Abstract
BACKGROUND:
Pesticides are used in agriculture to protect crops, but they pose a potential risk to farmers and environment. The aim of the present study is to investigate the relation between the occupational exposure to various pesticides and the presence of DNA damage.
MATERIALS AND METHODS:
Blood samples of 210 exposed workers (after a day of intense spraying) and 50 control subjects belonging to various districts of Punjab (India) were evaluated using Comet assay. Sixty workers who showed DNA damage were selected for follow up at 5-6 months after the first sampling during a low or null spraying period.
RESULTS:
Significant differences were found in DNA damage between freshly exposed workers and controls and freshly exposed and followed up cases. There was significant increase in the comet parameters viz. mean comet tail length and frequency of cells showing migration in exposed workers as compared to controls (72.22 ± 20.76 vs. 46.92 ± 8.17, P<0.001; 31.79 vs. 5.77, P<0.001). In the second samples, followed up cases showed significant decrease in frequency of damaged cells as compared to freshly exposed workers of first sampling (P<0.05). The confounding factors such as variable duration of pesticide exposure, age, smoking, drinking and dietary habits etc which were expected to modulate the damage, were instead found to have no significant effect on DNA fragmentation.
CONCLUSION:
The evidence of a genetic hazard related to exposure resulting from the intensive use of pesticides stresses the need for educational programs for agricultural workers to reduce the use of chemicals in agriculture.
Keywords: Agricultural workers, comet assay, DNA damage, genotoxicity, pesticides, Punjab
Introduction
Pesticides are extensively used all over the world to increase food production and control vector-borne diseases and in recent years their use was increased dramatically. Unfortunately, large amounts of these chemicals are released into the environment and many of them affect non-target organisms, being a potential hazard to human health. Fifty-six pesticides have been classified as carcinogenic to laboratory animals by the IARC.[1] Meta-analyses showed that pesticide-exposed farmers are at risk for specific tumors, including leukemia,[2–4] non-Hodgkin's lymphoma,[5] soft tissue sarcoma,[6] Parkinson disease,[7] multiple myeloma,[8] stomach and prostate malignancies.[9] Some selected pesticides have been tested individually by in vitro genotoxicity testing methods and considered as potential chemical mutagens.[10] However, the effective dose in many single tests is generally very high. As most occupational and environmental exposures are exposure to mixtures of pesticides, the genotoxic potential evaluated on single compounds could not be extrapolated to humans. Hence, the genotoxicological assessment in human populations is a useful tool to estimate the genetic risk from an integrated exposure to complex mixtures of pesticide. Several cytogenetic assays have been used to evaluate the potential genotoxicity of pesticide exposures in occupationally exposed populations. However, there are reports on positive genotoxic effects in populations exposed to pesticides[11–13] as well as negative findings.[14,15]
Alkylating abilities of the pesticide chemicals induce breaks in DNA[16] and thus affect DNA replicating ability and its ability to carry information.[17] DNA damage together with cellular response can establish genomic instability through multiple pathways[18] and can be considered as an effective strategy for risk assessment. Individuals occupationally exposed to pesticides have great genotoxic risk and assessment of this risk in exposed subjects can be used as fairly reliable biomarker of early biological alterations.[19] Biomarkers frequently used to assess genotoxic effects of pesticides include chromosomal aberration (CA), micronuclei formation (MN), sister chromatid exchanges (SCEs) and comet assay.
From the past few years, single cell gel electrophoresis (SCGE) or comet assay has been used as a sensitive, visual, reliable, rapid and inexpensive technique for measuring and analyzing DNA single and double-strand breaks, alkali-labile sites, DNA cross- linking and delayed repair-site detection in eukaryotic individual cells.[20,21] The sensitivity as well as the specificity of comet assay can be increased by incorporating an extra step of digestion with a lesion-specific endonuclease following lysis.[22] The relevance of technique lies in its requirement of small amount of blood sample and its ability to evaluate index of genetic damage in the non-proliferating cells. Though the technique is extensively used in fundamental DNA-repair studies, toxicology and biomonitoring studies, yet only a limited number of biomonitoring studies on the genotoxic effects due to occupational exposure to pesticides in sprayers have been reported.[23–29] These studies have revealed significant increase in DNA damage in exposed workers in comparison to control subjects. A few studies have also reported negative results.[30] Genetic polymorphism of the metabolic enzyme GSTP1 has been found to be associated with greater risk of DNA damage in pesticide exposed workers.[29] However, there is a scarcity of data pertaining to studies conducted on blood samples of individuals during intense spraying activity alternating with no spraying/very low spraying of pesticide. Moreover, in developing countries like India, most of the pesticide applicators are illiterate and untrained and do not use appropriate protective clothing / devices and have never been monitored for the genotoxic effects of pesticides they are being exposed to.
