Abstract
Background
Postoperative infections are frequent complications after liver resection and have significant impact on length of stay, morbidity and mortality. Surgical site infection (SSI) is the most common nosocomial infection in surgical patients, accounting for 38% of all such infections.
Objectives
This study aimed to identify predictors of SSI and organ space SSI after liver resection.
Methods
Data from the American College of Surgeons National Surgical Quality Improvement Program (ACS–NSQIP) database for patients who underwent liver resection in 2005, 2006 or 2007 in any of 173 hospitals throughout the USA were analysed. All patients who underwent a segmental resection, left hepatectomy, right hepatectomy or trisectionectomy were included.
Results
The ACS–NSQIP database contained 2332 patients who underwent hepatectomy during 2005–2007. Rates of SSI varied significantly across primary procedures, ranging from 9.7% in segmental resection patients to 18.3% in trisectionectomy patients. A preoperative open wound, hypernatraemia, hypoalbuminaemia, elevated serum bilirubin, dialysis and longer operative time were independent predictors for SSI and for organ space SSI.
Conclusions
These findings may contribute towards the identification of patients at risk for SSI and the development of strategies to reduce the incidence of SSI and subsequent costs after liver resection.
Keywords: hepatectomy, surgical site infection, organ space infection
Introduction
Postoperative infection is one of the most common complications after liver resection and has significant impact on length of stay (LoS), morbidity and mortality.1 Surgical site infections (SSIs) are the most common nosocomial infections in surgical patients, accounting for 38% of such infections. Because SSIs have a severe impact on overall morbidity and costs, in the USA, the Centers for Disease Control and Prevention (CDC), through the National Nosocomial Infection Surveillance System (NNISS), monitor the incidence of SSI. For this purpose, SSI is defined as an infection with either incisional involvement of skin alone or with involvement of subcutaneous tissue or organ space. The NNISS reported an overall incidence of SSI of 2.6% between 2002 and 2004,2 of which two-thirds were incisional and one-third were organ space infections.
During the last decade, a decreased incidence of perioperative septic complications has been reported as a result of the use of advanced surgical techniques, new suturing materials, better perioperative management and the implementation of infection surveillance.3 Overall, post-hepatectomy infection rates have been reported to vary from 4% to 20%1 and incidences of SSI after liver resection to range between 2.1% and 14.5%.4–7 These cases are associated with increases in morbidity, mortality and health care expenses.8
Previous studies have documented increased risk for SSI based on patient and operative characteristics. The goal of this study was to identify predictors of SSI after liver resection using data maintained by the American College of Surgeons National Surgical Quality Improvement Program (ACS–NSQIP) and including all patients who underwent hepatectomies performed at any of 173 centres in the USA between 2005 and 2007.
Materials and methods
The ACS–NSQIP is a robust reporting system designed to provide reliable, risk-adjusted surgical outcomes data to surgical services and administrators at medical centres throughout the private sector so that surgical quality can be assessed and improved on a national level.9,10
Data from the Participant Use Data File for all surgical cases submitted to the ACS–NSQIP in 2005, 2006 and 2007 by 173 hospitals throughout the USA were analysed.
The ACS–NSQIP collects data on 135 variables, including preoperative risk factors, intraoperative variables, and 30-day postoperative morbidity and mortality outcomes for a systematic and prospective sample of patients undergoing surgical procedures in both inpatient and outpatient settings. Data are collected in a standardized fashion according to strict definitions by dedicated surgical clinical nurse reviewers. Patients are followed throughout their hospital course and after discharge from hospital for up to 30 days postoperatively. Nurse reviewers collect data from computerized and paper patient medical records and doctor's office records, and through telephone interviews with patients. The accuracy and reproducibility of the data have been previously demonstrated.9,11,12
CPT-4® (Current Procedural Terminology) codes were used to select patients who had undergone liver resection, using primary procedure codes 47120 (segmental resection), 47122 (trisectionectomy), 47125 (left hepatectomy) and 47130 (right hepatectomy). The primary outcome examined was 30-day SSI in subcategories of superficial, deep or organ space SSI (Table 1). Length of hospital stay and 30-day mortality outcomes were also analysed. Outcomes were compared across type of resection using analysis of variance (anova) or chi-squared tests.
