Abstract
Major pelvic ganglion electrocautery (MPGE) and spinal cord injury in the rat induce bladder hypertrophy and a change in muscarinic receptor subtypes mediating bladder contraction from predominantly M3 to a combination of M2 and M3. To determine whether this is a result of bladder hypertrophy or denervation, we studied the following groups: sham-operated controls, urinary diversion (DIV), MPGE together with urinary diversion (DIV-DEN), bilateral MPGE (DEN), bladder outlet obstruction (BOO), and MPG decentralization (MPG-DEC). The degree of bladder denervation was determined by the maximal carbachol response normalized to the response to electric field stimulation. Receptor subtype density was determined by immunoprecipitation. The affinity of subtype-selective muscarinic antagonists for inhibition of carbachol-induced contractions was used to determine the subtype-mediating contraction. DEN, MPG-DEC, and BOO bladders were hypertrophic whereas DIV bladders were atrophic compared with sham operated. Bladder contraction in sham-operated, DIV, and DIV-DEN was mediated by the M3 receptor subtype, whereas the M2 subtype participated in contraction in the DEN, MPG-DEC, and BOO groups. The hypertrophied bladders had an increase in total and M2 receptor density while all experimental groups showed a reduction in M3 receptor density. Thus bladder hypertrophy, independent from bladder denervation, causes a shift in the muscarinic receptor subtype mediating bladder contraction from M3 toward M2.
Keywords: denervation, outlet obstruction, urinary diversion
Pharmacological data, based on the actions of subtype-selective antimuscarinic agents, can distinguish four subtypes of muscarinic acetylcholine receptors (M1–M4). Molecular techniques have identified five muscarinic receptor subtypes (M1–M5) arising from five separate genes (7, 8). Both M2 and M3 muscarinic receptor subtypes are found in most smooth muscles. The M2 receptor preferentially couples to the inhibition of adenylyl cyclase through the Gi family of proteins, while the M3 receptor preferentially couples to IP3 generation and calcium mobilization through the Gq family of proteins (7, 8). Pertussis toxin (PTX), which ADP ribosylates and therefore inactivates the Gi family of proteins, has no apparent effect on contraction (23). Even though the M2 muscarinic receptor density is greater than the M3 receptor density in bladder and other smooth muscles, the affinity of subtype-selective muscarinic receptor antagonist drugs indicates that contraction is mediated by the M3 receptor in most smooth muscles under normal conditions (7, 9).
A number of studies have shown that under certain conditions the M2 receptor subtype can contribute to the contractile response. This includes selective alkylation of M3 receptors in an environment of increased intracellular levels of cAMP in the rat urinary bladder (5, 16), guinea pig ileum (10), and trachea (29) or after alkylation without increasing intracellular cAMP levels in other tissues such as the guinea pig gallbladder (2) and colon (22). Other studies of smooth muscle contraction after experimentally induced pathologies, for example in a cat model of experimentally induced esophagitis (25), in the denervated rat bladder (4), and in a model of acute cholecystitis in the guinea pig gallbladder (2), also suggest that the M2 receptor participates in mediation of contraction. In addition, in otherwise normal tissues, the M2 receptor appears to mediate contraction after inhibition of the sarcoplasmic reticulum calcium ATPase, Gq, phosphatidylinositol-specific phospholipase C, phosphatidylcholine-specific phospholipase C, or protein kinase C (PKC; Refs. 2, 25). Additional evidence for an M2 receptor-mediated contractile pathway was demonstrated by the synergistic affects of M2- and M3-selective antagonists for inhibition of bladder contraction in normal bladders treated with thapsigargin and denervated bladders (6).
Our previous studies showed that both bilateral major pelvic ganglion electrocautery (DEN) and spinal cord injury (SCI) in the rat induce bladder hypertrophy and a change in muscarinic receptor subtype mediating bladder contraction from M3 toward M2 (1, 4). To determine whether this change is a result of bladder hypertrophy or denervation, additional experimental pathologies were studied. These include major pelvic ganglion decentralization (MPG-DEC), bladder outlet obstruction (BOO), urinary diversion (DIV), and urinary diversion with denervation (DIV-DEN).
METHODS
Materials
The following drugs or chemicals were obtained from the sources indicated: carbachol, methoctramine, and para-fluoro-hexahydro-sila-diphenidol (p-F-HHSiD) were from Sigma Chemical (St. Louis, MO), and darifenacin was a generous gift from Pfizer (Sandwich, UK).
Surgery
Rats (200–250 g female Sprague-Dawley rats from Ace Animals, Boyertown, PA) were anesthetized with 25 µg/kg buprenorphine and 2% isoflurane in oxygen, and a midline incision was made in the lower abdomen. The pelvic plexus was exposed. For bilateral denervation, both the left and right major pelvic ganglion were cauterized with a hand stitching pencil attached to a model SSE 2 solid-state electrosurgery device (Valleylab, Boulder, CO). For sham-operated animals, the plexus was exposed but left intact. For urinary diversions, both ureters were dissected free, cut, and sutured into the colon. For BOO, the urethra was exposed, a 21-gauge syringe needle was placed parallel to the urethra, a 3–0 silk suture was tied around both the needle and urethra, and the needle was then removed. For MPG-DEC, the nerve fibers entering the ganglion from the spinal cord were severed. The subcutaneous tissue, muscle, and skin were sutured. After surgery, urine was expressed with manual pressure on the lower abdomen twice daily for 3 days.