Earlier data suggests that endogenous and exogenous factors are involved in modulating the effects of pesticides and hence the individuals of different genetic constitution may respond differently to pesticide exposure. The present study is thus designed to evaluate the extent of possible DNA damage among freshly exposed workers during intense spraying season and follow up cases during low exposure period among the pesticide sprayers of Punjab (India) who were occupationally exposed to various pesticides for variable duration of exposure.
Materials and Methods
Study population
The study was carried out on a group of 210 male farm workers (Vegetables, Orchards, Cotton, Paddy and Wheat Sprayers) belonging to various districts of Punjab and being exposed to various pesticides. This was a longitudinal study in which each farmer was his own control. This approach removed problems generated by the choice of unexposed control and their similarity to exposed population for any epidemiological parameter except exposure. Still an effort was made to compare the exposed and non-exposed group. So the 50 age-matched healthy male individuals were selected as control from general population having no history of occupational exposure to pesticides, any serious medical problem and intake of drugs or other therapeutic medicines (at least from the past one year from the day of sampling). Both, the exposed workers and unexposed subjects were selected from the same region. In Punjab, agricultural work is in the hand of males mainly and participation of females is least. So females were excluded from the study. A follow up study was conducted at 5–6 months after the first sampling, in a low exposure period. A total of 60 samples were selected from the exposed group who showed any DNA damage during intense spraying period. Blood samples were collected from December, 2003 to January, 2006. Approximately 0.5 ml of blood sample was collected from each subject in an Eppendorf tube containing one drop of the anticoagulant EDTA.K2 solution. A gentle prick was given on the fingertip using a sterilized lancet. Tubes were serially numbered and were brought to the laboratory in an air tight ice container for analysis. Samples were transported to the laboratory at or below 8°C and were processed within 1–8 h of collection. Before comet assay, total cell count and cell viability were evaluated using the Trypan blue exclusion method. The cell viability was found to be about 98% in all samples.
All participants signed a written consent before sampling. Complete information regarding sex, age, marital status, medical history, life style (smoking, drinking, drug intake habits etc.) along with the occupational history regarding various aspects of pesticides, duration of exposure, working hours/day, name and class of pesticides, protective measures used etc. was enquired from the workers and recorded in the questionnaire. In all cases, individuals who smoked more than five cigarettes per day for at least one year were considered as smokers.
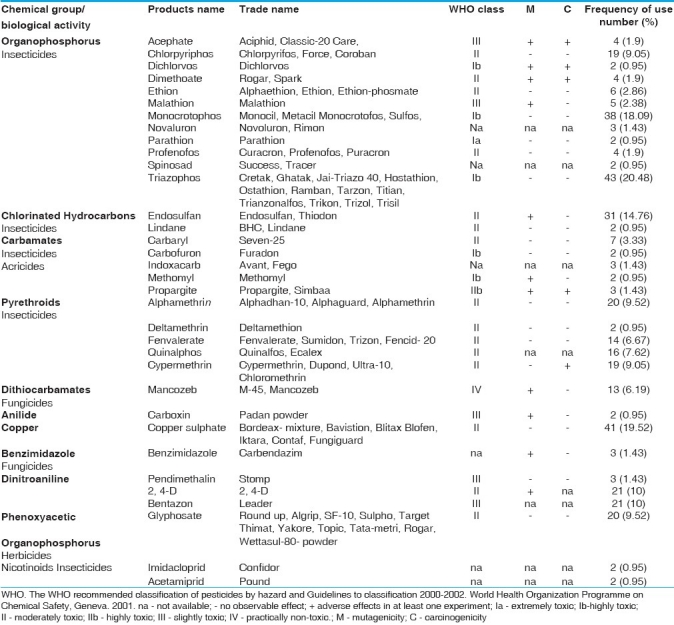
The exposed group workers and control have an average age of 32.92 + 11.03 years (17- 45 years) and 26.34 + 7.41 (18- 50 years) respectively. The duration of exposure to pesticides varied from 1 to 25 years in exposed workers with an average of 10.75 + 7.92 years. The exposed group handled pesticides throughout the year and the average number of hours that the workers had been directly involved in handling these chemicals were approximately 6 h. The demographic characteristics of the studied group are described in Table 1. Workers worked in open fields and pesticides were applied above the head. Very few workers used some kind of protective measures (gloves, shoes, mask etc.) during the preparation and application of pesticides. Carbamates, organophosphates and pyrethroids were the most used families of the pesticides. A list of various pesticides used by the farm workers along with their frequency is given in Table 2.
Table 1.
