Abstract
PURPOSE:
This study was designed to evaluate the levels of hepcidin in the serum of patients with chronic obstructive pulmonary disease (COPD).
METHODS:
In the study, 74 male patients (ages 45-75) in a stable period for COPD were grouped as Group I: Mild COPD (n:25), Group II: Moderate COPD (n:24), and Group III: Severe COPD (n:25). Healthy non-smoker males were included in Group IV (n:35) as a control group. The differences of hepcidin level among all the groups were examined. Also, in the patient groups with COPD, hepcidin level was compared with age, body mass index, cigarette (package/year), blood parameters (iron, total iron binding capacity, ferritin, hemoglobin, hematocrit [hct]), respiratory function tests, and arterial blood gas results.
RESULTS:
Although there was no difference between the healthy control group and the mild COPD patient group (P=0.781) in terms of hepcidin level, there was a difference between the moderate (P=0.004) and the severe COPD patient groups (P=0.002). The hepcidin level of the control group was found to be higher than the moderate and severe COPD patient groups. In the severe COPD patients, hepcidin level increased with the increase in serum iron (P=0.000), hct (P=0.009), ferritin levels (P=0.012), and arterial oxygen saturation (SaO2, P=0.000).
CONCLUSION:
The serum hepcidin level that is decreased in severe COPD brings into mind that it may play a role in the mechanism to prevent hypoxemia. The results suggest that serum hepcidin level may be a useful marker in COPD. Larger prospective studies are needed to confirm our findings between hepcidin and COPD.
Keywords: Chronic obstructive pulmonary disease, hepcidin, hypoxemia
Chronic Obstructive Pulmonary Disease (COPD) is characterized by progressive air flow obstruction that is not completely reversible, and is related to an abnormal inflammatory response in the lungs against harmful particles and gases. The prevalence, morbidity, and mortality of the disease have been increasing in recent years.[1] According to the World Health Organization (WHO), COPD has become the fourth most common cause of deaths in the world.[2] The inflammatory response found in the lungs is also present in peripheral airways, parenchyma, and in the walls of pulmonary veins. In COPD, an inflammatory response similar to that in the lungs is also observed in systemic circulation.[3] Local and systemic inflammation, together with arterial hypoxemia and oxygen delivery to tissue, are known to influence the prognosis of COPD.[4]
In many chronic inflammatory diseases, changes occur in iron metabolism. Hepcidin, a recently discovered hormone responsible for regulating iron homeostasis, is becoming increasingly important as a possible inflammatory marker. Hepcidin (hepatic bactericidal protein) was discovered by Park et al.,[5] is synthesized in the liver, has a role in iron metabolism, and is coded by human hepcidin gene (HAMP; OMIM 606464) located on chromosome 19q13.1.[6,7] Hepcidin has a regulatory role in inflammation, the immune system, and iron metabolism. It has been shown that hepcidin and proinflammatory cytokine interleukin 6 (IL-6) which is an important inducer of hepcidin synthesis during infection and inflammation.[7–9] Human hepcidin is classified as a type 2 acute phase protein.[10] Hepcidin is suppressed by both anemia and hypoxemia.[11]
The hepcidin level increase during inflammation is directly correlated with ferroportin in macrophages, hepatocytes, and duodenal enterocytes, and stimulates cellular ingestion. Thus, it is involved in cellular iron retention and prevention of iron loss into the plasma.
In this study, the serum hepcidin levels of patients with mild, moderate, and severe COPD, as classified according to criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2009[1] were measured and compared with each other and with a control group. The aim of this study was to examine a possible relationship between serum hepcidin level and severity of COPD.
Methods
Seventy-four male patients aged 45 to 75 years who were diagnosed with COPD according to the GOLD 2009 criteria between June 2010 and December 2010, and who were in stable condition were included in this study, which was done at the Diskapı Yildirim Beyazit Trainig and Research Hospital Chest Diseases Clinic. The patients were divided into three groups according to the GOLD criteria as follows: Group I: Mild COPD (n:25), Group II: Moderate COPD (n:24), and Group III: Severe COPD (n:25). Thirty-five healthy non-smoker males above 40 years of age with no chronic diseases were used as the control group (Group IV, n:35). COPD was excluded in the control group by physical examination and respiratory function tests.