Immediately before bladder harvesting of the MPG-DEC group, they were tested to ensure that spinal stimulation was ineffective in inducing a bladder contraction while MPG stimulation caused an increase in bladder pressure. For this determination, the bladder was catheterized per urethra with PE-50 tubing connected to a pressure transducer. The bladder was emptied and filled manually until an intravesical pressure of 5 cm H2O was induced. A unipolar pith electrode grounded to the abdomen was inserted into the spinal column between the L2 and L3 vertebral bodies and stimulated with a 2- to 5-s train of square wave pulses at 2 V, 30 Hz, 1-ms duration delivered by a Grass S-88 stimulator (Astro-Med, West Warwick, RI). The major pelvic ganglion was stimulated with bipolar electrodes separated by 4 mm with the same stimulation parameters. These stimulations consistently induced a marked bladder contraction in neurally intact animals. Any MPG-DEC animals that showed a bladder contraction to the spinal stimulation or did not show a bladder contraction to the MPG stimulation were not used.
Muscle strips
Urinary bladders were removed from rats euthanized by decapitation. The urinary bladder body (tissue above the ureteral orifices) was dissected free of the serosa and surrounding fat. The bladder was divided in the midsagittal plane, then cut into longitudinal smooth muscle strips (~4 × 10 mm). The muscle strips were then suspended with 1 g of tension in tissue baths containing 15 ml of modified Tyrode solution (125 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 1.8 mM CaCl2, 0.5 mM MgCl2, 23.8 mM NaHCO3, and 5.6 mM glucose) and equilibrated with 95 O2-5% CO2 at 37°C.
Carbachol dose response
After equilibration to the bath solution for 30 min, bladder strips were incubated for 30 min in the presence or absence of antagonist. Dose-response curves were derived from the peak tension developed after cumulative addition of carbachol (10 nM to 300 µM final bath concentration). Only one concentration of antagonist was used for each muscle strip (n = 6–8 strips per antagonist concentration). Dose ratios were determined based on the average of the responses of antagonist free strips. An EC50 value was determined for each strip using a sigmoidal curve fit of the data (Origin, Originlab Northampton, MA). The EC50 values determined in the presence of antagonist were used to generate Schild plots to calculate pA2 values for each antagonist. If the slope of the Schild plot was not significantly different from unity (95% confidence interval), the slope of the Schild plot was constrained to unity to calculate the pKb value. To construct the Schild plot for methoctramine and p-F-HHSiD, doses of 0.3 and 3.0 µM were used. The Schild plot for 4-diphenacetoxy-N-methylpiperidine methiodide (4-DAMP) was done using 3.0, 10.0, and 30.0 nM. Because higher doses of darifenacin appeared unsurmountable and lower doses did not produce a significant shift in the concentration- response curve, a single dose of 30 nM darifenacin was used. The estimated pKb for darifenacin was calculated using the formula pKb = − [log(darifenacin concentration) − log(dose ratio − 1)].
Immunoprecipitations
Immunoprecipitations were performed using antibodies as previously described. (4, 30). Protein concentration in the solubilized receptor preparation was determined by a dye binding assay (Bio-Rad). Muscarinic receptor density is reported as mean ± SE femtomoles per milligram protein in this solubilized receptor preparation. Total muscarinic receptor levels were determined by desalting over Sephadex G-50 minicolumns. At least four determinations were performed on two different pools of bladders for all groups.
Statistical and data analysis
For Fig. 1, contractile force is presented as absolute millinewtons of force generated. Tension displayed in Fig. 2, B and C, is normalized to cross-sectional area defined as weight divided by length (which assumes a tissue density of 1). Statistical analysis of multiple group comparison was performed by ANOVA with a post hoc Newman-Keuls test or a Student’s t-test where appropriate (GB-STAT, Dynamic Microsystems, Silver Spring, MD). Statistically significant differences in the affinity values and departure from unity in the slopes derived from the Schild plots were determined using the 95% confidence intervals.
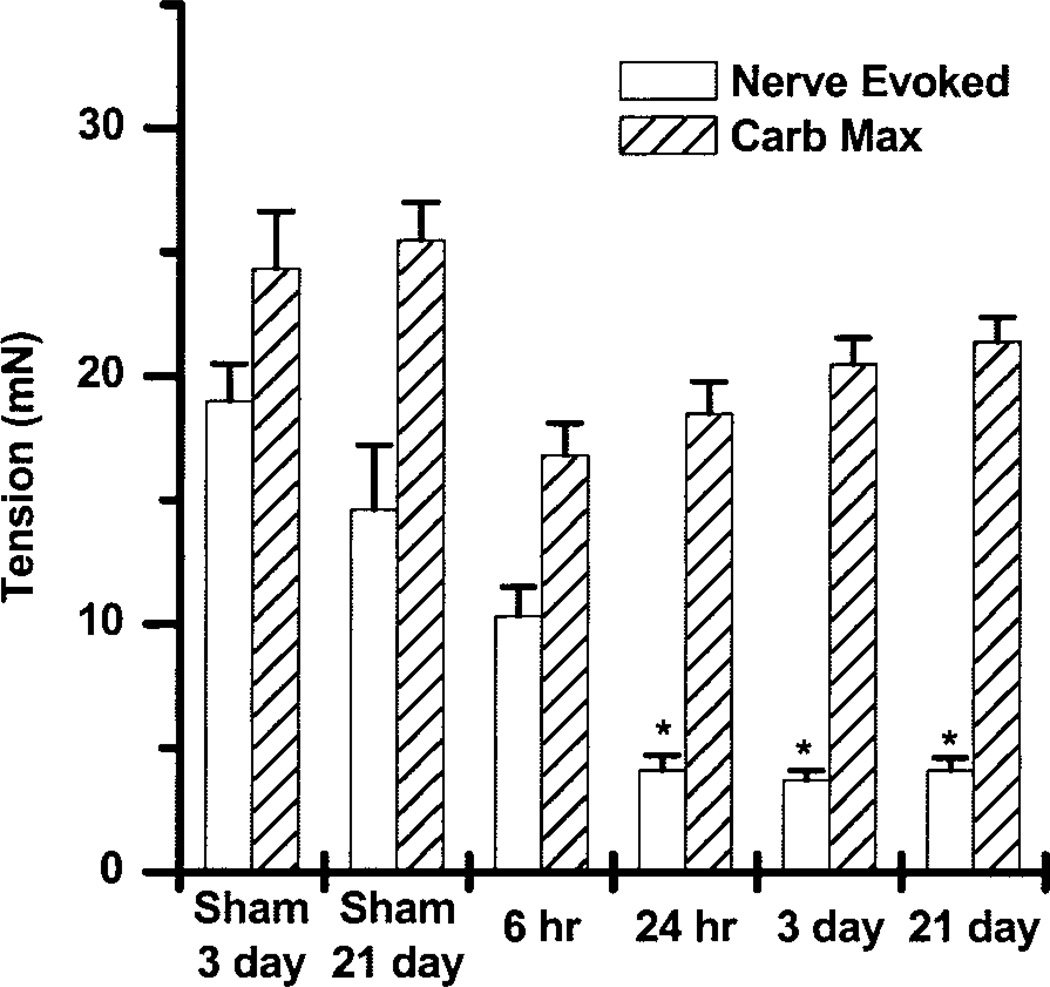
Fig. 1.