Table 1.
Definition of surgical site infection (SSI) according to the National Surgical Quality Improvement Program (NSQIP)
| SSI classification | Definition according to NSQIP |
|---|---|
| Superficial incisional SSI | Infection that occurs within 30 days after the operation, involves only the skin or subcutaneous tissue of the incision and shows at least one of the following characteristics: purulent drainage with or without laboratory confirmation from the superficial incision; organisms isolated from an aseptically obtained culture of fluid or tissue from the superficial incision; at least one sign of infection; superficial incision that is deliberately opened by the surgeon unless incision is culture-negative, or a diagnosis of superficial incisional SSI made by the surgeon or attending doctor |
| Deep incisional SSI: | Infection that occurs within 30 days after the operation, appears to be related to the operation, involves deep soft tissues of the incision and shows at least one of the following characteristics: purulent drainage from the deep incision but not from the organ space of the surgical site; a deep incision that spontaneously dehisces or is deliberately opened by a surgeon when the patient has either fever (>38 °C), located pain or tenderness unless culture is negative; an abscess or other evidence of infection involving the deep incision on direct examination, during reoperation or by histopathologic or radiologic examination, or the diagnosis of a deep incision SSI made by the surgeon or attending doctor |
| Organ space SSI | Infection that occurs within 30 days after the operation, appears to be related to the operation and involves any part of the anatomy other than the incision, which was opened or manipulated during an operation, and shows at least one of the following characteristics: purulent drainage from the drain that is placed through a stab wound into the organ space; organisms isolated from an aseptically obtained culture of fluid or tissue from the organ space; an abscess or other evidence of infection involving the organ space that is found on direct examination, during reoperation or by histopathologic or radiologic examination, or the diagnosis of an organ space SSI made by the surgeon or attending doctor |
A total of 37 patient demographic, history, review of system and laboratory values were compared between patients who developed SSI and those who did not, using chi-squared tests (for binary variables) or t-tests (for continuous variables). Because of the increased risk for type I error, a threshold for significance in these analyses was set at <0.001. Intraoperative variables [operative duration, transfusion (yes/no) and dirty/infected wound class] were similarly compared. Independent risk factors for any SSI and for organ space SSI only were calculated using multivariable logistic regressions. Preoperative risk variables were considered for inclusion in a forward stepwise fashion (P-value for entry: P < 0.05; P-value for exit: P > 0.10). After independent preoperative risks had been identified, independent intraoperative variables were identified in a similar forward stepwise fashion adjusted for the presence of preoperative variables.
Results
The ACS–NSQIP database contained 2332 patients who underwent an open or laparoscopic hepatectomy between 2005 and 2007. Trauma and paediatric cases were excluded from the analysis. The mean ± standard deviation patient age was 58.3 ± 13.5 years; 1150 patients (49.3%) were male. Eighteen procedures (0.8%) were emergent. In 103 patients (4.4%), a bilioenteric anastomosis was performed. A total of 347 patients (14.9%) underwent concomitant gastrointestinal resection including the oesophagus, stomach, small bowel, colon and anus.
Surgical site infection rates varied significantly across primary procedures, ranging from 9.7% in segmental resection patients to 18.3% in trisectionectomy patients (P = 0.001) (Table 2). The overall incidence of SSI was 11.5%. Univariate analyses (Table 3) showed SSI occurred more frequently in patients who were male, smokers, had a preoperative open wound, reduced serum albumin, elevated bilirubin and elevated alkaline phosphatase. Intraoperatively, operative duration and transfusion were associated with SSI occurrence. Development of SSI was associated with a 6.1-day increase in LoS (13.6 days vs. 7.5 days; P < 0.001), a 12.5% increase in the rate of return to the operating room within 30 days (16.0% vs. 3.5%; P < 0.001) and trended towards a 3.1% increase in mortality (5.2% vs. 2.1%; P = 0.002) (Table 4).
Table 2.