The severity of the COPD was categorized into mild (forced expiratory volume in one second/forced vital capacity [FEV1/FVC<0.70, FEV1≥80% predicted), moderate (FEV1/FVC<0.70, 50%≤FEV1<80% predicted), and severe (FEV1/FVC<0.70, 30%≤FEV1<50% predicted).
The treatment of our patient's protocols was explained to GOLD criteria.[1] Anemia was defined as Hb<12 g/dl in male. Baseline arterial oxygen saturation was defined ≤88%. All of the patient groups and control group included in the study had C-reactive protein (CRP) levels within the normal limits (upper normal level, 10 mg/l).
The study included patients with COPD over 40 years of age, having a diagnosis of COPD according to the GOLD 2009, being at a stable phase of COPD and volunteering for the study. Our exclusion criteria were an acute COPD exacerbations in the last 3 months (increased cough, dyspnea, sputum production, and/or purulence),[1] other infections, a blood transfusion in the last 6 months, anti-inflammatory therapy (oral, parenteral systemic glucocorticosteroids) within the last 3 months, and anemia within the last 6 months.
Demographic information and the smoking history of patients were recorded. Body mass indexes (BMI; kg/m2) were calculated for all groups. Respiratory function tests were performed in all patients at the respiration laboratory of our clinic using a Jaeger spirometer according to Thoracic Society (ATS) guidelines. FEV1, FVC, and FEV1/FVC (Tiffeneau index) parameters were measured at least three times and the values were recorded.
The lung graphs of the patients were evaluated. The patient groups and control group also underwent physical examinations.
In all patient groups and control group included in the study, blood and serum samples for full blood testing were taken in the morning on an empty stomach. Serum iron, total iron binding capacity, ferritin, hemoglobin, and hematocrit (hct) levels were assessed using standard laboratory methods. Serum samples for hepcidin were centrifuged for 10 minutes at 3000 rpm and stored at –20°C.
A hepcidin prohormone enzyme immunoassay kit (RE 54051, IBL) was used for serum hepcidin measurement. Also, a sample from the radial artery for arterial blood gas analysis was obtained.
The study was planned in accordance with the suggestions of the Helsinki Document and Dışkapı Yıldırım Beyazıt Research and Education Hospital's ethic commission. Signed consent forms were obtained from all the patients who volunteered.
The statistical method
SPSS 15.0 (SPSS Inc; Chicago, III) package program was used for statistical analysis of the data. Mean±SD or median and range (min-max) were used to present continuous variables. Continuous variables which were normally distributed were reported as mean and SD and those which were not normally distributed were reported as median and range. The Kruskall-Wallis test was used to compare medians of the hepcidin levels of the COPD patient groups. There was said to be a statistically significant difference between groups when P<0.005. In that case, difference groups were determined by the Mann-Whitney U test and Bonferroni correction. The relationship of hepcidin levels with other variables was examined using the Spearman Correlation coefficient. Correlations in which P<0.05 were interpreted as normal. The median level was used in order to present some data which were normally distributed.
Results
Demographic information and laboratory results of the patient groups and control group are shown in Table 1.
Table 1.
Demographic information and laboratory characteristics of COPD patient groups and control group
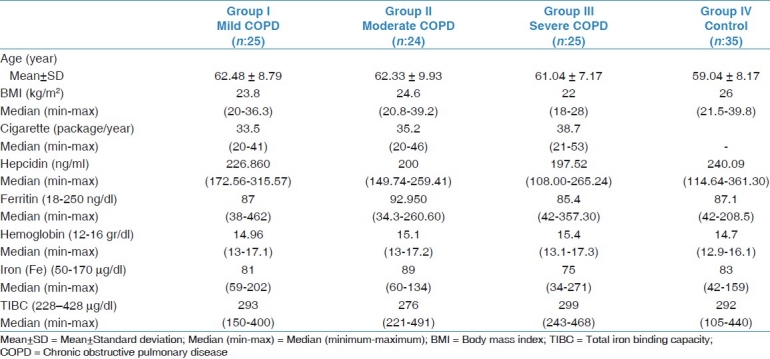
Age (year)
The ages of the 74 male COPD patients included in the study were between 45 and 75 years. The patients in the lowest age group were found to have severe COPD patient group (61.04 ± 7.17 years). The highest age group had mild COPD patient group (62.48 ± 8.79 years). The mean age of the moderate COPD patient group were found to be 62.33 ± 9.93 years. The mean age of the control group was 59.04 ± 8.17 years.