Demographic characteristics of the study group and control
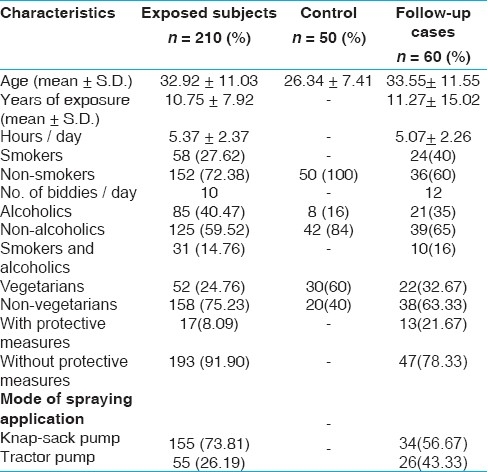
Table 2.
Pesticides used by the study group along with their WHO classification and frequency of use
Experimental design
The alkaline single cell gel electrophoresis assay was carried out according to the technique given by Singh et al,[20] with slight modification incorporated later on in the original technique by Ahuja and Saran[31] to access DNA damage. Silver staining method was used to get the results. Slides were prepared in triplicates per subject. Clean and dry glass slides were coated by putting a small amount of 1% normal melting point agarose (NMPA) (40-42°C) and dried at 37°C for 2-3 h. An aliquot of 25 μl of whole blood was mixed with 0.5% of 75 μl low melting point agarose (LMPA 37°C). Second layer of 100 μl of this mixture was pipetted onto the precoated slides and covered with coverslips. These slides were allowed to solidify at 4°C for 30 min. After solidification, coverslips were removed and a third layer of 100 μl of LMPA was pipetted onto the slides and made to spread properly with the help of coverslip. These slides were again kept at 4°C for another 30 min. LMPA and NMPA were prepared in phosphate buffer saline (136 mM NaCl, 2.68 Mm KCl, 8.10Mm Na2HPO4, 1.47 Mm KH2PO4 and pH 7.4). After removing coverslips, slides were immersed in freshly prepared cold lysing solution [2.5 M NaCl, 100 mM Na2EDTA, 10mM tris-HCl, pH 10, 1% Trition X-100 and 10% DMSO (added just before use)] for 2 h.
Slides were then placed in alkaline buffer (300 mM NaOH and 1 mM Na2EDTA, pH 13) in a horizontal electrophoretic chamber for 20 min to allow the unwinding of the DNA and expression of alkali-labile sites. Electrophoresis was conducted for 30-35 min at 25 V (0.66 V/cm) and 300 mA current. The current was adjusted to 300 mA by raising or lowering the buffer level in the tank. Electrophoresis procedure and the efficiency of each electrophoresis run were checked using negative controls, consisting of whole human unmodified blood collected in the laboratory. Each electrophoresis run was considered as valid only if the negative controls yielded the expected results. Slides were dried, placed on a tray and washed thrice for 10 min each with neutralization buffer (0.4 M tris-HCl, pH 7.5). The whole procedure was carried out in dim light to avoid additional DNA damage. The gel was dried for 1 h at room temperature and fixed for 10 min in fixing solution (15w/v trichloroacetic acid, 5% zinc sulfate, 5% glycerol) and dried at 37°C for 1 h. The staining solution was prepared fresh before use by mixing 34 ml of solution A (0.2% ammonium nitrate, 0.2% silver nitrate, and 0.5% formaldehyde) with 66 ml of solution B (5% sodium carbonate) and poured over the samples very gently. The slides were immersed for 30 min and shook until grey color appeared. After staining, slides were washed three to four times with deionized water, air dried and viewed under a trinocular Zeiss-microscope. All slides were coded to blind analysis and were scored by one person, to avoid inter-scorer variability. These slides were examined at 100X magnification using 10X objective and 10X eyepiece. A total of 100 cells were scanned per subject. Undamaged cells have intact nuclei without a tail and appear as a ‘halo’and damaged cells have the appearance of comets. The length of DNA migration in the comet tail gives an estimate of the extent of DNA damage and was measured with an ocular micrometer calibrated with the help of a stage micrometer at 100X magnification. Each division on the micrometer scale was equivalent to 15 μm DNA migration length. DNA damage for each cell was quantified as follows: Comet tail length (μm) = Maximum total length - head diameter
Mean tail length in μm was calculated by taking the average of the measurements obtained for all the comets. Frequency of cells showing migration (number of cells with comets/ total cells scored × 100) was also determined in each group. These comets were randomly selected for taking measurements from each individual by avoiding the edges and the damaged parts of the gel and the superimposed comets. The DNA damage was also assessed by visual scoring, but we found measurements of tail length more reliable and therefore considered only tail lengths and frequency of cells showing DNA damage as a tool to quantify DNA damage.
Statistical analysis
Mean and standard deviation (mean ±S.D) were calculated for each parameter studied. The statistical analysis of differences in DNA damage, as measured by the comet assay, was carried out using t-test. The selection of t-test was made after finding variables to lie in the normal distribution curve. χ2- test and multifactor analysis of variance (ANOVA) were used to check the significant differences. Correlations between different variables were determined by Spearman rank correlation test. The critical level for rejection of the null hypothesis was considered to be a P-value of 5%. All analyses were performed with the SPSS 10.0 version software packages.
