Abstract
BACKGROUND:
Ischemic stroke descent has a genetic basis. Stroke represents a complex trait, which is assumed to be polygenic. On this topic, the role of a wide number of candidate genes has been investigated in stroke through association studies.
MATERIALS AND METHODS:
We performed a literature-based systematic review of genetic association studies in stroke abound several populations. Odds ratios (ORs) and 95% confidence intervals (CIs) were determined for each gene-disease association. Following a review of 300 manuscripts, five candidate gene variants were analyzed among 152,797 individuals (45,433 cases and 107,364 controls).
RESULTS:
For these five candidate genes studied, the prothrombin OR is 1,57 (1,23-2,89), the factor V Leiden OR is 1,43 (0,67-6,24), the mean OR of angiotensin I converting enzyme (ACE) insertion/deletion (I/D) polymorphism is 1,11 (1,02-1,25), the summary OR for the C677T variant of 5,10-methylenetetrahydrofolate reductase (MTHFR) is 1,23 (0,61-1,47) and the pooled OR for the apolipoprotein E (APOE) gene is 0,95 (0,77-1,14) .
CONCLUSION:
These data suggest the genetic associations of some genes with ischemic stroke and it is necessary to compete with other genes. Our findings could represent an epidemiological base and a useful tool to address further molecular investigations and to realize more detailed meta-analyses.
Keywords: Angiotensin I converting enzyme, apolipoprotein E, factor V Leiden, factor II prothrombin, meta-analysis, MTHFR, stroke
Introduction
Stroke is believed to be a complex multifactorial and polygenic disease arising from a wide number of gene-gene and gene-environment interactions. Genetic factors could act by predisposing to conventional risk factors. Studies on stroke genetics present some methodological difficulties. Therefore, the major line of multifactorial stroke investigation is the candidate-gene approach, which consists of identifying molecular variants within a functional relevant gene and establishing its function in stroke risk by association case- control. Following some reports of positive association ischemic disease, a wide number of candidate genes have been investigated in stroke, even if, so far, only a few polymorphisms have been consistently associated with stroke occurrence. Most of these studies have been criticized for some bias related to small sample size, lack of classification by stroke phenotype or subtype, use of ethnically different populations, and unmatched controls (Flossmann et al., 2004[1]; Dichgans and Markus et al., 2005[2]). The differences in patient's characteristics together with the heterogeneity in study design, could explain much of the inconsistency between studies (Dichgans et al., 2007[3]).
Although association studies are considered as a powerful instrument to identify risk factors, both methodological and appropriate studies and replication of results are necessary to demonstrate a causal relationship between a genetic marker and stroke. Therefore, the results of existing studies identified a list of possible candidate genes associated with stroke (Meschia et al., 2005[4]). In our work, we want to provide a meta analysis reporting studies published on genetic of stroke, and we analyzed only studies in which genotype frequency was reported. In our literature revision, we focused on ischemic stroke, excluding association analyses on hemorrhagic stroke patients.
The aim of this work is to give a panel of possible genes associated with ischemic stroke risk that could be useful both for future epidemiological data and for deciding new research strategies for stroke genetics studies.
Materials and Methods
Electronic databases (MEDLINE, EMBASE, PUBMED, SCOPUS…) were searched up until 2010 for all case-control studies evaluating any candidate gene and stroke. The medical subject headings terms and text words used for the search were cerebrovascular disease, stroke, cerebral ischemia, and brain infarction in combination with polymorphism(s), mutation, genetic, genotype, or genes. All languages were searched initially, but only English language articles were selected. The references of all computer- identified publications were searched for any additional studies, and the MEDLINE option related articles was used for all relevant articles. In addition, a search to identify previous genetic meta-analyses in stroke was also performed. Studies were selected if neuroimaging (magnetic resonance imaging or computed tomography) had been used to confirm the diagnosis of ischemic stroke. Studies were excluded if the patients were children (aged 18 years), quantitative traits or intermediate phenotypes were being investigated, or genotype frequency was not reported. For duplicate publications, the smaller data set was discarded. First search generated 300 relevant articles, of which 60 met the inclusion criteria. Data for analysis were extracted and entered into separate databases by two of us (K.H. and A.T).