Time course of denervation-induced changes in the maximal carbachol response and the nerve-evoked responses to electric field stimulation (EFS) of 8 V, 30 Hz, 1 ms presented as means ± SE. There were no significant differences in the carbachol-evoked maximum (Carb Max). However, by 24 h the nerve-evoked contractions were significantly reduced. *Significantly different from control, P < 0.05.
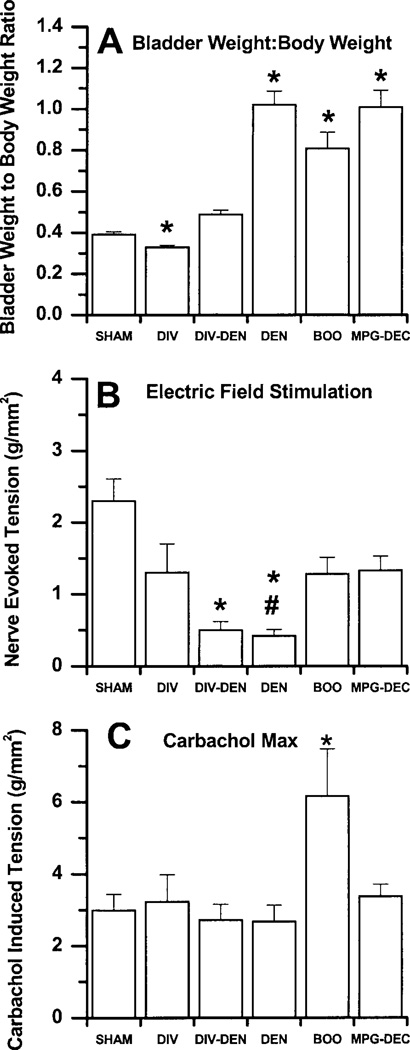
Fig. 2.
DIV, urinary diversion; DIV-DEN, major pelvic ganglion (MPG) electrocautery (MPGE) together with urinary diversion; DEN, bilateral MPGE; BOO, bladder outlet obstruction; MPG-DEC; MPG decentralization. A: effect of experimental pathologies on bladder hypertrophy presented as means ± SE (mg/g). *DEN, BOO, and MPG-DEC are significantly greater than sham operated while DIV is significantly less than sham operated (n = 9–24 rats/group).B: nerve-evoked contractile response to EFS of 8 V, 30 Hz, 1 ms presented as means ± SE. *DIV-DEN and DEN are significantly less than sham operated. #DIV-DEN is significantly greater than DEN (n = 40–60 muscle strips/group). C: maximal carbachol contractile response presented as means ± SE. *BOO is significantly greater than every other group (n = 40–60 muscle strips/group).
RESULTS
Hypertrophy
The time course of denervation-induced changes reveals no increase in bladder weight after 6 h but a statistically significant increase after 24 h, which continues to increase at 3 days and 3 wk (data not shown). Even though there is a large increase in bladder weight, no differences in the carbachol-induced maximal contraction are seen at any time point (Fig. 1). Because the rat bladder has no intramural ganglion cells (14), after denervation and degeneration of the nerve terminals in the bladder wall, no response to electric field stimulation (EFS) was expected. At 6 h postdenervation, the nerve terminals are apparently still intact because the muscle strips are able to contract to EFS (8 V, 30 Hz, 1 ms). A significant reduction in the EFS contractile response is seen at 24 h, the next time point measured. No further reduction is seen at time points after 24 h. The change in affinity of muscarinic receptor subtype-selective antagonists is not evident at 24 h postsurgery but occurs at 3 days postsurgery (data not shown). Consequently, the 3-day postsurgery time point was chosen for all subsequent studies.
Neither DIV nor DIV-DEN induces hypertrophy as evidenced by an increase in bladder weight-to-body weight ratio compared with sham-operated controls; however, DEN, BOO, and MPG-DEC induce significant hypertrophy by 3 days postsurgery. Interestingly, DIV bladders are significantly smaller than sham-operated or DIV-DEN bladders (Fig. 2A).