Results
Results are expressed as mean + S.D. A complete history including age, duration of exposure, smoking habits etc. are given in Table 1. Most of the workers used cocktail of two or more pesticides belonging to different chemical groups. There were 58 (27.62%) smokers in the exposed group and no smoker in the control group. The average biddi (locally made cigarette) consumption of smokers was nearly 10 biddies per day. All control individuals in this study were selected to be non-smokers in order to eliminate confounding effect of smoking. About 40.47% of the workers were alcoholic. Regarding the protective measures, only 8.09% workers used some kind of protection during the preparation and application of pesticides.
Comet assay analysis of the 210 exposed workers revealed DNA damage in 35.71% of the cases, while in controls, the damage was detected in only 8% of the cases [Table 3]. These differences were highly significant (P<0.001). Only 25% of the followed up cases exhibited DNA damage which was significantly lower than fresh cases (P<0.05). In the first samples of intense spraying, there was significant increase in the comet parameters viz. mean comet tail length and frequency of cells showing migration in exposed workers as compared to control (72.22 ± 20.76 vs. 46.92 ± 8.17, P<0.001; 31.79 vs. 5.77, P<0.001). In the second samples, of low or no spray, followed up cases showed significant decrease in frequency of damaged cells as compared to first samples of freshly exposed workers (P <0.05) [Table 4]. Negative control for each electrophoresis demonstrated negative results.
Table 3.
Frequency of DNA damage in the total sample and control

Table 4.
Comet parameters in the freshly exposed cases, followed up cases and controls.

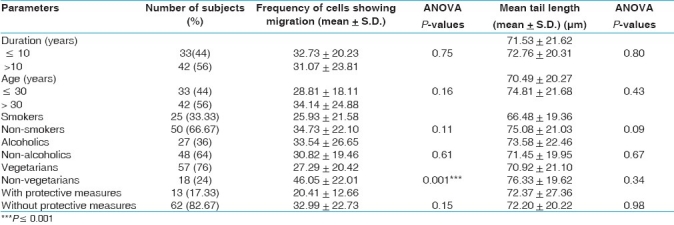
Effects of variables such as age, smoking, drinking and dietary habits and duration of exposure and use of protective measures were evaluated in the exposed group of first samples only. None of these confounding factors, except dietary habits, revealed a significant influence over the comet parameters or exhibited any association with increased DNA damage. The frequency of cells showing migration was significantly high in the non-vegetarian exposed cases [Table 5].
Table 5.
Comet parameters in 75 freshly exposed cases showing DNA damage in relation to duration of exposure, age, smoking, drinking and dietary habits
Discussion
Base line genetic damage is influenced by various intrinsic and extrinsic factors, but it is not yet clear how an individual's inborn genetic constitution may influence yield of such damage. For this reason, assessment of level of DNA damage in 210 occupationally exposed Punjab farmers along with 50 matched control subjects was done by taking two quantitative exposure parameters i.e. intense pesticide spraying season alternating with periods of reduced or null exposure, to determine the effects of immediate exposure as well as accumulated exposure. Results have shown a significant increase in the level of DNA damage in the exposed workers as compared to controls [Table 1]. Previous studies both in vivo and in vitro are in agreement with the present findings except the comet tail length seems to be comparatively more in the present workers.[27–29,32,33] This may be due to unwise and indiscriminate use of pesticides by these workers. Moreover, in majority of the cases, no protective measure was taken by these workers and cocktail of two or more pesticides were used, belonging to different chemical groups and which may probably be causing some antagonestic or synergic effect also. Some investigators had studied DNA damage in the farmers who were recurrently exposed to pesticides,[19,23,24] whereas others had studied DNA damage in pesticide manufacturing workers who were continuously exposed to pesticides.[25,27,34] There are a few studies in which no significant increase in DNA damage in exposed workers in comparison to control was found[30,35] which could be due to the differences in work conditions like use of varied quality of protective equipment, variable duration of exposure etc.