Data synthesis:
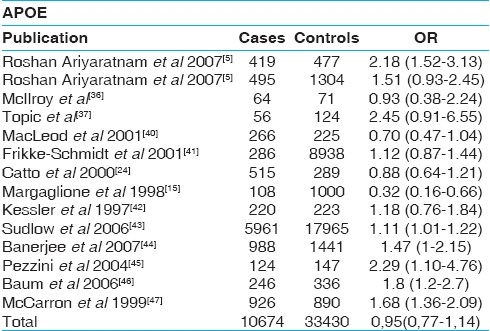
Thirty two candidate gene case-control studies in which the presence or absence of stroke were analyzed. In total, 5 polymorphisms in 5 genes were identified. From the 5 polymorphisms analyzed in detail for a total of 152797 individuals (representing 45433 cases and 107364 controls), the mean number of studies per candidate gene was 12. The ACE I/D polymorphism was evaluated in 12 studies (7709 cases and 17284 controls), and a summary OR of 1.11 (95% CI, 1.02-1.25; P = .002), under a fixed-effects model, was observed for individuals homozygous for the D allele compared with heterozygous (D/I) and homozygous (I/I) individuals combined [Table 1]. The prothrombin G20210A mutation was evaluated in 12 studies, with a total of 5644 cases and 13459 controls. The summary OR under a fixed-effects model showed that carriers of the mutation were 1.57 times more likely to develop stroke (95% CI, 1.23- 2.89; P = .005) [Table 2]. The Factor V Leiden G1691A mutation was evaluated in 12 studies, with a total of 7749 cases and 26101 controls. The summary OR under a fixed-effects model showed that carriers of the mutation were 1.43 times more likely to develop stroke (95% CI, 0.67- 6.24; P=.005) [Table 3]. A total of 12 studies (13657 cases and 17090 controls) were identified that evaluated the polymorphism in the gene encoding methylenetetrahydrofolate reductase where cytosine is replaced by thymidine at base position 677 of the gene (MTHRFC677T). A summary OR, under the fixed-effects model, of 1.23 (95% CI, 0.61-1.47; P=.001) was observed for individuals homozygous for the T allele compared with C allele carriers (C/T plus C/C) [Table 4]. The apolipoprotein E genotype has been by far the most investigated, with 12 studies that included 10674 cases and 33430 controls. Carriers of the factor ApoE E4 allele were 0.95 times to develop stroke (95% CI, 0.77-1.14; P=.002) [Table 5].
Table 1.
Results of published studies of the association between the ACE I/D polymorphism and ischemic stroke. Odds ratios for the outcome compared individuals homozygous for the D allele with those with the heterozygous (D/I) plus wild type (I/I). CI indicates confidence interval.

Table 2.
Results of published studies of the association between the prothrombin G20210A polymorphism and ischemic stroke. Odds ratios for the outcome compared carriers of the A allele with those with wild type (G/G). CI indicates confidence interval.
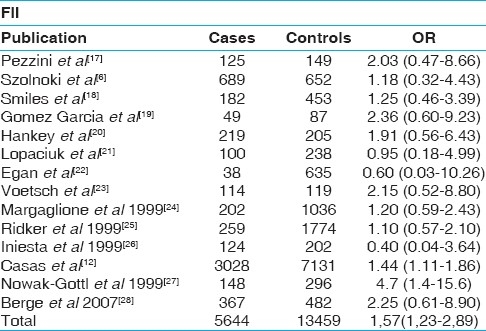
Table 3.