Body mass index (BMI, kg/m2)
Of the COPD patients, the highest median BMI levels were seen in the moderate COPD patient group (median: 24.6 (20.8-39.2)). The BMI of the mild COPD patient group were 23.8 (20-36.3), for the severe COPD patient group were 22 (1828), and for the control group was 26 (21.5-39.8).
Cigarette consumption per year (cigarette package/year)
The average cigarette pack consumption per year in severe COPD patient group was the highest 38.7 (21-53), whereas it was the lowest in mild COPD patient group and the control group consisted of non-smokers.
Serum hepcidin level
Among the COPD patient groups, the hepcidin level in the mild COPD patient group had the highest median level (226.860 ng/ml (172.56-315.57)), and the severe COPD patient group had the lowest median hepcidin level (197.52 ng/ml (108.00-265.24)). The median level of the moderate COPD patient group was found to be (200 ng/ml (149.74-259.41)). The median level in control group was 240.09 ng/ml (114.64-361.30). The serum hepcidin level in the mild COPD patient group was higher than the moderate and severe COPD patient groups. There was no difference in terms of hepcidin levels between the moderate and severe COPD patient group (P=0.624) and there was difference in hepcidin levels between mild and severe COPD patient groups (P=0.003). Each COPD patient group was compared with the normal group using the nonparametric statistical method, the Mann-Whitney U test. There was no difference between the healthy control group and the mild COPD patient group (P=0.781), whereas there was a difference between the control group and the moderate (P=0.004) and severe (P=0.002) COPD patient groups. The hepcidin level of the control group was higher than the moderate and severe COPD patient groups. Figure 1shows the box plot graphs of the serum hepcidin levels in COPD patients and control group. Serum hepcidin levels were lower in the COPD patients than the control group, and decreased by severity of COPD.
Figure 1.
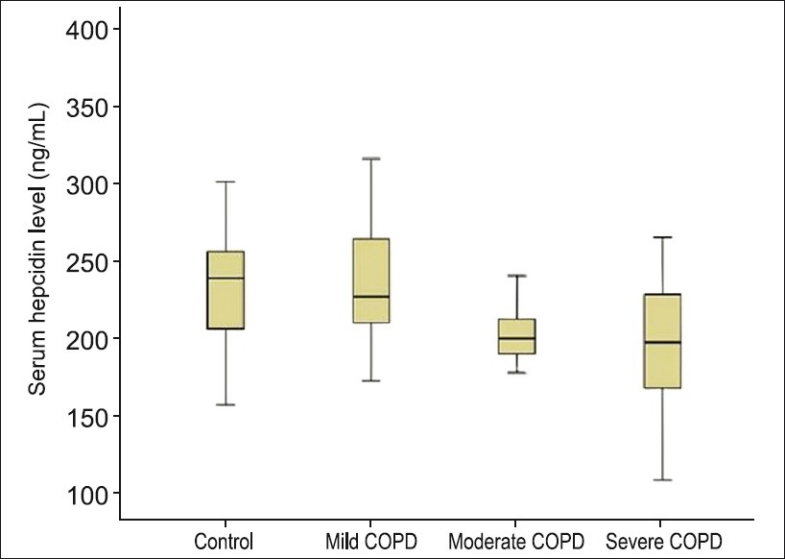
Serum hepcidin level in patient with COPD and control group
Blood parameters related to iron (iron, total iron binding capacity, ferritin, hemoglobin, hematocrit)
There was no anemia in all groups. Of the COPD patient groups, blood parameters related to iron were evaluated and median levels were found to be within normal limits.
The correlations between the serum hepcidin levels of the COPD patient groups and age, BMI, FEV1/FVC level, and laboratory parameters are shown in Table 2.
Table 2.