Surgical site infection (SSI) and other outcomes in 2332 hepatectomy patients by primary procedure
| Intraoperative variables | Primary procedure | Chi-squared P-value | ||||
|---|---|---|---|---|---|---|
| 47120 SR | 47130 RH | 47125 LH | 47122 TS | All hepatectomies | ||
| Cases, n | 1357 | 512 | 233 | 230 | 2332 | |
| Any SSI at ≤30 days postoperatively | 9.7 | 13.3 | 11.2 | 18.3 | 11.5 | 0.001 |
| Superficial SSI, % | 4.8 | 4.7 | 6.4 | 8.7 | 5.3 | 0.074 |
| Deep SSI, % | 0.9 | 1.2 | 0.9 | 1.3 | 1.0 | 0.896 |
| Organ space SSI, % | 4.6 | 7.8 | 5.2 | 10.9 | 6.0 | <0.001 |
| 30-day mortality, % | 1.8 | 3.7 | 0.9 | 5.2 | 2.5 | 0.002 |
| LoS, days, mean ± SD | 7.5 ± 8.2 | 9.4 ± 11.0 | 7.8 ± 6.0 | 10.3 ± 8.5 | 8.2 ± 8.8 | <0.001 |
LH, left hepatectomy; RH, right hepatectomy; SR, segmental resection; TS, trisectionectomy; LoS, length of stay; SD, standard deviation.
Table 3.
Preoperative and intraoperative variables by occurrence of surgical site infection (SSI)
| Variable | All hepatectomy patients (n = 2332) | Patients without SSI (n = 2064, 88.5%) | Patients with SSI (n = 268, 11.5%) | P-value |
|---|---|---|---|---|
| Age, years, mean ± SD | 58.3 ± 13.5 | 58.3 ± 13.7 | 59.0 ± 12.6 | 0.399 |
| Male, % | 49.3 | 48.1 | 58.6 | 0.001 |
| ASA physical status class, % | 0.003 | |||
| ASA Class III | 59.6 | 58.7 | 66.4 | |
| ASA Class IV | 5.0 | 4.7 | 7.5 | |
| ASA Class V | 0.0 | 0.0 | 0.0 | |
| Smoked within 1 year prior to surgery, % | 15.3 | 14.6 | 20.5 | 0.011 |
| Pack-years smoked, mean ± SD | 12.4 ± 22.2 | 11.6 ± 21.4 | 18.4 ± 26.4 | <0.001 |
| Medically treated hypertension, % | 45.6 | 44.8 | 51.5 | 0.039 |
| BMI class, kg/m2, % | 0.002 | |||
| ≤18.50 | 2.7 | 2.6 | 3.4 | |
| 18.51–25.00 | 33.1 | 33.6 | 28.6 | |
| 25.01–30.00 | 33.1 | 33.6 | 28.9 | |
| 30.01–35.00 | 19.8 | 19.4 | 22.6 | |
| 35.01–40.00 | 7.1 | 6.4 | 12.8 | |
| >40.00 | 4.2 | 4.3 | 3.8 | |
| Preoperative functional status, % | 0.020 | |||
| Partially dependent | 1.5 | 1.3 | 3.4 | |
| Fully dependent | 0.4 | 0.4 | 0.7 | |
| History severe COPD, % | 3.0 | 2.7 | 5.2 | 0.023 |
| Disseminated cancer, % | 41.2 | 41.9 | 35.8 | 0.059 |
| Recent loss of > 10% of body weight, % | 5.4 | 5.0 | 8.2 | 0.031 |
| Recent chemotherapy, % | 7.9 | 7.9 | 7.5 | 0.783 |
| Recent radiotherapy, % | 1.2 | 1.2 | 0.7 | 0.503 |
| Preoperative open wound/infection, % | 6.2 | 0.0 | 54.1 | <0.001 |
| Preoperative systemic inflammation, % | 0.054 | |||
| SIRS | 2.0 | 2.0 | 2.2 | |
| Sepsis | 0.3 | 0.2 | 1.1 | |
| Septic shock | 0.3 | 0.3 | 0.0 | |
| Laboratory values | ||||
| Preoperative serum albumin, g/dl, mean ± SD | 4.03 ± 0.57 | 4.04 ± 0.55 | 3.92 ± 0.67 | 0.001 |
| Bilirubin > 1.0, % | 11.3 | 10.6 | 16.8 | 0.003 |
| Bilirubin, mg/dl, mean ± SD | 0.75 ± 1.02 | 0.71 ± 0.90 | 1.04 ± 1.62 | <0.001 |
| Alkaline phosphatase | 123 ± 96 | 120 ± 89 | 149 ± 133 | <0.001 |
| Haematocrit < 38%, % | 33.6 | 32.9 | 38.4 | 0.074 |
| WBC, n/mm3, mean ± SD | 6963 ± 2874 | 6902 ± 2844 | 7430 ± 3062 | 0.005 |
| Sodium > 145 mmol/l, % | 1.0 | 0.9 | 2.2 | 0.037 |
P < 0.001.