Results of published studies of the association between the factor V Leiden mutation and ischemic stroke. Odds ratios for the outcome compared carriers of the Gln506 allele vs wild type (Arg/Arg). CI indicates confidence interval.
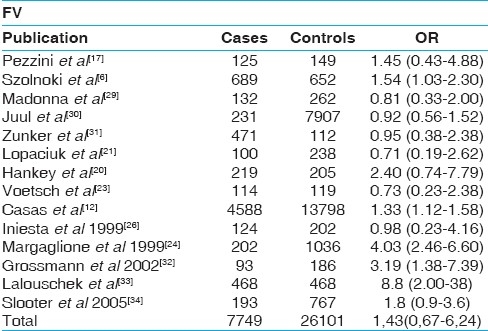
Table 4.
Results of published studies of the association between the methylenetetrahydrofolate reductase C677T polymorphism and ischemic stroke. Odds ratios for the outcome compared individuals homozygous for the T allele (T/T) with those heterozygous individuals (C/T) plus wild type (C/C). CI indicates confidence interval.
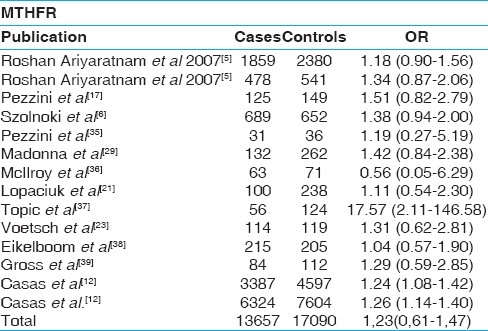
Table 5.
Results of published studies of the association between the apolipoprotein E polymorphism and ischemic stroke. Odds ratios for the outcome compared carriers of the ε4 allele with those with the ε3 and ε2 alleles. CI indicates confidence interval.
Comments
In this comprehensive meta-analysis, 3 (47%) of the 5 candidate polymorphisms analyzed significantly increased the risk of stroke. In 24 of these studies (ACE I/D, factor V Leiden, MTHFR C677T, prothrombin G20210A, and apolipoprotein E), the number of cases included per gene was more than 1000, allowing more precise estimates to be made of the effect of these genes than from any single study. Most candidate genes assessed in stroke thus far have been evaluated initially for their potential role in ischemic disease. Therefore, up to now, most genetic studies have focused on genes involved in thrombosis and coagulation (factor V Leiden, prothrombin, and MTHFR, also genes regulating other well-established risk factors for stroke (e.g., hypertension, diabetes mellitus, and hyperlipidemia (ACE and apolipoprotein E) are studied.
The factor V Leiden mutation causes activated protein C resistance. Activated protein C limits clot formation by proteolytic inactivation of factors Va and VIIIa, and the single point mutation in the gene for facto rV(1691G→A) studied predicts replacement of arginine by glycine at position 506 in the activated protein C cleavage site. After activation, the mutated factor V is less efficiently degraded by activated protein C than normal factor V, resulting in increased thrombin generation and a hypercoagulable state, with a 5-10-fold increased risk of thrombosis in heterozygotes and a 50-100-fold increased risk in homozygotes which may explain the increased risk of stroke in carriers of this mutation observed in this study (OR=1.43 (0.67-6.24)). In our study, the risk factor is varying from 0.19 found by Lopaciuk et al[21] to 38 found in the series of Lalouschek et al.[33] Those results can be explained by the large difference of the A1691G allelic frequency in several populations.
A sequence variation in the 3_untranslated region of the prothrombin gene (G20210A), which alters messenger RNA stability is associated with elevated prothrombin levels and thrombin formation and may similarly lead to a procoagulant state. A carrier frequency around 3.0% has been detected in southern Europe, whereas the prothrombin variant is very rare in non-Caucasians (OR=1.57 (1.23-2.89)), the extreme values of OR are unregistered in same the work of Egan et al[9] 2000 (OR (0.03-10.26).