Genetic susceptibility has been reported to modulate the level of genotoxic risk. Many studies have shown an association between DNA damage and glutathione-S-transferase polymorphisms.[29] Inheritance of unfavorable genes has been shown to cause reduced detoxification and elimination of environmental mutagen as pesticides. When they are not efficient in detoxification, the metabolic sub-products accumulate, contributing to the tumorigenic process.[36,37] Certain pesticides (parathion, carbaryl, chlorpyrifos etc.) are known to inhibit P450 enzyme system and results in free radical production, which causes DNA damage.[38,39] In the present work, only 35.71% sprayers showed DNA damage and the remaining 64.29% sprayers who did not show any damage might have some intrinsic protective mechanism working against the pesticide exposure or else they might be having oxidative DNA damage which we could not detect with the standard comet assay as this technique detects only strand breaks and alkali-labile sites. DNA damage was significantly decreased in the followed up cases in comparison to fresh cases. Similar findings of reduced DNA damage in the followed up cases have also been revealed by Garaj-Vrhovac and Zeljezic.[19] Cytogenetic studies have also reported decrease in genetic damage during the period of low exposure.[40–41] The decrease in DNA damage in followed up cases could be attributed to three independent physiological processes: repair of DNA damage, elimination of cells due to death of highly damaged cells, dilution of cells carrying DNA damage by the production of undamaged lymphocytes from the stem cell pool.[42–44] Earlier followed up studies have revealed that comet assay is more efficient in detecting ongoing exposure rather than accumulated exposure.[23,45,46] Non-exposed farmers were not compared with the follow up cases as longitudinal study on the same subjects is a powerful mean to observe modification than a comparison between two different populations.
Some other factors like age, smoking, drinking and dietary habits were also analyzed while interpreting the results. No relationship between DNA damage and duration of exposure was found [Table 5]. Earlier cytogenetic studies of pesticide sprayers have shown similar results.[12,41] However, a few studies have reported positive relationship.[19,25,29]
No significant increase in DNA damage with increase in age was observed in the present sprayers. Similarly no association of DNA damage with age was observed in earlier studies.[23,25,27,28,47] There are evidences that certain vegetables and fruits contain anticlastogenic agents and have antioxidant properties.[48,49] Non-significant increase in non-vegetarian workers was observed and was in agreement with the results of Giovannelli et al[50] and Dhawan et al.[51] No significant relationship has been detected between DNA damage and smoking. These findings are in accordance with the findings of the other workers.[28,48,52] The damage was rather slightly higher (non-significant) in the non-smokers than smokers. Similar findings have been reported by some other authors.[23,28,46,53–55] Non-significant increase in DNA damage was observed in alcoholics. Non-significant differences in DNA damage was reported in the farmers who used protective measures [Table 5]. This may be due to large number of workers who did not use any safety measure while spraying in this study. Several studies have reported less DNA damage in workers who used some kind of protective measures.[40,52]
It is concluded that pesticides did cause DNA damage irrespective of duration of exposure. The damage caused by pesticides seems to be repaired as the follow up cases which were studied during null or low period of spraying did reveal significantly lower frequency of DNA damage in comparison to fresh cases. The confounding factors including age, smoking and diet were expected to modulate the genotoxic effect of xenobiotics, but the absence of any positive correlation between these factors and comet parameters suggest that the DNA damage was probably caused by pesticides only.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Monographs on the evaluation of carcinogenic risk to human. Vol. 5. Lyon (France): IARC; 2003. IARC; p. 53. [Google Scholar]
- 2.Zahm SH, Ward MH, Blair A. Pesticides and cancer. Occup Med. 1997;12:269–89. [PubMed] [Google Scholar]