SD, standard deviation; ASA, American Society of Anesthesiologists; BMI, body mass index; COPD, chronic obstructive pulmonary disease; SIRS, systemic inflammatory response syndrome; WBC, white blood cell count.
Table 4.
Intraoperative characteristics, length of stay and mortality by surgical site infection (SSI) occurrence
| Variable | All hepatectomy patients (n = 2332) | Patients without SSI (n = 2064, 88.5%) | Patients with SSI (n = 268, 11.5%) | P-value |
|---|---|---|---|---|
| Wound class dirty/infected, % | 5.5 | 5.1 | 8.2 | 0.038 |
| Operative duration, min, mean ± SD | 253 ± 122 | 247 ± 120 | 299 ± 132 | <0.001 |
| Transfused intraoperatively | <0.001 | |||
| 1–2 units PRBCs, % | 14.1 | 14.2 | 13.9 | |
| ≥3 units PRBCs, % | 14.8 | 13.6 | 24.0 | |
| Length of stay, days, mean ± SD | 8.2 ± 8.8 | 7.5 ± 7.6 | 13.6 ± 14.1 | <0.001 |
| Return to OR within 30 days of surgery, % | 5.0 | 3.5 | 16.0 | <0.001 |
| Mortality, % | 2.5 | 2.1 | 5.2 | 0.002 |
P < 0.001.
SD, standard deviation; PRBCs, packed red blood cells; OR, operating room.
Independent preoperative predictors of SSI from the forward regression model were (by order of entry) preoperative open wound/infection, serum sodium > 145 mmol/l, serum albumin (continuous variable g/dl), total bilirubin > 1.0 mg/dl and operative duration in minutes. Primary procedure, wound class and transfusion were not independently significant intraoperative predictors. The same variables in the same entry order were independent predictors of organ space SSI only, with the addition of dialysis and smoking (Table 5). Rates of organ space SSI by hour of operative duration are graphed in Fig. 1; the chi-squared test for linear trend was significant (P < 0.001).
Table 5.
Independent predictors of surgical site infection (SSI) in 2332 hepatectomy patients
| Variable | Multivariate odds ratio (95% confidence interval, P-value) | |
|---|---|---|
| Superficial, deep or organ space SSI (n = 268, 11.5%) | Organ space SSI only (n = 140, 6.0%) | |
| Preoperative open wound/infection | All patients with this variable had SSIa | 1.81 (1.03–3.20, P = 0.039) |
| Serum sodium > 145 mmol/l | 6.05 (2.30–15.93, P < 0.001) | 6.25 (2.36–16.55, P < 0.001) |
| Serum albumin, per mg/dl from mean | 0.67 (0.49–0.91, P = 0.011) | 0.70 (0.52–0.95, P = 0.022) |
| Total bilirubin > 1.0 | 1.70 (1.05–2.77, P = 0.032) | 1.87 (1.19–2.95, P = 0.007) |
| On dialysis | Not in model | 4.46 (1.14–17.45, P = 0.032) |
| Smoker within 1 year of procedure | Not in model | 1.66 (1.08–2.54, P = 0.021) |
| Operative time, per min from mean | 1.003 (1.001–1.004, P < 0.001) | 1.003 (1.002–1.004, P < 0.001) |
Because all patients with this variable experienced SSI, the odds ratio was extremely large (>100). No precise odds ratio is estimable from this patient population; however, it is clearly a strong predictor.
Figure 1.