Plasma and intracellular levels of ACE have been shown to be partly determined by the presence of the ACE I/D polymorphism in healthy individuals and in patients with stroke. Individuals homozygous for the D allele have a 56% increase in ACE activity compared with I allele homozygotes. 121 Angiotensin-converting enzyme converts angiotensin I to angiotensin II, which is known to be involved in vascular hypertrophy, vasoconstriction, and atherosclerotic processes (OR=1.11 (1.02-1.25)). In the 12 works studied in our meta-analysis, the OR is sensibly varying between 0.5 and 2.
Long-term differences of 5 μmol/L in the serum concentration of homocysteine are associated with a 59% increase in the risk of stroke. The C677T mutation in the MTHFR gene, which encodes an amino acid substitution (A222V), renders the enzyme thermolabile and reduces metabolism of homocysteine (OR=1.23 (0.61-1.47)). The MTHFR remains the most controversial genetics factor with an average of OR from 0.05 in McIlroy et al,[36] to 146.58 in the work of Topic et al.[37] Carriers of the apolipoprotein E ε4 allele, which affects serum cholesterol, and which has been associated with a moderate increase in the risk of coronary heart disease, are at a substantially higher risk of stroke (OR=0.95 (0.77-1.14)).
The results of our meta-analysis about the five stroke genes are summarized in Figure 1.[47]
Figure 1.
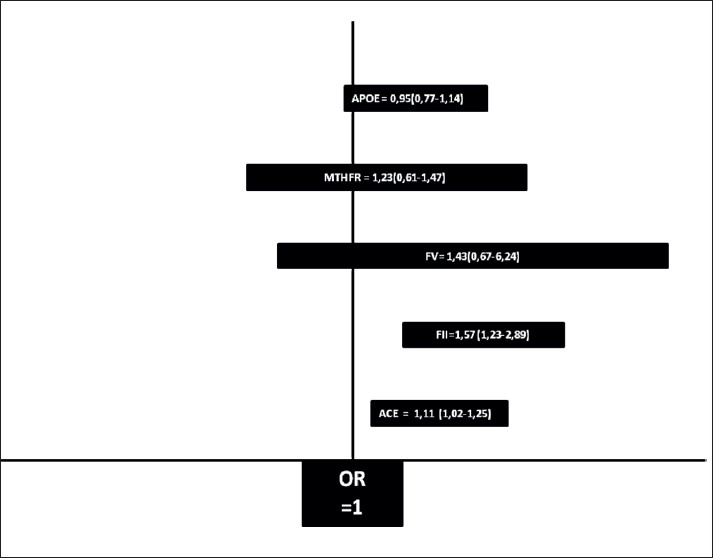
summary of the results of the five genes studies in the meta analysis (ACE, MTHFR, FVL, FII AND APOE).
The limits of the box presents the extreme values of OR
Acknowledgments
The authors express their thanks for all the staff of the Laboratory of Human Genetics, Medical School of Casablanca for their technical advice and for their contributions to this study. This work is supported by the academy Hassan II of sciences and technology - Stroke project.
Footnotes
Source of Support: Academy Hassan II of sciences and technology - Stroke project,
Conflict of Interest: None declared.