EFS responses
To determine the effect of short-term hypertrophy on EFS contractile force, the contractile force normalized to cross-sectional area was determined for each group. Figure 2B shows that no differences in the EFS contraction to a submaximal stimulation of 8 V, 30 Hz, 1 ms is seen with DIV, BOO, or MPG-DEC compared with sham-operated control bladders. DIV-DEN and DEN bladders contract less to EFS than sham-operated controls. Unexpectedly, DIV-DEN bladders contract greater than DEN bladders to EFS, suggesting that the nerve terminals in these bladders are not completely degenerated at 3 days (Fig. 2B).
Carbachol responses
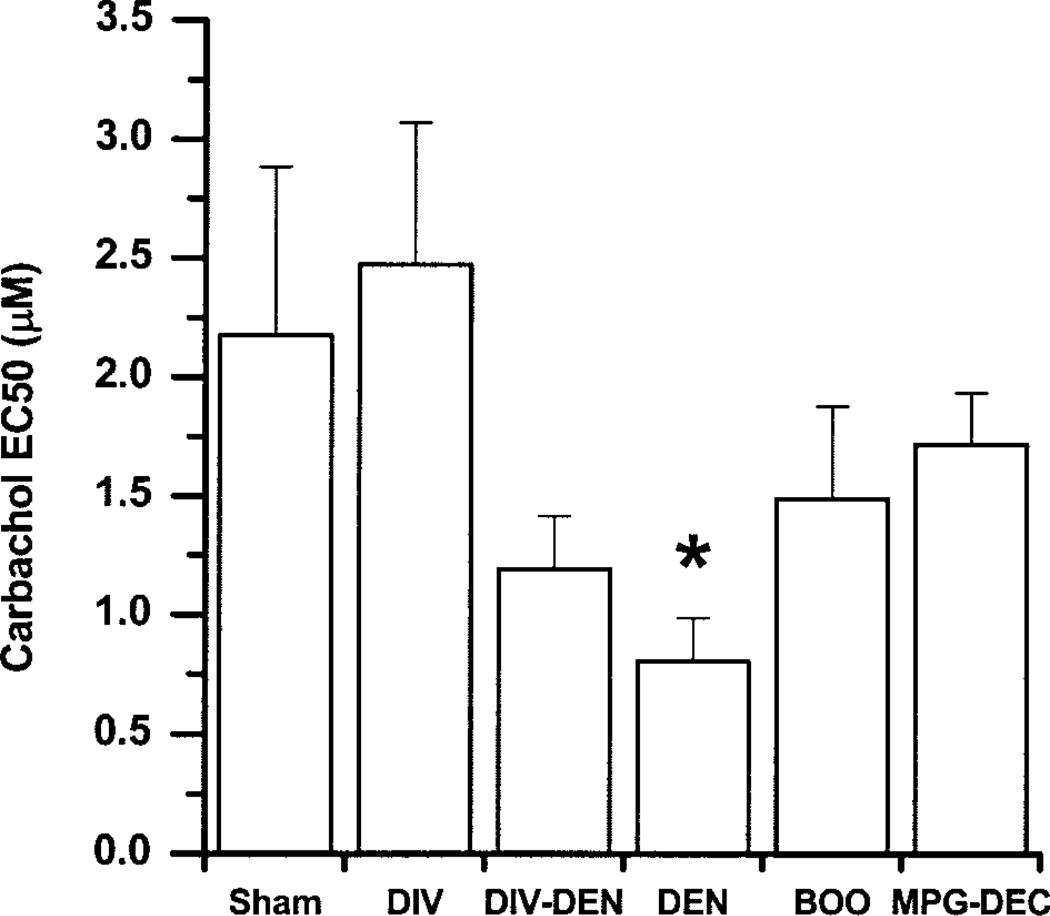
The carbachol-induced maximal contraction (Fig. 2C) is not different between any groups except that the BOO group contracts significantly greater than every other group. As the nerves degenerate or are unable to induce a maximal contraction, the ratio of the carbachol maximum contractile response to the EFS contractile response will increase. This can be used as a measure of functional denervation. DEN, DIV-DEN, MPG-DEC, and BOO bladders are functionally denervated compared with sham-operated bladders. DEN bladders are significantly more functionally denervated than the DIV-DEN, BOO, and MPG-DEC bladders. DIV-DEN bladders are functionally denervated similar to BOO, both of which are more functionally denervated than the MPG-DEC bladders. Comparing the carbachol potency between the groups, a lower carbachol EC50 is found in the DEN bladders compared with sham operated (Fig. 3).
Fig. 3.
Effect of experimental pathologies on the potency of carbachol to induce contraction presented as means ± SE. *DEN is significantly less than sham operated, DIV, and MPG-DEC (n = 7–36 muscle strips/group).