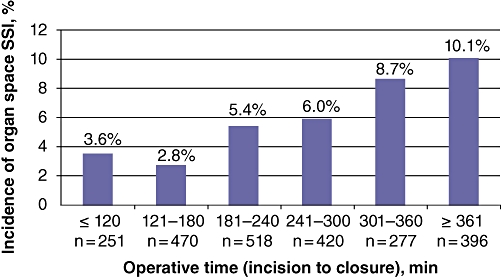
Incidence of organ space surgical site infection by hour of operative duration
Because the presence of a preoperative open wound was strongly predictive of SSI in hepatectomy patients, this variable, for which no specific aetiology is given in the ACS–NSQIP, was further analysed. The NSQIP describes three common sources of wound for this variable: (i) a prior operation wound left open and packed; (ii) a diabetes ulcer, and (iii) a decubitus ulcer. The ACS–NSQIP did not track data on prior surgery within 30 days until 2006. An examination of hepatectomy cases with preoperative open wounds in 2006 and 2007 showed three of 16 patients had undergone prior surgery, six of 16 were diabetes patients, and two of 16 were completely functionally dependent. These data suggest a range of preoperative open wound aetiologies.
Discussion
Nosocomial infection continues to represent a major cause of morbidity, mortality and resource utilization. It is estimated to occur in around 2.1 million cases per year in the USA.13 Surgical site infections account for approximately 20% of these instances.14
The last decade has seen a marked increase in interest in predictive factors for infectious complications in general surgery. Most studies have included a variety of different surgical procedures,15–17 some have analysed many different types of complication or infection,1,18 and only a few have focused on SSI in patients undergoing liver resection.3,5
Our study includes 2332 patients who underwent liver resection in 173 hospitals throughout the USA. Some of the strengths of the ACS–NSQIP system refer to the large number of patients included, the strict definition of each and every variable, and the prospective nature of the database. This dataset records a significant amount of procedures performed in a short period of time, which adds homogeneity to this series.
One interesting finding concerns the correlation between preoperative open wounds and SSI. In order to establish the origin of the preoperative open wound, we investigated patients included in our series and found that prior open surgery leaving an open packed wound, decubitus ulcers and diabetes ulcers were equally distributed. Other related factors identified in previous series as predictors of SSI after different types of surgical procedures, such as diabetes mellitus and advanced age, were not significantly associated with SSI. Our data suggest that the presence of an open wound, whether infected or not, anywhere on the body is strongly associated with the occurrence of SSI in the postoperative period after hepatectomy. Pessaux et al. reported an increased risk for SSI in patients diagnosed with a preoperative cutaneous abscess or skin necrosis after a non-colorectal abdominal procedure.19 They proposed that a skin infection represents a source of bacteria that may lead to a secondary deeper infection.19 Our findings support the recommendation that any open wound should be treated prior to surgery to decrease the incidence of SSI and subsequent costs. In some instances, such as in patients with cancer, the timing of the liver resection could be essential and a risk : benefit approach should be used.
Several previous reports have addressed the relationship between operative time and SSI in a wide range of surgical procedures in general surgery.8,15 In this study, we found a linear relation between operative time and SSI, suggesting that the shorter the surgical procedure, the lower the probability of developing an SSI.
We have previously shown that smoking is an important determinant of resource utilization after liver resection and is associated with a longer LoS, longer intensive care unit stay and increased perioperative mortality.20 This study confirmed a strong relationship between smoking and the occurrence of SSI, specifically with organ space SSI.