References
- 1.Flossmann E, Schulz UG, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke. 2004 Jan;35(1):212–27. doi: 10.1161/01.STR.0000107187.84390.AA. [DOI] [PubMed] [Google Scholar]
- 2.Dichgans M, Markus HS. Genetic association studies in stroke: methodological issues and proposed standard criteria. Stroke. 2005 Sep;36(9):2027–31. doi: 10.1161/01.STR.0000177498.21594.9e. [DOI] [PubMed] [Google Scholar]
- 3.Dichgans M. Genetics of ischaemic stroke. Lancet Neurol. 2007 Feb;6(2):149–61. doi: 10.1016/S1474-4422(07)70028-5. [DOI] [PubMed] [Google Scholar]
- 4.Meschia JF, Brott TG, Brown RD., Jr Genetics of cerebrovascular disorders. Mayo Clin Proc. 2005 Jan;80(1):122–32. doi: 10.1016/S0025-6196(11)62969-8. [DOI] [PubMed] [Google Scholar]
- 5.Ariyaratnam R, Casas JP, Whittaker J, Smeeth L, Hingorani AD, Sharma P. Genetics of ischaemic stroke among persons of non-European descent: a meta-analysis of eight genes involving approximately 32,500 individuals. PLoS Med. 2007 Apr;4(4):e131. doi: 10.1371/journal.pmed.0040131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szolnoki Z, Somogyvari F, Kondacs A, Szabo M, Fodor L. Evaluation of the interactions of common genetic mutations in stroke subtypes. J Neurol. 2002;249:1391–7. doi: 10.1007/s00415-002-0848-4. [DOI] [PubMed] [Google Scholar]
- 7.Peterlin B, Petrovic D, Zorc M, Keber I. Deletion/insertion polymorphism in the angiotension-converting enzyme gene as a risk factor in the Slovenian patients with coronary heart disease. Pflugers Arch. 2000;439(suppl):R40–1. [PubMed] [Google Scholar]
- 8.Markus H. Risk of stroke in asymptomatic carotid stenosis. Lancet. 1995 Mar;18(345)(8951):721. [PubMed] [Google Scholar]
- 9.Kostulas K, Huang WX, Crisby M, Jin YP, He B, Lannfelt L, Eggertsen G, Kostulas V, Hillert J. An angiotensin-converting enzyme gene polymorphism suggests a genetic distinction between ischaemic stroke and carotid stenosis. Eur J Clin Invest. 1999 Jun;29(6):478–83. doi: 10.1046/j.1365-2362.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- 10.Pfohl M, Fetter M, Koch M, Barth CM, Rüdiger W, Häring HU. Association between angiotensin I-converting enzyme genotypes, extracranial artery stenosis, and stroke. Atherosclerosis. 1998 Sep;140(1):161–6. doi: 10.1016/s0021-9150(98)00100-2. [DOI] [PubMed] [Google Scholar]
- 11.Zee RY, Ridker PM, Stampfer MJ, Hennekens CH, Lindpaintner K. Prospective evaluation of the angiotensin-converting enzyme insertion/deletion polymorphism and the risk of stroke. Circulation. 1999 Jan 26;99(3):340–3. doi: 10.1161/01.cir.99.3.340. [DOI] [PubMed] [Google Scholar]
- 12.Casas JP, Hingorani AD, Bautista LE, Sharma P. Meta-analysis of genetic studies in ischemic stroke: Thirty-two genes involving approximately 18,000 cases and 58,000 controls. Arch Neurol. 2004;61:1652–61. doi: 10.1001/archneur.61.11.1652. [DOI] [PubMed] [Google Scholar]
- 13.Zhang XF, Attia J, D’Este C, Yu XH. Prevalence and magnitude of classical risk factors for stroke in a cohort of 5092 Chinese steelworkers over 13.5 years of follow-up. Stroke. 2004 May;35(5):1052–6. doi: 10.1161/01.STR.0000125305.12859.ff. Epub 2004 Apr 8. [DOI] [PubMed] [Google Scholar]