Muscarinic receptor-subtype protein density
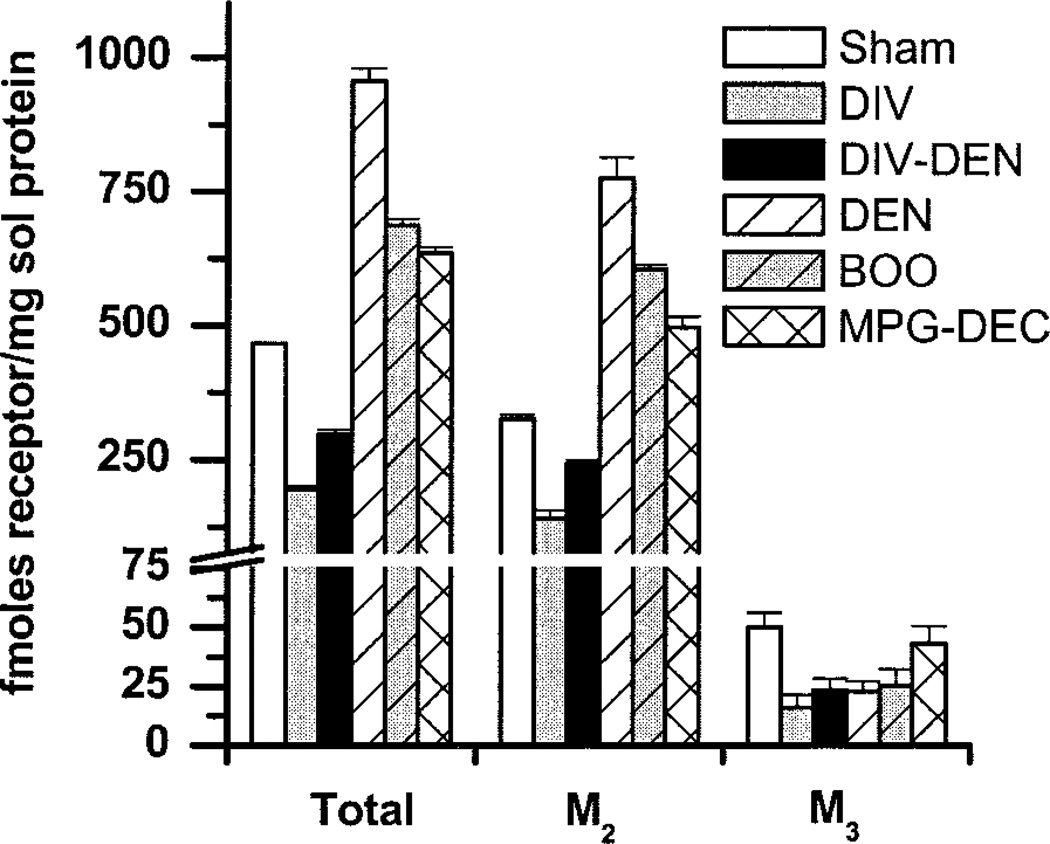
Total, M2, and M3 receptor protein density was determined by subtype-selective immunoprecipitation (Fig. 4). Sham-operated bladders have a significantly different total receptor density (~470 fmol/mg solubilized protein) than every group, with an M2-to-M3 ratio of ~6:1. Total muscarinic receptor density in the DIV and DIV-DEN bladders is significantly less than sham operated with M2-to-M3 ratios of about 9:1 and 10:1, respectively. DEN, BOO, and MPG-DEC bladders have greater total receptor densities than sham-operated with M2-to-M3 ratios of about 34:1, 24:1, and 11:1, respectively.
Fig. 4.
Effect of experimental pathologies on the density of urinary bladder muscarinic receptor subtypes. M2 and M3 receptors were labeled with [3H]QNB and solubilized as described in Luthin et al. (19). Data shown are average fmol (±SE) of receptor/mg solubilized protein from sham operated, DIV, DIV-DEN, DEN, BOO, and MPG-DEC (n = 4–5 determinations from 6–18 bladders). Protein concentration in the solubilized receptor preparation was ~8% of the protein concentration in the crude homogenate. Compared with filtration binding, ~50% of the muscarinic receptors were solubilized (data not shown). Group differences were determined using ANOVA with a post hoc Newman-Keuls test. For total receptor density all groups are different from each other. For M2 receptor density, all groups are different from each other. For M3 receptor density, sham operated is different from DIV, DIV-DEN, DEN, and BOO.
The hypertrophied bladders (DEN, BOO) have an increase in M2 receptors, a decrease in M3 receptors, and an increase in total receptor density, while the hypertrophied MPG-DEC bladders have an increase in M2 and total receptors, with no change in M3 receptors compared with sham operated. The atrophied bladders (DIV) have a decrease in both M2 and M3 receptors, which is reflected in a decrease in total receptor density. The DIV-DEN bladders, which are neither hypertrophied nor atrophied, have a decrease in M2, M3, and total receptor density.
Correlation of muscarinic receptor-subtype density with functional denervation
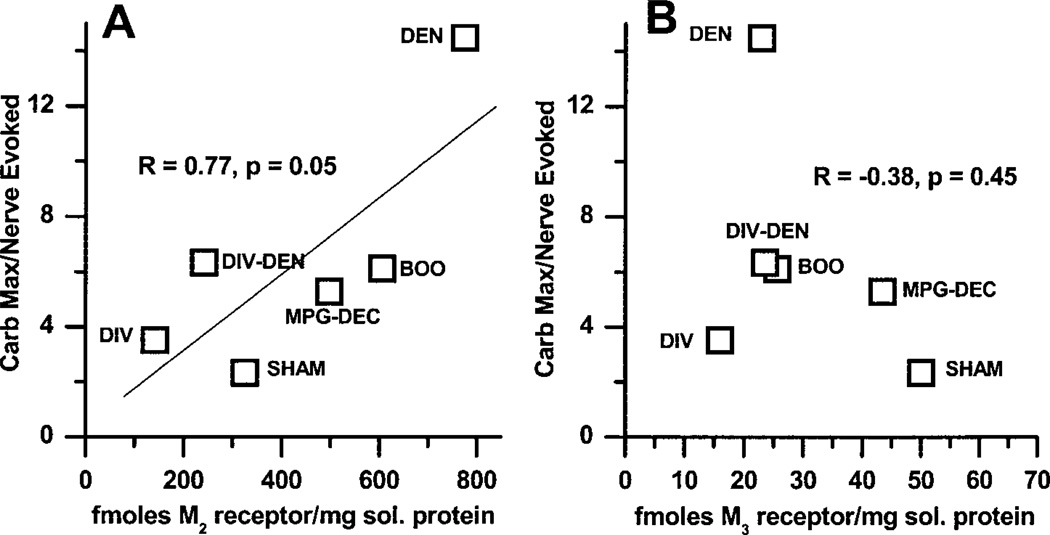
As can be seen in Fig. 5, the density of the M2 receptor protein correlates (R = 0.77, P = 0.05) with the degree of functional denervation. However, there is no correlation between the density of the M3 subtype and the degree of functional denervation.