- 3.Zahm SH, Ward MH. Pesticides and childhood cancer. Environ Health Perspect. 1998;106:893–908. doi: 10.1289/ehp.98106893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniels JL, Olshan AF, Savitz DA. Pesticides and childhood cancers. Environ Health Perspect. 1997;105:1068–77. doi: 10.1289/ehp.971051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng T, Zahm SH, Cantor KP, Weisenburger DD, Zhang Y, Blair A. Agricultural exposure to carbamate pesticides and risk of non-Hodgkin lymphoma.J Occup. Environ Med. 2001;43:641–9. doi: 10.1097/00043764-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Kogevinas M, Kauppinen T, Winkelmann R, Becher H, Bertazzi PA, Bueno-de-Mesquita HB, et al. Soft tissue sarcoma and non-Hodgkin's lymphoma in workers exposed to phenoxy herbicides, chlorophenols, and dioxins: Two nested case-control studies. Epidemiology. 1995;6:396–402. [PubMed] [Google Scholar]
- 7.Woodward G. Autism and Parkinson's disease. Med Hypotheses. 2001;56:246–9. doi: 10.1054/mehy.2000.1189. [DOI] [PubMed] [Google Scholar]
- 8.Viel JF, Challier B, Pitard A, Pobel D. Brain cancer mortality among French farmers: The vineyard pesticide hypothesis. Arch Environ Health. 1998;53:65–70. doi: 10.1080/00039899809605690. [DOI] [PubMed] [Google Scholar]
- 9.Bolognesi C. Genotoxicity of pesticides: A review of human biomonitoring studies. Mutat Res. 2003;543:251–72. doi: 10.1016/s1383-5742(03)00015-2. [DOI] [PubMed] [Google Scholar]
- 10.Dearfield KL, McCarroll NE, Protzel A, Stack HF, Jackson MA, Waters MD. A survey of EPA/OPP and open literature on selected pesticide chemicals. II. Mutagenicity and carcinogenicity of selected chloroacetanilide and related compounds. Mutat Res. 1999;443:181–221. doi: 10.1016/s1383-5742(99)00019-8. [DOI] [PubMed] [Google Scholar]
- 11.Bolognesi C, Parrini M, Bonassi S, Ianello G, Salanitto A. Cytogenetic analysis of a human population occupationally exposed to pesticides. Mutat Res. 1993;285:239–49. doi: 10.1016/0027-5107(93)90112-s. [DOI] [PubMed] [Google Scholar]
- 12.Kourakis A, Mouratidou M, Barbouti A, Dimikiotou M. Cytogenetic effects of occupational exposure in the peripheral blood lymphocytes of pesticide sprayers. Carcinogenesis. 1996;17:99–101. doi: 10.1093/carcin/17.1.99. [DOI] [PubMed] [Google Scholar]
- 13.Antonucci GA, de Syllos Colus IM. Chromosomal aberrations analysis in a Brazilian population exposed to pesticides. Teratog Carcinog Mutagen. 2000;20:265–72. [PubMed] [Google Scholar]
- 14.Scarpato R, Migliore L, Angotzi G, Fedi A, Miligi L, Loprieno N. Cytogenetic monitoring of a group of Italian floriculturists: No evidence of DNA damage related to pesticide exposure. Mutat Res. 1996;367:73–82. doi: 10.1016/0165-1218(95)00071-2. [DOI] [PubMed] [Google Scholar]
- 15.Lucero L, Pastor S, Suarez S, Durban R, Gomez C, Parron T, et al. Cytogenetic biomonitoring of Spanish greenhouse workers exposed to pesticides: Micronuclei analysis in peripheral blood lymphocytes and buccal epithelial cells. Mutat Res. 2000;464:255–62. doi: 10.1016/s1383-5718(99)00200-4. [DOI] [PubMed] [Google Scholar]
- 16.Velazquez AN, Xamena A, Marcos R. Indication for weak mutagenicity of the organophosphorous insecticide, dimethoate in drosophila melanogaster. Mutat Res. 1986;172:237–43. doi: 10.1016/0165-1218(86)90061-3. [DOI] [PubMed] [Google Scholar]
- 17.Islas-Gonzalez K, Gonzalez-Horta C, Sanchez-Ramirez B, Reyes-Aragon E, Levario-Carrillo M. In vitro assessment of the genotoxicity of ethyl paraoxon in newborns and adults. Human Exp Toxicol. 2005;24:319–24. doi: 10.1191/0960327105ht534oa. [DOI] [PubMed] [Google Scholar]
- 18.Gontijo AM de MC, Elias FN, Salvadori DM, de Oliveira ML, Correa LA, Goldberg J, et al. Single-cell gel (Comet) assay detects primary DNA damage in non-neoplastic urothelial cells of smokers and ex-smokers. Cancer Epidemiol Biomark Prev. 2001;10:987–93. [PubMed] [Google Scholar]
- 19.Garaj-Vrhovac V, Zeljezic D. Cytogenetic monitoring of Croation population occupationally exposed to a complex mixture of pesticides. Toxicology. 2001;165:153–62. doi: 10.1016/s0300-483x(01)00419-x. [DOI] [PubMed] [Google Scholar]
- 20.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(Suppl 1):184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 21.Rojas E, Lopez MC, Valverde M. Single cell gel electrophoresis assay: Methodology and applications. J Chromatogr B Biomed Sci Appl. 1999;722:225–54. doi: 10.1016/s0378-4347(98)00313-2. [DOI] [PubMed] [Google Scholar]