- 14.Ueda T, Hatakeyama T, Sakaki S, Ohta S, Kumon Y, Uraoka T. Changes in coagulation and fibrinolytic system after local intra-arterial thrombolysis for acute ischemic stroke. Neurol Med Chir (Tokyo) 1995 Mar;35(3):136–43. doi: 10.2176/nmc.35.136. [DOI] [PubMed] [Google Scholar]
- 15.Cato AC, Wade E. Molecular mechanisms of anti-inflammatory action of glucocorticoids. Bioessays. 1996 May;18(5):371–8. doi: 10.1002/bies.950180507. Review. [DOI] [PubMed] [Google Scholar]
- 16.Kario K, Kanai N, Saito K, Nago N, Matsuo T, Shimada K. Ischemic stroke and the gene for angiotensin-converting enzyme in Japanese hypertensives. Circulation. 1996 May 1;93(9):1630–3. doi: 10.1161/01.cir.93.9.1630. [DOI] [PubMed] [Google Scholar]
- 17.Pezzini A, Del Zotto E, Magoni M, Costa A, Archetti S, Grassi M, et al. Inherited thrombophilic disorders in young adults with ischemic stroke and patent foramen ovale. Stroke. 2003;34:28–33. doi: 10.1161/01.str.0000046457.54037.cc. [DOI] [PubMed] [Google Scholar]
- 18.Smiles AM, Jenny NS, Tang Z, Arnold A, Cushman M, Tracy RP. No association of plasma prothrombin concentration or the G20210A mutation with incident cardiovascular disease: Results from the Cardiovascular Health Study. Thromb Haemost. 2002;87:614–21. [PubMed] [Google Scholar]
- 19.Gomez Garcia EB, van Goor MP, Leebeek FW, Brouwers GJ, Koudstaal PJ, Dippel DW. Elevated prothrombin is a risk factor for cerebral arterial ischemia in young adults. Clin Neurol Neurosurg. 2002;104:285–8. doi: 10.1016/s0303-8467(01)00202-5. [DOI] [PubMed] [Google Scholar]
- 20.Hankey GJ, Eikelboom JW, van Bockxmeer FM, Lofthouse E, Staples N, Baker RI. Inherited thrombophilia in ischemic stroke and its pathogenic subtypes. Stroke. 2001;32:1793–9. doi: 10.1161/01.str.32.8.1793. [DOI] [PubMed] [Google Scholar]
- 21.Lopaciuk S, Bykowska K, Kwiecinski H, Mickielewicz A, Czlonkowska A, Mendel T, et al. Factor V Leiden, prothrombin gene G20210A variant, and methylenetetrahydrofolate reductase C677T genotype in young adults with ischemic stroke. Clin Appl Thromb Hemost. 2001;7:346–50. doi: 10.1177/107602960100700418. [DOI] [PubMed] [Google Scholar]
- 22.Egan RA, Kuyl JM, Press R, Lutsep HL. Lack of prothrombin gene mutation in young stroke patients. J Stroke Cerebrovasc Dis. 2000;9:229–31. [Google Scholar]
- 23.Voetsch B, Damasceno BP, Camargo EC, Massaro A, Bacheschi LA, Scaff M, et al. Inherited thrombophilia as a risk factor for the development of ischemic stroke in young adults. Thromb Haemost. 2000;83:229–33. [PubMed] [Google Scholar]
- 24.Margaglione M, D’Andrea G, Colaizzo D, Cappucci G, del Popolo A, Brancaccio V, Ciampa A, Grandone E, Di Minno G. Coexistence of factor V Leiden and Factor II A20210 mutations and recurrent venous thromboembolism. Thromb Haemost. 1999 Dec;82(6):1583–7. [PubMed] [Google Scholar]
- 25.Ridker PM. Duration and intensity of anticoagulation among patients with genetic predispositions to venous thrombosis. Curr Cardiol Rep. 1999 Jul;1(2):88–90. doi: 10.1007/s11886-999-0064-2. [DOI] [PubMed] [Google Scholar]