Hypoalbuminaemia, a well-known marker of liver dysfunction, protein-calorie malnutrition and active acute-phase response, has been associated with higher incidences of sepsis and postoperative infection after surgery.8,17,21 Previous studies have assessed the effect of hypoalbuminaemia as a major predictor of SSI, including deep and organ space infection, in patients undergoing general surgery procedures.8 In this study, hypoalbuminaemia was identified as an independent factor associated with the presence of SSI after hepatectomy, underlining the impact of malnutrition and possible liver dysfunction in the development of septic complications. Other variables related to hypoalbuminaemia, such as portal hypertension, oesophageal varices, ascites or prolonged international normalized ratio (INR) do not seem to predispose to SSI. Hypoalbuminaemia has been shown to be the only laboratory value associated with an increased risk for infection and overall morbidity and mortality in patients undergoing liver resection.1,22 Interestingly, SSI was also associated with the presence of jaundice, which concords with findings in other series in which higher morbidity and mortality rates were reported after hepatectomy in part because of infection-related complications associated with the preoperative use of biliary stents.23
Although our study did not analyse the increase in costs related to SSI in patients undergoing liver resection, we found a strong association between SSI and prolonged LoS and surgical revision, which represent major components of health care expenditure. Hospital discharge was delayed by > 6 days and mortality and reoperation rates were increased substantially, by 3% and 12%, respectively, in the presence of SSI. Weber et al. compared the costs of hospital care in a group of patients with and without SSI and identified a statistically significant difference between the groups which was mainly related to longer postoperative LoS and antibiotics administration.24 However, Weber et al.24 included only in-hospital incidences of SSI and thereby excluded patients diagnosed in an outpatient setting who did not require hospitalization. Alfonso et al. performed a similar study including both in-hospital and extra-hospital costs and thus both the direct and indirect costs of SSI to show its impact on resource utilization.25
There are several limitations to this study. Firstly, our data did not include information on the use of prophylactic antibiotics. Although the utilization of prophylactic antibiotics is considered standard in this type of surgery and was probably applied in most cases, the type and duration of medication were not recorded. Although the use of i.v. antibiotics prior to skin incision has been related to a lower incidence of wound infection, its applicability as prophylaxis in deep SSI has not been proven. We were able to demonstrate several factors strongly associated with superficial and deep SSI using a multicentre design with a large number of patients, which may offset any site-specific factors and thereby accurately reflect national trends. Secondly, the NSQIP database was developed to evaluate a wide variety of different surgical interventions and does not include data on procedure-specific details, such as the utilization of stents or drains prior to surgery, which may be associated with an increased risk for infection.
In summary, the present study has identified the preoperative presence of an open wound, hypernatraemia, hypoalbuminaemia, elevated serum bilirubin, dialysis and longer operative time as independent predictors for SSI and for organ space SSI in patients undergoing liver resection. The intention to correct these factors prior to surgery should impact on the incidence of SSI and decrease mortality, LoS and ultimately costs.
Conflicts of interest
None declared.
References
- 1.Garwood RA, Sawyer RG, Thompson L, Adams RB. Infectious complications after hepatic resection. Am Surg. 2004;70:787–792. [PubMed] [Google Scholar]
- 2.Reilly J, Allardice G, Bruce J, Hill R, McCoubrey J. Procedure-specific surgical site infection rates and post-discharge surveillance in Scotland. Infect Control Hosp Epidemiol. 2006;27:1318–1323. doi: 10.1086/509839. [DOI] [PubMed] [Google Scholar]
- 3.Togo S, Matsuo K, Tanaka K, Matsumoto C, Shimizu T, Ueda M, et al. Perioperative infection control and its effectiveness in hepatectomy patients. J Gastroenterol Hepatol. 2007;22:1942–1948. doi: 10.1111/j.1440-1746.2006.04761.x. [DOI] [PubMed] [Google Scholar]
- 4.Uchiyama K, Ueno M, Ozawa S, Kiriyama S, Kawai M, Hirono S, et al. Risk factors for postoperative infectious complications after hepatectomy. J Hepatobiliary Pancreat Sci. 2011;18:67–73. doi: 10.1007/s00534-010-0313-1. [DOI] [PubMed] [Google Scholar]