Fig. 5.
Correlation of muscarinic receptor subtype density with functional denervation. For each individual muscle strip the ratio of the carbachol maximum:EFS-induced contraction was determined. The average of the individual muscle strips for each experimental group for functional denervation is plotted vs. the immunoprecipitation data for M2 receptor density (A) and M3 receptor density (B). The ratio of the average carbachol maximum to the average EFS contraction displayed in Fig. 2 does not equal the average of the individual ratios for the strips. The density of M2 receptors correlates with functional denervation. No correlation between the M3 receptor density and functional denervation is seen.
Affinity of antagonists for inhibition of carbachol-induced contractions
Based on the affinity of a series of muscarinic receptor antagonists for inhibition of carbachol-induced contractions, sham-operated bladder contractions are mediated by the M3 receptor subtype (Table 1). This is based on a high affinity for the M3-selective antagonists p-F-HHSiD (7.7 ± 0.2), 4-DAMP (9.1 ± 0.2), and darifenacin (8.5 ± 0.1) and a low affinity for the M2-selective antagonist methoctramine (6.2 ± 0.2). The affinities in DIV and DIV-DEN are also consistent with M3 receptors mediating contraction. The M3-selective antagonists have a lower affinity in the hypertrophied bladders (DEN, MPG-DEC, and BOO), which is consistent with participation of the M2 receptor in mediation of contraction. Paradoxically, the M2-selective antagonist methoctramine has a low affinity for inhibition of contraction in all groups, which seems to preclude participation of M2 receptors in contraction. This phenomenon was also seen in our earlier studies (1, 4), which prompted speculation of the existence of two contractile pathways, one mediated by the M3 receptor subtype, the other mediated by the M2 subtype. Further evidence for the existence of two pathways was provided by studies in which antagonists showed superadditive inhibitory effects in blocking bladder contraction (6).
Table 1.
Affinity (95% confidence interval) values of subtype-selective muscarinic receptor antagonists for inhibition of carbachol-induced bladder contraction and their reported affinity for the M2 and M3 receptor subtypes
| Group | Methoctramine | 4-DAMP | p-F-HHSiD | Darifenacin |
|---|---|---|---|---|
| Sham operated | 6.0–6.4 | 8.9–9.1 | 7.6–7.8 | 8.3–8.7 |
| DIV | 6.0–6.4 | 7.2–7.6 | 8.7–9.1 | |
| DIV-DEN | 6.1–6.5 | 7.2–7.6 | 8.6–9.2 | |
| DEN | 6.3–6.7 | 8.3–8.7 | 6.8–7.2 | 7.5–8.5 |
| BOO | 5.9–6.5 | 6.5–7.1 | 7.8–8.4 | |
| MPG-DEC | 4.4–5.6 | 6.5–7.1 | 7.5–7.9 | |
| M2* | 7.8–8.3 | 8.0–8.4 | 6.0–6.9 | 7.0–7.4 |
| M3* | 6.3–6.9 | 8.9–9.3 | 7.8–7.9 | 8.4–8.9 |
Values are 95% confidence intervals; 6–8 muscle strips were used per antagonist concentration. Nonoverlapping confidence intervals denote a statistically significant difference (P < 0.05). The affinities for 4-DAMP, methoctramine, and p-F-HHSiD were determined by Schild analysis. Concentrations of 0.3 and 3.0 µM methoctramine and p-F-HHSiD were used, while 3.0, 10.0, and 30.0 nM 4-DAMP were used. The estimated pKb for darifenacin was determined based on a single 30 nM darifenacin concentration.
Adapted from Caulfield (7) and Caulfield and Birdsall (8), which include both functional and ligand binding studies. DIV, urinary diversion; DIV-DEN, major pelvic ganglion (MPG) electrocautery (MPGE) together with urinary diversion; DEN, bilateral MPGE; BOO, bladder outlet obstruction; MPG-DEC, MPG decentralization; 4-DAMP, 4-diphenacetoxy-N-methylpiperidine methiodide; p-F-HHSiD, para-fluoro-hexahydrosila-diphenidol.
DISCUSSION
Hypertrophy occurs rapidly after either DEN, MPG-DEC, or BOO in the rat urinary bladder. The bladder-to-body weight ratios (mg/g) in these groups increased by over twofold within 3 days. These results are in general agreement with previous published increases in bladder weight after 1 wk of denervation, decentralization, or outlet obstruction (11. 13). Similarly, nerve terminals in the denervated bladders degenerate rapidly as evidenced by an almost complete absence of EFS-induced contraction by 24 h after surgery. Although there is virtually no response to EFS at 24 h postsurgery and significant hypertrophy, there is no reduction in the maximal response to carbachol in the DEN and the MPG-DEC bladders. However, the BOO bladders, while having no reduction in EFS-induced contractility, have a significantly greater response to carbachol with no change in agonist affinity. As expected, using the ratio of the maximal carbachol contraction to the EFS-induced contraction as a measure of functional denervation, the DEN and DIV-DEN bladders are functionally denervated. Unexpectedly though, the MPG-DEC and the BOO bladders are also somewhat functionally denervated. It is possible that the increased bladder pressures in these groups leads to local ischemia and thus degeneration of nerve terminals. The DIV-DEN group, while functionally denervated compared with sham-operated animals, has a greater response to EFS than DEN alone, providing evidence that the nerve terminals do not degenerate as rapidly in this group where the bladder is not exposed to increased mechanical stress. In addition, because bladders from the DIV-DEN group do not atrophy as do the DIV bladders, an intact innervation appears to be required for atrophy to occur. This may indicate a role for bladder innervation in control of hypertrophy, possibly via the release of paracrine factors that act in opposition to bladder hypertrophy and thereby induce atrophy.