Correlation between serum hepcidin levels of COPD patient groups with other laboratory parameters
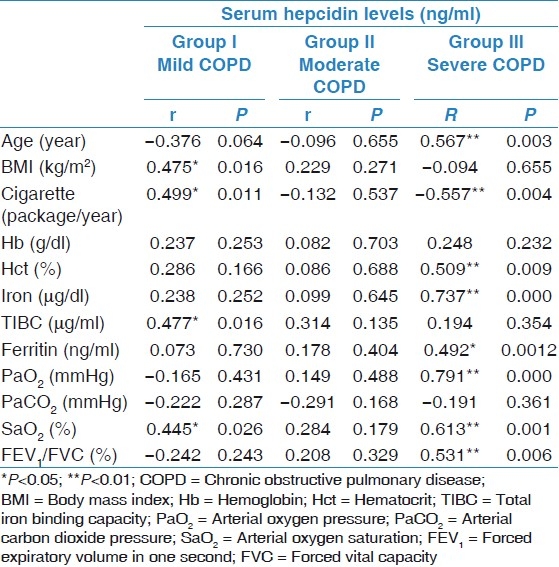
Correlations found to be significant between the mild chronic obstructive pulmonary disease patient group serum hepcidin level and other variables
A positive correlation was found between serum hepcidin levels and BMI (r=47, P=0.01) and hepcidin level and arterial oxygen saturation (SaO2, %) (r=0.44, P=0.02).
Serum hepcidin level in the moderate chronic obstructive pulmonary disease patient group
There was no significant correlation between the serum hepcidin level and any other variables.
Significant correlations found between the severe chronic obstructive pulmonary disease patient group serum hepcidin level and other variables
There was a negative correlation between serum hepcidin level and the ages of patients (r=0.57, P=0.003), packs of cigarettes consumed per year (r=0.55, P=0.004). There was a positive correlation between serum hepcidin level and hct (r=0.51, P=0.009), iron (r=0.74, P=0.000), ferritin (r=0.49, P=0.001), Pa02 (r=0.79, P=0.000), SaO2(r=0.61, P=0.001), and FEV1/FVC level (r=0.53, P=0.006).
Discussion
The serum level of hepcidin that increases with inflammation and decreases with hypoxia and anemia brings into mind that COPD can be controlled by a complex alarm network. Hepcidin is a liver-produced peptide implicated in the anemia of inflammation.[10] Inflammation increases hepcidin expression. Also, serum hepcidin was abnormally increased in patients with inflammation (CRP>10 mg/l), in patients with multiple myeloma[12,13] and in patients with chronic kidney disease[14] without associated inflammatory disorders. In a study, it was found that hepcidin was expressed by airway epithelial cells and was induced by both interferon gamma and IL-6 in a cell-specific pattern andmay serve as a protective factor through its direct antimicrobial effects.[15]
In another study it was shown that hepcidin is also produced in mouse macrophages infected with intracellular Mycobacteria.[16] However, there are conflicting findings about serum hepcidin level related to acute and chronic inflammation in the literature. In a previous study, the pro-hepcidin concentration was significantly higher in the patients with active rheumatoid artritis (RA) than those with inactive to moderate RA.[17]
On the other hand, in another study, it is reported that RA patients have higher serum concentration of pro-hepcidin than patients with systemic lupus erythematosus and healthy volunteers, but the pro-hepcidin concentration does not correlate with RA disease activity scores, tumor necrosis factor-alpha (TNF-α), or IL-6.[18] All of our patient groups were in stabilface of the COPD. Our patient groups and control group had no anemia and high serum CRP levels. Hepcidin plays a crucial role in the anemia of chronic disease. We think that in our severe COPD patient group, the decrease in serum hepcidin levels is related to hypoxemia rather than inflammation. In our study, serum hepcidin levels were significantly different between the healthy control group and the moderate COPD patient group (P=0.004), between healthy control group and severe (P=0.002) COPD patient group, between the mild and moderate COPD (P=0.001) patient groups, and between the mild and severe COPD (P=0.003) patient groups. These findings suggest that hepcidin could be an important marker which could be used to evaluate the disease state. In COPD patients, tissue hypoxia occurs when the partial oxygen pressure (Pa02) falls below 60 mmHg which causes systemic effects. Serious complications may occur when vital organs cannot receive sufficient oxygen. Hypoxemia is seen more in the severe COPD patient group than in the mild and moderate COPD patient groups. Systemic hypoxia reduced hepcidin production in the liver. However, the molecular mechanisms in which hypoxemia plays a role to repress hepcidin production has not yet been fully understood.[19] In our severe COPD patient group, there was positive correlation between serum hepcidin levels and Pa02 (P=0.000). This result demonstrates that there is a correlation between the degree of hypoxemia and the serum hepcidin level in the severe COPD patient group.