- 26.Iniesta JA, Corral J, González-Conejero R, Rivera J, Vicente V. Prothrombotic genetic risk factors in patients with coexisting migraine and ischemic cerebrovascular disease. Headache. 1999 Jul-Aug;39(7):486–9. doi: 10.1046/j.1526-4610.1999.3907486.x. [DOI] [PubMed] [Google Scholar]
- 27.Nowak-Göttl U, Sträter R, Heinecke A, Junker R, Koch HG, Schuierer G, von Eckardstein A. Lipoprotein (a) and genetic polymorphisms of clotting factor V, prothrombin, and methylenetetrahydrofolate reductase are risk factors of spontaneous ischemic stroke in childhood. Blood. 1999 Dec 1;94(11):3678–82. [PubMed] [Google Scholar]
- 28.Berge E, Haug KB, Sandset EC, Haugbro KK, Turkovic M, Sandset PM. The factor V Leiden, prothrombin gene 20210GA, methylenetetrahydrofolate reductase 677CT and platelet glycoprotein IIIa 1565TC mutations in patients with acute ischemic stroke and atrial fibrillation. Stroke. 2007 Mar;38(3):1069–71. doi: 10.1161/01.STR.0000258076.04860.8e. [DOI] [PubMed] [Google Scholar]
- 29.Madonna P, de Stefano V, Coppola A, Cirillo F, Cerbone AM, Orefice G, et al. Hyperhomocysteinemia and other inherited prothrombotic conditions in young adults with a history of ischemic stroke. Stroke. 2002;33:51–6. doi: 10.1161/hs0102.100483. [DOI] [PubMed] [Google Scholar]
- 30.Juul K, Tybjaerg-Hansen A, Steffensen R, Kofoed S, Jensen G, Nordestgaard BG. Factor V Leiden: The Copenhagen City Heart Study and 2 meta-analyses. Blood. 2002;100:3–10. doi: 10.1182/blood-2002-01-0111. [DOI] [PubMed] [Google Scholar]
- 31.Zunker P, Hohenstein C, Plendl HJ, Zeller JA, Caso V, Georgiadis D, et al. Activated protein C resistance and acute ischaemic stroke: Relation to stroke causation and age. J Neurol. 2001;248:701–4. doi: 10.1007/s004150170117. [DOI] [PubMed] [Google Scholar]
- 32.Grossmann R, Geisen U, Merati G, Müllges W, Schambeck CM, Walter U, Schwender S. Genetic risk factors in young adults with ‘cryptogenic’ischemic cerebrovascular disease. Blood Coagul Fibrinolysis. 2002 Oct;13(7):583–90. doi: 10.1097/00001721-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Lalouschek W, Aull S, Serles W, Schnider P, Mannhalter C, Lang T, et al. Genetic and nongenetic factors influencing plasma homocysteine levels in patients with ischemic cerebrovascular disease and in healthy control subjects. J Lab Clin Med. 1999;133:575–82. doi: 10.1016/s0022-2143(99)90187-7. [DOI] [PubMed] [Google Scholar]
- 34.Slooter AJ, Rosendaal FR, Tanis BC, Kemmeren JM, van der Graaf Y, Algra A. Prothrombotic conditions, oral contraceptives, and the risk of ischemic stroke. J Thromb Haemost. 2005 Jun;3(6):1213–7. doi: 10.1111/j.1538-7836.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- 35.Pezzini A, Del Zotto E, Archetti S, Negrini R, Bani P, Albertini A, et al. Plasma homocysteine concentration, C677T MTHFR genotype, and 844ins68bp CBS genotype in young adults with spontaneous cervical artery dissection and atherothrombotic stroke. Stroke. 2002;33:664–9. doi: 10.1161/hs0302.103625. [DOI] [PubMed] [Google Scholar]
- 36.McIlroy SP, Dynan KB, Lawson JT, Patterson CC, Passmore AP. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke. 2002;33:2351–6. doi: 10.1161/01.str.0000032550.90046.38. [DOI] [PubMed] [Google Scholar]