- 22.Collins AR, Duthie SJ, Dobson VL. Direct enzymeic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogenesis. 1993;14:1733–5. doi: 10.1093/carcin/14.9.1733. [DOI] [PubMed] [Google Scholar]
- 23.Lebailly P, Vigreux C, Lechevrel C, Ledemeney D, Godard T, Sichel F, et al. DNA damage in mononuclear leukocytes of farmers measured using the alkaline comet assay: discussion of critical parameters and evaluation of seasonal variations in relation with pesticide exposure. Cancer Epidemiol Biomark Prev. 1998a;7(Suppl 10):917–27. [PubMed] [Google Scholar]
- 24.Lebailly P, Vigreux C, Lechevrel C, Ledemeney D, Godard T, Sichel F, et al. DNA damage in mononuclear leukocytes of farmers measured using the alkaline comet assay: modifications of DNA damage levels after a one-day field spraying period with selected pesticides. Cancer Epidemiol Biomark Prev. 1998;7(Suppl 10):929–40. [PubMed] [Google Scholar]
- 25.Grover P, Danadevi K, Mahboob M, Rozati R, Saleha Banu B, Rahman MF. Evaluation of genetic damage in workers employed in pesticide production utilizing Comet assay. Mutagenesis. 2003;18(Suppl 2):201–5. doi: 10.1093/mutage/18.2.201. [DOI] [PubMed] [Google Scholar]
- 26.Jamil K, Shaik AP, Mahboob M, Krisna D. Effect of organophosphorus and organochlorine pesticides (monochrotophos, chlorpyriphos, dimethoate and endosulfan) on lymphocytes in vitro. Drug Chem Toxicol. 2004;27(Suppl 2):133–44. doi: 10.1081/dct-120030725. [DOI] [PubMed] [Google Scholar]
- 27.Bhalli JA, Khan QM, Nasim A. DNA damage in Pakistani pesticide manufacturing workers assayed using the Comet assay. Environ Mol Mutagen. 2006;47(Suppl 8):587–93. doi: 10.1002/em.20232. [DOI] [PubMed] [Google Scholar]
- 28.Castillo-Cadena J, Tenorio-Vieyra LE, Quintana-Carabia AI, Garcia-Fabila MM, Ramirez-San Juan E, Madrigal-Bujaidar E. Determination of DNA damage in floriculturists exposed to a mixture of pesticides. J Biomed Biotech. 2006;97896:12. doi: 10.1155/JBB/2006/97896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YJ, Huang PL, Chang YF, Chen YH, Chiou YH, Xu ZL, et al. GSTPI genetic polymorphism is associated with a higher risk of DNA damage in pesticide-exposed fruit growers. Cancer Epidemiol Biomark Prev. 2006;15(Suppl 4):659–66. doi: 10.1158/1055-9965.EPI-05-0617. [DOI] [PubMed] [Google Scholar]
- 30.Piperakis SM, Kontogianni K, Siffel C, Piperakis MM. Measuring the effects of pesticides on occupationally exposed humans with the comet assay. Environ Toxicol. 2006;21(Suppl 4):355–9. doi: 10.1002/tox.20191. [DOI] [PubMed] [Google Scholar]
- 31.Ahuja YR, Saran R. Alkaline single cell gel electrophoresis assays I. Protocol J Cytol Genet. 1999;34(Suppl 1):57–62. [Google Scholar]
- 32.Paz-Y-Mino C, Arevalo M, Sanchez ME, Leone PE. Chromosome and DNA damage analysis in individuals occupationally exposed to pesticides with relation to genetic polymorphism for CYP1A1 gene in Ecuador. Mutat Res. 2004;562(Suppl 1-2):77–89. doi: 10.1016/j.mrgentox.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Bajpayee M, Pandey AK, Zaidi S, Musarrat J, Parmar D, Mathur N, et al. DNA damage and mutagenicity induced by endosulfan and its metabolites. Environ Mol Mutagen. 2006;47:682–92. doi: 10.1002/em.20255. [DOI] [PubMed] [Google Scholar]
- 34.Shadnia S, Azizi E, Hosseini R, Khoei S, Fouladdel S, Pajoumand A, et al. Evaluation of oxidative stress and genotoxicity in organophosphorus insecticide formulators. Hum Exp Toxicol. 2005;24(Suppl 9):439–45. doi: 10.1191/0960327105ht549oa. [DOI] [PubMed] [Google Scholar]
- 35.Piperakis SM, Petrakou E, Tsilimigaki S, Sagnou M, Monogiudis E, Haniotakis G, et al. Biomonitoring with the comet assay of Greek greenhouse workers exposed to pesticides. Environ Mol Mutagen. 2003;41:104–10. doi: 10.1002/em.10143. [DOI] [PubMed] [Google Scholar]
- 36.Hodgson E, Silver IS, Butler LE, Lawton MP, Levi PE. Metabolism. In: Hayes WJ, Laws ER, editors. Handbook of Pesticides Toxicology. Vol. 1. San Diego CA: Academic Press; 1991. pp. 106–67. [Google Scholar]
- 37.Au WW, Sierra-Torres CH, Cajas-Salazar N, Shipp BK, Legator MS. Cytogenetic effects from exposure to mixed pesticides and the influence from genetic susceptibility. Environ Health Perspect. 1999;107:501–5. doi: 10.1289/ehp.99107501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler AM, Murray M. Inhibition and inactivation of constitutive cytochrome P450 in rat liver by parathion. Mol Pharmacol. 1993;43:902–8. [PubMed] [Google Scholar]