Along with the age, a decrease in lung and chest wall compliance, an increase in prevalence of a ventilation-perfusion disorder, low arterial oxygen pressure, and an increase in physical dead space make hypoxemia more apparent. In our study, in the severe COPD patient group, serum hepcidin levels decreased with aging (P=0.003). However, this relation may come from multifactorial reasons and more likely to be related to hepatic function.
The hepatic synthesis of hepcidin increases when serum iron concentration goes up. In contrast, hepcidin synthesis decreases when there is iron deficiency. Our results showed that the serum hepcidin level increased with increasing hct (P=0.009) and serum iron (P=0.000) levels in the severe COPD patient group. Hepcidin production also correlated with the serum ferritin level. It was found that the serum hepcidin level increased when the serum ferritin level increased (P=0.012) in the severe COPD patient group.
Further studies are needed to clarify this issue and the role of inflammatory and iron regulatory pathways in hepcidin synthesis in COPD patients.
BMI has a negative effect on the quality of life of patients with COPD, and previous studies have found a relationship between poor prognosis, mortality, and BMI.[20,21] In one study, no relationship was found between TNF-α, interferon-γ (IFN-γ) and hepcidin.[9] A number of studies have found a relationship between low BMI and TNF-α.[22,23] Also, The correlation between serum hepcidin levels and BMI was shown in some studies on obesity.[24–26] All of our patients had normal or low BMI. In our study, no relationship was found between BMI and serum hepcidin levels in the moderate and severe COPD patient groups. Only a positive correlation was found between hepcidin levels and BMI (P=0.01) in mild COPD patient group.
Impaired pulmonary function is a strong risk factor for the development of COPD and a marker of disease severity. In our study, no relationship between respiratory function parameters and hepcidin in the mild and moderate COPD patient groups was found, whereas there was an increase in the hepcidin level (P=0.006) with the increase in the FEV1/FVC level in the severe COPD patient group.
Limitation of the study
The limitation of the study is that many of the patients admitted to our clinic for COPD were either in an inflammation stage or had additional diseases. Therefore, we think that more studies using a larger number of patients need to be done.
Conclusion
As far as our best knowledge from the literature, our study is the first study that shows the relationship between serum hepcidin level and hypoxemia in the COPD patients. The relationship between the increase in the severity of the disease and the decrease of the hepcidin level was found to be significant. We suggest that hepcidin may play an important role in COPD. Our clinical findings are in conformity with recent experimental studies defining hepcidin as a useful marker in COPD.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Global strategy for agnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2009. Global Initiative for Chronic Obstructive Lung Disease (GOLD) Scientific Committe. [Last accessed on 2011 June 9]. Available from: http://www.goldcopd.org .
