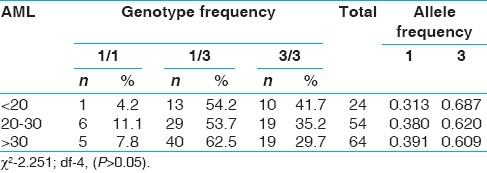
Table 5.
CYP3A5*3 polymorphism and age at onset in all groups
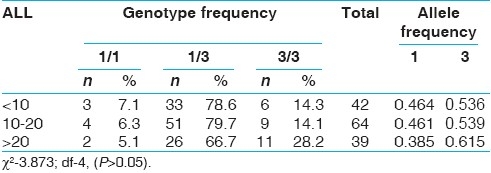
Table 4.
CYP3A5*3 polymorphism and sex in AML group
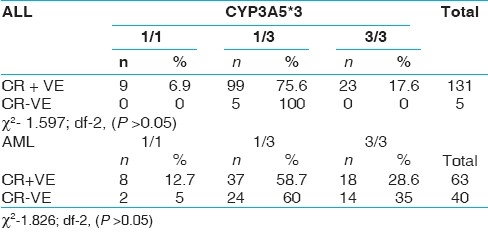
When the data was analyzed with respect to clinical variables, there was a considerable elevation in mean WBC count, blast% and LDH level and reduction in mean DFS of ALL patients with 3/3 genotype [Table 7]. Similar increasing trend in the values of WBC, blast% and LDH were observed in AML patients with 3/3 genotype without any difference in DFS values [Table 8]. 35% of AML patients with 3/3 genotype failed to achieve complete remission as compared to 5% of patients with 1/1 genotype [Table 9].
Table 7.
Mean values of clinical variables with respect to CYP3A5*3 polymorphism in all groups
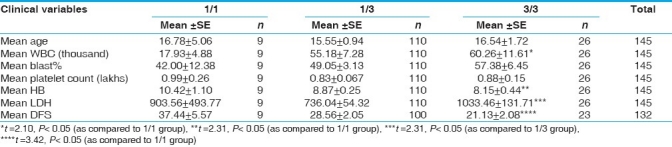
Table 8.
Mean values of clinical variables with respect to CYP3A5*3 polymorphism in AML group
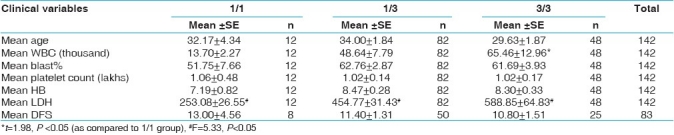
Table 9.
CYP3A5*3 polymorphism and complete remission rates in all and AML groups
There was no variation with respect to platelet count, Hb in both ALL and AML patients.
Discussion
Polymorphic variants in the CYP3A5 gene have functional effect on the activation of environmental pollutants. Accordingly, the significant association of CYP3A5*3 allele with ALL and AML observed in the present study indicates the importance of proper detoxification mechanism in preventing carcinogenesis. Elevation in the frequency of homozygous genotype 3/3 and 3 allele indicated that CYP3A5*3 genotype with lower CYP expression might result in accumulation of xenobiotics leading to leukemogenesis. Further, it might not be metabolizing the antileukemic drugs effectively which would result in drug toxicity and poor survival.
Previous studies reported the lack of association of CYP3A5*3 with development of myeloid leukemia (CML, AML).[8] No significant association was reported between CYP3A5*3 polymorphism and morbidity of acute leukemia patients, but it was closely associated with the chemotherapeutic effect and prognosis.[9]
The results also indicated that selection might be operating in the population against *3 allele, since loss of CYP3A5 enzyme activity is associated with defective detoxification of xenobiotics. These selective forces might be responsible for deviation from Hardy–Weinberg equilibrium in the control group. Hence, increased frequency of *3 allele in disease group and also the lack of deviation of the same from Hardy–Weinberg equilibrium indicated the importance of selection in elimination of ineffective allele. However, sample size and method of sampling also contribute to deviation from equilibrium.
In conclusion, our results suggested that the CYP3A5*3 polymorphism might confer the risk to develop ALL or AML. The 3/3 genotype can be considered as a risk genotype to develop acute leukemia and also influences clinical variables such as WBC count, blast%, LDH levels and contribute to poorer survival.
Acknowledgment
This work was supported by financial assistance from UGC and Department of Medical Oncology, Nizams institute of Medical Sciences, Hyderabad, India.
Footnotes
Source of Support: UGC and Department of Medical Oncology, Nizams institute of Medical Sciences, Hyderabad, India.
Conflict of Interest: None declared.
References
- 1.Friche E, Danks MK, Beck WT. Characterization of tumor cell resistance to 4‘-deoxy-4’-iododoxorubicin developed in Ehrlich ascites cells in vivo. Cancer Res. 1992;52:5701–6. [PubMed] [Google Scholar]
- 2.Ingelman-Sundberg M. Genetic susceptibility to adverse effects of drugs and environmental toxicants: The role of the CYP family of enzymes. Mutat Res. 2001;482:11–9. doi: 10.1016/s0027-5107(01)00205-6. [DOI] [PubMed] [Google Scholar]
- 3.Felix CA, Walker AH, Lange BJ, Williams TM, Winicki NJ, Cheung NV, et al. Association of CYP3A4 genotype with treatment-related leukemia. Proc Natl Acad Sci. 1998;95:13176–81. doi: 10.1073/pnas.95.22.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith G, Stanley LA, Sim E, Strange RC, Wolf CR. Metabolic polymorphisms and cancer susceptibility. Cancer Surv. 1995;25:27–65. [PubMed] [Google Scholar]
- 5.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucl Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 7.Hustert E, Haberl M, Burk O, Wolbold R, He YQ, Klein K, et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics. 2001;11:773–9. doi: 10.1097/00008571-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Liu AY, True LD, LaTray L, Nelson PS, Ellis WJ, Vessella RL, et al. Cell-cell interaction in prostate gene regulation and cyto-differentiation. Proc Natl Acad Sci USA. 1997;94:10705–10. doi: 10.1073/pnas.94.20.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen LJ, Chen FY, Wang T, Wang HR, Zhong JH, Han JY, et al. Polymorphisms of CYP3A5 gene in acute leukemia patients and their role in chemotherapy and prognosis. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16:26–30. [PubMed] [Google Scholar]