- 39.Dinsdale D, Verschoyle RD. Cell-specific loss of cytochrome P450 2B1 in rat lung following treatment with pneumotoxic and non-pneumotoxic trialkylphosphorothioates. Biochem Pharmacol. 2001;61:493–501. doi: 10.1016/s0006-2952(00)00572-4. [DOI] [PubMed] [Google Scholar]
- 40.Carbonell E, Valbuena A, Xamena N, Creus A, Marcos R. Temporary variations in chromosomal aberrations in a group of agricultural workers exposed to pesticides. Mutat Res. 1995;344:127–34. doi: 10.1016/0165-1218(95)00051-8. [DOI] [PubMed] [Google Scholar]
- 41.Lander B, Knudsen L, Gamborg M, Jarventaus H, Norppa H. Chromosome aberrations in pesticide-exposed greenhouse workers. Scand J Work Environ Health. 2000;26:436–42. doi: 10.5271/sjweh.565. [DOI] [PubMed] [Google Scholar]
- 42.Obe G, Beck B. The human leukocyte test system. In: De Serres FJ, Hollaender A, editors. Chemical Mutagens. Vol. 7. New York, NY: Plenum Press; 1982. pp. 337–400. [Google Scholar]
- 43.Biological Dosimetry: Chromosome Aberration Analysis for Dose assessment, International Atomic Energy Technical Reports Series no. 260. Vienna: IAEA; 1986. IAEA; pp. 1–69. [Google Scholar]
- 44.Carrano A, Natarajan A. Consideration for population monitoring using cytogenetic techniques. Mutat Res. 1988;204:379–406. doi: 10.1016/0165-1218(88)90036-5. [DOI] [PubMed] [Google Scholar]
- 45.Vaghef H, Nygren P, Edling C, Bergh J, Hellman B. Alkaline single-cell gel electrophoresis and human biomonitoring for genotixicity: A pilot study on breast cancer patients undergoing chemotherapy including cyclophosphamide. Mutat Res. 1997;395:127–38. doi: 10.1016/s1383-5718(97)00157-5. [DOI] [PubMed] [Google Scholar]
- 46.Lebailly P, Devaux A, Pottier D, De Meo M, Andre V, Baldi I, et al. Urine mutagenicity and lymphocytes DNA damage in fruit growers occupationally exposed to the fungicide captan. Occup Environ Med. 2003;60(Suppl 12):910–7. doi: 10.1136/oem.60.12.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kopjar N, Zeljezic D, Garaj-Vrhovac V. Evaluation of DNA damage in white blood cells of healthy human volunteers using the alkaline comet assay and the chromosome aberration test. Acta Biochimica Polonica. 2006;53(Suppl 2):321–36. [PubMed] [Google Scholar]
- 48.Hoffmann H, Isner C, Hogel J, Speit G. Genetic polymorphisms and the effect of cigarette smoking in the comet assay. Mutagenesis. 2005;20(Suppl 5):359–64. doi: 10.1093/mutage/gei049. [DOI] [PubMed] [Google Scholar]
- 49.Staruchova M, Collins AR, Volkovova K, Mislanová C, Kovacikova Z, Tulinska J, et al. Occupational exposure to mineral fibres: Biomarkers of oxidative damage and antioxidant defence and associations with DNA damage and repair. Mutagenesis. 2008;23:249–60. doi: 10.1093/mutage/gen004. [DOI] [PubMed] [Google Scholar]
- 50.Giovannelli L, Saieva C, Masala G, Testa G, Salvini S, Pitozzi V, et al. Nutritional and lifestyle determinants of DNA oxidative damage: A study in a Mediterranean population. Carcinogenesis. 2002;23(Suppl 9):1483–9. doi: 10.1093/carcin/23.9.1483. [DOI] [PubMed] [Google Scholar]
- 51.Dhawan A, Mathur N, Seth PK. The effect of smoking and eating habits on DNA damage in Indian population as measured in the Comet assay. Mutat Res. 2001;474(Suppl 1-2):121–8. doi: 10.1016/s0027-5107(00)00171-8. [DOI] [PubMed] [Google Scholar]
- 52.Undeger U, Basaran N. Assessment of DNA damage in workers occupationally exposed to pesticide mixtures by the alkaline comet assay. Arch Toxicol. 2002;76:430–6. doi: 10.1007/s00204-002-0355-5. [DOI] [PubMed] [Google Scholar]
- 53.Moretti M, Villarini M, Scassellati-Sforzolini G, Monarca S, Libraro M, Fatigoni C, et al. Biological monitoring of genotoxic hazard in workers of the rubber industry. Environ Health Perspect. 1996;104:543–5. doi: 10.1289/ehp.96104s3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laffon B, Pasaro E, Mendez J. Evaluation of genotoxic effects in a group of workers exposed to low levels of styrene. Toxicology. 2002;171:175–86. doi: 10.1016/s0300-483x(01)00572-8. [DOI] [PubMed] [Google Scholar]
- 55.Garaj-Vrhovac V, Kopjar N. Investigation into possible DNA damaging effects of ultrasound in occupationally exposed medical personnel - the alkaline comet assay study. J Appl Toxicol. 2005;25:184–92. doi: 10.1002/jat.1035. [DOI] [PubMed] [Google Scholar]