- 37.Topic E, Simundic AM, Ttefanovic M, Demarin V, Vuković V, Lovrencić-Huzjan A, et al. Polymorphism of apoprotein E (APOE), methylenetetrahydrofolate reductase (MTHFR) and paraoxonase (PON1) genes in patients with cerebrovascular disease. Clin Chem Lab Med. 2001;39:346–50. doi: 10.1515/CCLM.2001.054. [DOI] [PubMed] [Google Scholar]
- 38.Eikelboom JW, Hankey GJ, Anand SS, Lofthouse E, Staples N, Baker RI. Association between high homocysteine and ischemic stroke due to large- and small artery disease but not other etiologic subtypes of ischemic stroke. Stroke. 2000;31:1069–75. doi: 10.1161/01.str.31.5.1069. [DOI] [PubMed] [Google Scholar]
- 39.Gross B, Antebi A, Cassel A, Honigman S. Is a mutation in the enzyme MTHFR a risk factor for stroke in young adults [abstract]? Neurology. 2000;54(suppl3):A142. [Google Scholar]
- 40.MacLeod RS, Punske B, Yilmaz B, Shome S, Taccardi B. The role of heart rate in myocardial ischemia from restricted coronary perfusion. J Electrocardiol. 2001;34(Suppl):43–51. doi: 10.1054/jelc.2001.28825. [DOI] [PubMed] [Google Scholar]
- 41.Frikke-Schmidt R, Nordestgaard BG, Thudium D, Moes Grønholdt ML, Tybjaerg-Hansen A. APOE genotype predicts AD and other dementia but not ischemic cerebrovascular disease. Neurology. 2001 Jan 23;56(2):194–200. doi: 10.1212/wnl.56.2.194. [DOI] [PubMed] [Google Scholar]
- 42.Kessler C, Spitzer C, Stauske D, Mende S, Stadlmüller J, Walther R, Rettig R. The apolipoprotein E and beta-fibrinogen G/A-455 gene polymorphisms are associated with ischemic stroke involving large-vessel disease. Arterioscler Thromb Vasc Biol. 1997 Nov;17(11):2880–4. doi: 10.1161/01.atv.17.11.2880. [DOI] [PubMed] [Google Scholar]
- 43.Sudlow C, Martínez González NA, Kim J, Clark C. Does apolipoprotein E genotype influence the risk of ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage? Systematic review and meta-analyses of 31 studies among 5961 cases and 17,965 controls. Stroke. 2006 Feb;37(2):364–70. doi: 10.1161/01.STR.0000199065.12908.62. Epub 2005 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banerjee I, Gupta V, Ganesh S. Association of gene polymorphism with genetic susceptibility to stroke in Asian populations: a meta-analysis. J Hum Genet. 2007;52(3):205–19. doi: 10.1007/s10038-006-0098-x. Epub 2006 Dec 14. [DOI] [PubMed] [Google Scholar]
- 45.Pezzini A, Grassi M, Del Zotto E, Bazzoli E, Archetti S, Assanelli D, Akkawi NM, Albertini A, Padovani A. Synergistic effect of apolipoprotein E polymorphisms and cigarette smoking on risk of ischemic stroke in young adults. Stroke. 2004 Feb;35(2):438–42. doi: 10.1161/01.STR.0000112973.00867.98. [DOI] [PubMed] [Google Scholar]
- 46.Baum L, Ng HK, Wong KS, Tomlinson B, Rainer TH, Chen X, Cheung WS, Tang J, Tam WW, Goggins W, Tong CS, Chan DK, Thomas GN, Chook P, Woo KS. Associations of apolipoprotein E exon 4 and lipoprotein lipase S447X polymorphisms with acute ischemic stroke and myocardial infarction. Clin Chem Lab Med. 2006;44(3):274–81. doi: 10.1515/CCLM.2006.047. [DOI] [PubMed] [Google Scholar]
- 47.McCarron MO, Delong D, Alberts MJ. APOE genotype as a risk factor for ischemic cerebrovascular disease: a meta-analysis. Neurology. 1999 Oct 12;53(6):1308–11. doi: 10.1212/wnl.53.6.1308. [DOI] [PubMed] [Google Scholar]