- 5.Okabayashi T, Nishimori I, Yamashita K, Sugimoto T, Yatabe T, Maeda H, et al. Risk factors and predictors for surgical site infection after hepatic resection. J Hosp Infect. 2009;73:47–53. doi: 10.1016/j.jhin.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Gotohda N, Nakagohri T, Takahashi S, Konishi M, Kinoshita T. Risk factors of surgical site infection after hepatectomy for liver cancers. World J Surg. 2009;33:312–317. doi: 10.1007/s00268-008-9831-2. [DOI] [PubMed] [Google Scholar]
- 7.Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. Am J Med. 1991;91 (Suppl.):152–157. doi: 10.1016/0002-9343(91)90361-z. [DOI] [PubMed] [Google Scholar]
- 8.Haridas M, Malangoni MA. Predictive factors for surgical site infection in general surgery. Surgery. 2008;144:496–501. doi: 10.1016/j.surg.2008.06.001. discussion 501–503. [DOI] [PubMed] [Google Scholar]
- 9.Khuri SF, Daley J, Henderson W, Hur K, Demakis J, Aust JB, et al. The Department of Veterans Affairs' NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg. 1998;228:491–507. doi: 10.1097/00000658-199810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khuri SF, Henderson WG, Daley J, Jonasson O, Jones RS, Campbell DA, et al. The patient safety in surgery study: background, study design, and patient populations. J Am Coll Surg. 2007;204:1089–1102. doi: 10.1016/j.jamcollsurg.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Daley J, Khuri SF, Henderson W, Hur K, Gibbs JO, Barbour G, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:328–340. [PubMed] [Google Scholar]
- 12.Khuri SF, Daley J, Henderson W, Hur K, Gibbs JO, Barbour G, et al. Risk adjustment of the postoperative mortality rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185:315–327. [PubMed] [Google Scholar]
- 13.Nguyen GT, Proctor SE, Sinkowitz-Cochran RL, Garrett DO, Jarvis WR. Status of infection surveillance and control programs in the United States, 1992–1996. Association for Professionals in Infection Control and Epidemiology, Inc. Am J Infect Control. 2000;28:392–400. doi: 10.1067/mic.2000.110298. [DOI] [PubMed] [Google Scholar]
- 14.Malone DL, Genuit T, Tracy JK, Gannon C, Napolitano LM. Surgical site infections: reanalysis of risk factors. J Surg Res. 2002;103:89–95. doi: 10.1006/jsre.2001.6343. [DOI] [PubMed] [Google Scholar]
- 15.Campbell DA, Jr, Henderson WG, Englesbe MJ, Hall BL, O'Reilly M, Bratzler D, et al. Surgical site infection prevention: the importance of operative duration and blood transfusion – results of the first American College of Surgeons–National Surgical Quality Improvement Program Best Practices Initiative. J Am Coll Surg. 2008;207:810–820. doi: 10.1016/j.jamcollsurg.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Fiorio M, Marvaso A, Vigano F, Marchetti F. Incidence of surgical site infections in general surgery in Italy. Infection. 2006;34:310–314. doi: 10.1007/s15010-006-6632-0. [DOI] [PubMed] [Google Scholar]
- 17.Neumayer L, Hosokawa P, Itani K, El-Tamer M, Henderson WG, Khuri SF. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1178–1187. doi: 10.1016/j.jamcollsurg.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Aloia TA, Fahy BN, Fischer CP, Jones SL, Duchini A, Galati J, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford) 2009;11:510–515. doi: 10.1111/j.1477-2574.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pessaux P, Msika S, Atalla D, Hay JM, Flamant Y. Risk factors for postoperative infectious complications in non-colorectal abdominal surgery: a multivariate analysis based on a prospective multicentre study of 4718 patients. Arch Surg. 2003;138:314–324. doi: 10.1001/archsurg.138.3.314. [DOI] [PubMed] [Google Scholar]
- 20.Gedaly R, McHugh PP, Johnston TD, Jeon H, Ranjan D, Davenport DL. Obesity, diabetes, and smoking are important determinants of resource utilization in liver resection: a multicentre analysis of 1029 patients. Ann Surg. 2009;249:414–419. doi: 10.1097/SLA.0b013e31819a032d. [DOI] [PubMed] [Google Scholar]
- 21.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 22.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708. doi: 10.1097/01.sla.0000141195.66155.0c. discussion 708–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber WP, Zwahlen M, Reck S, Feder-Mengus C, Misteli H, Rosenthal R, et al. Economic burden of surgical site infections at a European university hospital. Infect Control Hosp Epidemiol. 2008;29:623–629. doi: 10.1086/589331. [DOI] [PubMed] [Google Scholar]
- 25.Alfonso JL, Pereperez SB, Canoves JM, Martinez MM, Martinez IM, Martin-Moreno JM. Are we really seeing the total costs of surgical site infections? A Spanish study. Wound Repair Regen. 2007;15:474–481. doi: 10.1111/j.1524-475X.2007.00254.x. [DOI] [PubMed] [Google Scholar]