The only group with denervation-induced supersensitivity is the DEN group, not the MPG-DEC or the DIV-DEN groups (Fig. 3). While the DIV-DEN group does not have increased bladder pressure because of the diversion, the MPG-DEC does. However, the MPG-DEC group has intact innervation from the major pelvic ganglion to the bladder. This suggests that, for at least the 3-day postoperative time point, both a complete absence of nerve terminals and increased pressure with concomitant hypertrophy are required for development of carbachol supersensitivity. Muscarinic agonist supersensitivity after 1–3 wk of urinary diversion or MPG decentralization has been reported (12), but we found that this does not occur at the 3-day time point. Our results are somewhat different from previously reported effects of bladder hypertrophy for 1 wk where no increase in the methacholine-induced contraction was seen but supersensitivity to methacholine was noted. These differences may be attributed to the duration of hypertrophy or possibly the method of inducing the hypertrophy [urethral ligature used here vs. paraffin injected into the lumen of the bladder used by Ekstrom et al. (13)]. The duration of the hypertrophy is more likely the reason for the differences, because the supersensitivity was transient and not present 4 wk after paraffin injection (13).
Analysis of the density of muscarinic receptors reveals an increase in total receptor density in the hypertrophied bladders regardless of whether they are denervated. All of this increase is accounted for by an increase in the M2 subtype. All experimental groups except MPG-DEC have a significantly lower density of the M3 subtype. The nonhypertrophied groups (DIV and DIV-DEN) have a decrease in total, M2, and M3 receptor density. Therefore, hypertrophy leads to an increase in density of total and M2 receptors while urinary diversion results in a decrease in density of total and M2 receptors regardless of whether the diversion is accompanied by tissue atrophy (DIV group) or not (DIV-DEN group). Our results are consistent with those reported by Nilvebrant et al. (20), who showed increases in total muscarinic receptor density after 1 wk of denervation and decreases in total receptor density after 1 wk of diversion or diversion with denervation. While a trend for an increase in total receptor density was reported in the bladders induced to hypertrophy by paraffin injection, the increase was not significant (20), whereas we found a significant increase in total receptor density as a result of hypertrophy induced by outlet obstruction. This discrepancy may be the result of either the duration of hypertrophy or the method used to induce the hypertrophy.
In the DIV and DIV-DEN groups, despite decreased M3 receptor density (Fig. 4), the contractions are mediated by the M3 subtype (Table 1) and there is no change in agonist potency (Fig. 3). In other words there is no correlation between M3 receptor density and the potency of agonist or antagonist. The potency of carbachol is increased only in the DEN group (Fig. 3), which also has the largest increase in M2 receptor density (Fig. 4). The potency of carbachol is not increased in the BOO and MPG-DEC groups despite bladder hypertrophy and increased M2 receptor density. Thus the potency of carbachol for inducing contraction is not related to total receptor density or the density of the M2 or M3 subtype. This finding is similar to that reported by Nilvebrant et al. (20), who found that total receptor density does not correlate with supersensitivity in denervated, hypertrophied, diverted, or diverted and denervated bladders.
The significance of alterations in receptor density is not completely clear. However, the density of the M2 receptor subtype correlates with functional denervation. The greater the degree of functional denervation, the greater is the density of the M2 receptor subtype (Fig. 5A). No such correlation exists for the M3 subtype (Fig. 5B). However, all groups with bladder hypertrophy have an increase in M2 receptor density and low affinities for M3-selective antagonists, which suggests an M2-mediated component of contraction. The DIV and DIV-DEN bladders, which are not hypertrophied, have higher affinities for these M3-selective antagonists, suggesting that the M3 subtype mediates bladder contraction. Another report on the effect of BOO on muscarinic receptor-mediated bladder contraction found no differences in carbachol-mediated contraction (18). The differences in results reported by this group and our results may be due to the use of males as opposed to females or the duration of obstruction (4 wk as opposed to 3 days in our study).
Prejunctional autoreceptors have been identified on the nerves innervating the rat bladder (3, 26, 28). Activation of the M1 subtype increases acetylcholine release and contraction while activation of the M2 subtype reduces acetylcholine release and inhibits contraction. Somogyi and de Groat (27) have shown that the prejunctional facilitatory mechanism is upregulated after chronic spinal transection; furthermore, this facilitation appears to be primarily mediated by M3 receptors as opposed to M1 receptors in normal animals. Thus previous evidence exists for plasticity in the neural mechanism governing bladder contraction, and our results provide evidence for plasticity in the smooth muscle mechanism mediating bladder contraction.
The in vitro results reported in this manuscript, namely an M2 receptor-mediated component of contraction, is superficially in agreement with in vivo experiments implicating the M2 receptor subtype in bladder contraction. The amplitude of volume-induced bladder contractions in the urethane-anesthetized rat is inhibited by subtype-selective muscarinic antagonists with potencies that correlate most favorably with pKi estimates of these compounds at human recombinant M2 receptors (16). In addition, the M2-and M4-selective antagonist AQ-RA 741, similar to the nonselective antagonist tolterodine, has a greater selectivity for inhibition of bladder contraction than for inhibition of salivation in the α-chloralose-anesthetized cat, suggesting M2 receptor involvement in bladder contraction (15). Intravenous injection of AF-DX116 (M2-selective antagonist) reduces contraction pressure but not frequency or the duration of bladder contraction (21). In these in vivo studies of systemic administration of antagonists, the sites of action of the antagonists are not known and inhibition of bladder contraction or salivation could be due to additional effects on the central or the peripheral nervous system and not strictly due to effects of the antagonist on the end organ itself.