- 2.WHO (World Health Organization) World Health Statistics. 2008. [Last accessed on 2011 June 9]. Available from: http://www.who.int/topics/chronic_obstructive_pulmonary_disease/en/
- 3.Agusti A, Thomas a. Neff lecture: Chronic obstructive pulmonary disease—a systemic disease. Proc Am Thorac Soc. 2006;3:478–81. doi: 10.1513/pats.200603-058MS. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami Y, Kishi F, Yamamoto H, Miyamoto K. Relation of oxygen delivery, mixed venous oxygenation, and pulmonary hemodynamics to prognosis in chronic obstructive pulmonary disease. N Engl J Med. 1983;308:1405–7. doi: 10.1056/NEJM198305053081801. [DOI] [PubMed] [Google Scholar]
- 5.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthsized in the liver. J Biol Chem. 2001;276:7806–10. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 6.Kemna EH, Tjalsma H, Willems HL, Swinkles DW. Hepcidin: From discovery to differential diagnosis. Haematologica. 2008;93:90–7. doi: 10.3324/haematol.11705. [DOI] [PubMed] [Google Scholar]
- 7.Ganz T, Nemeth E. Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta. 2006;1763:690–9. doi: 10.1016/j.bbamcr.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Nemeth E, Rivera S, Gabayan V. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–6. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kemna E, Pickkers P, Nemeth E, Van Der Hoeven H. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–6. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 10.Nemeth E, Valore EV, Territo M, Schiller G. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute phase protein. Blood. 2003;101:2461–3. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 11.Nicolas G. The gene encoding then iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma S, Nemeth E, Chen YH. Involvement of hepcidin in the anemia of multiple myeloma. Clin Cancer Res. 2008;14:3262–7. doi: 10.1158/1078-0432.CCR-07-4153. [DOI] [PubMed] [Google Scholar]
- 13.Lauta VM. A review of the cytokine network in multiple myeloma: Diagnostic, prognostic, and therapeutic implications. Cancer. 2003;97:2440–52. doi: 10.1002/cncr.11072. [DOI] [PubMed] [Google Scholar]
- 14.Tomosugi N, Kabawata H, Wakatabe R. Detection of serum hepcidin in renal failure and inflammation by using Protein Chip System. Blood. 2006;108:1381–87. doi: 10.1182/blood-2005-10-4043. [DOI] [PubMed] [Google Scholar]
- 15.Frazier MD, Mamo LB, Ghio AJ, Turi JL. Hepcidin expression in human airway epithelial cells is regulated by interferon-gamma. Respir Res. 2011;12:100. doi: 10.1186/1465-9921-12-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sow FB, Florence WC, Satoskar AR, Schlesinger LS, Zwilling BS, Lafuse WP. Expression and localization of hepcidin in macrophages: A role in host defense against tuberculosis. J Leukoc Biol. 2007;82:934–45. doi: 10.1189/jlb.0407216. [DOI] [PubMed] [Google Scholar]
- 17.Kim HR, Kim KW, Yoon SY, Kim SH, Lee SH. Serum pro-hepcidin could reflect disease activity in patients with rheumatoid arthritis. J Korean Med Sci. 2010;25:348–52. doi: 10.3346/jkms.2010.25.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koca SS, Isik A, Ustundag B, Metin K, Aksoy K. Serum pro-hepcidin levels in rheumatoid arthritis and systemic lupus erythematosus. Inflammation. 2008;31:146–53. doi: 10.1007/s10753-008-9060-8. [DOI] [PubMed] [Google Scholar]
- 19.Ganz T. Hepcidin-a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18:171–82. doi: 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 20.Prescott E, Almdal T, Mikkelsen KL. Prognostic value of weight change in chronic obstructive pulmonary disease: Results from the Copenhagen City Heart Study. Eur Respir J. 2002;20:539–44. doi: 10.1183/09031936.02.00532002. [DOI] [PubMed] [Google Scholar]
- 21.Lanbo C, Prescott E, Lange P. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–61. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 22.Eid AA, Ionescu AA, Nixon LS. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1414–8. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- 23.Wouters EF, Creutzberg EC, Schols AM. Systemic effects in COPD. Chest. 2002;121(5 Suppl):127–30. doi: 10.1378/chest.121.5_suppl.127s. [DOI] [PubMed] [Google Scholar]
- 24.Amato A, Santoro N, Calobro P. Effect of body mass index reduction on serum hepcidin levels and iron status in obese children. Int J Obes. 2010;34:1772–4. doi: 10.1038/ijo.2010.204. [DOI] [PubMed] [Google Scholar]
- 25.Aeberli I, Hurell R. Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight children. Int J Obes. 2009;33:1111–7. doi: 10.1038/ijo.2009.146. [DOI] [PubMed] [Google Scholar]
- 26.Tussing-Humphreys LM, Nemeth E, Fantuzzi G. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity. 2010;18:1449–56. doi: 10.1038/oby.2009.319. [DOI] [PubMed] [Google Scholar]