Intracerebroventricular injection of the M3-selective antagonist 4-DAMP inhibits both the amplitude and the duration of volume-induced bladder contractions, whereas AF-DX116 decreases contraction frequency while prolonging the duration of contraction (21). On the other hand, intracerebroventricular injection of darifenacin, an M3-selective antagonist, has no effect on voiding parameters in normal conscious rats, while intracerebroventricular injection of tolterodine, a non-subtype-selective antagonist, decreases voiding pressure and increases bladder capacity (17). Thus evidence exists for the central nervous system control of bladder contraction by both M2 and M3 muscarinic receptor subtypes. Because the prejunctional facilitatory autoreceptors have been shown to change from M1 to predominantly M3 after spinal cord transection in the rat (27), it may also be possible that pathophysiological conditions could induce alterations in the central mechanisms governing micturition.
The question arises as to how M2 receptors directly mediate smooth muscle contraction. M2 receptors are traditionally thought to preferentially couple to PTX-sensitive G proteins such as the Gi subfamily, resulting in inhibition of adenylyl cyclase, while M3 receptors preferentially couple to Gq and stimulation of phosphoinositol hydrolysis leading to an increase in cytosolic calcium. Pharmacological studies demonstrate that most smooth muscle contraction is mediated by the M3 subtype (7, 9). However, an M2-mediated contractile response in bladder muscle can be demonstrated after the majority of M3 receptors are inactivated in an environment of increased intracellular cAMP such as during stimulation with a β-adrenergic agonist (5, 16). This pathway has been proposed to mediate contraction indirectly, merely by blocking β-adrenergic agonist-induced relaxation via increased cAMP (9). However, M2 receptors acting through Gi may also stimulate bladder contraction directly via PKC activation as previously found in the cat lower esophageal sphincter smooth muscle. A low degree of muscarinic stimulation and, consequently, a low degree of calcium mobilization result in activation of PKC, whereas PKC activation is inhibited at higher intracellular calcium concentrations (24). Thus in the face of normal calcium mobilization mediated by the M3 receptor subtype, the signal transduction pathway mediated by the M2 subtype may be inhibited. One hypothesis to explain the shift in muscarinic receptor subtype mediating contraction from M3 to M2 in the hypertrophied bladders is a deficit in calcium mobilization.
We have previously shown quantitative evidence for an interaction between the second messenger systems activated by the M2 and the M3 receptor subtypes in the denervated rat bladder (6). The simultaneous action of M2-selective and M3-selective antagonists induces a synergistic inhibition of contraction in denervated bladders, which indicates a facilitatory interaction of the two subtypes in inducing contraction. However, results in the normal rat bladder show no facilitatory interaction between M2 and M3 subtypes for inducing contraction. Only after either denervation or blocking the sarcoplasmic reticulum calcium pump with thapsigargin does this interaction become facilitatory. These two results provide further support for an interaction between subtypes mediating contraction as previously reported in the guinea pig colon where the M2 and M3 receptor subtypes are thought to interact in a facilitatory manner to mediate contraction (23). However, our results in the normal rat bladder do not support such a facilitatory interaction; actually, the opposite appears to occur, namely that the M3 pathway seems to inhibit the M2 pathway, possibly via calcium mobilization. It is possible that the interaction between subtypes is different in the guinea pig colon or that after 3 days of in vivo PTX treatment (23), the interaction between subtypes becomes altered.
Experimental pathologies that interfere with the normal functioning of the bladder induce a decrease in density of the M3 receptor subtype. Conditions that lead to hypertrophy induce an increase in density of the M2 receptor subtype and a shift in the mechanism of contraction such that the M2 subtype can be shown to at least partially mediate contraction.
Acknowledgments
DISCLOSURES
This work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grant R01-DK-4333.
REFERENCES
- 1.Braverman A, Legos J, Young W, Luthin G, Ruggieri M. M2 receptors in genito-urinary smooth muscle pathology. Life Sci. 1999;64:429–436. doi: 10.1016/s0024-3205(98)00582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braverman AS, Bartula LL, Myers SI, Parkman HP, Ruggieri MR. Inflammation changes the muscarinic receptor subtype and signal transduction pathway that mediates gallbladder contraction. Gastroenterology. 2000;118:A197. [Google Scholar]
- 3.Braverman AS, Kohn IJ, Luthin GR, Ruggieri MR. Prejunctional M1 facilitory and M2 inhibitory muscarinic receptors mediate rat bladder contractility. Am J Physiol Regul Integr Comp Physiol. 1998;274:R517–R523. doi: 10.1152/ajpregu.1998.274.2.r517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braverman AS, Luthin GR, Ruggieri MR. M2 muscarinic receptor contributes to contraction of the denervated rat urinary bladder. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1654–R1660. doi: 10.1152/ajpregu.1998.275.5.R1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braverman AS, Ruggieri MR. Selective alkylation of rat urinary bladder muscarinic receptors with 4-DAMP mustard reveals a contractile function for the M2 muscarinic receptor. J Recept Signal Transduct Res. 1999;19:819–833. doi: 10.3109/10799899909042875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braverman AS, Tallarida RJ, Ruggieri MR., Sr Interaction between muscarinic receptor subtype signal transduction pathways mediating bladder contraction. Am J Physiol Regul Integr Comp Physiol. 2002;283:R663–R668. doi: 10.1152/ajpregu.00116.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caulfield MP. Muscarinic receptors—characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 8.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 9.Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- 10.Ehlert FJ, Thomas EA. Functional role of M2 muscarinic receptors in the guinea pig ileum. Life Sci. 1995;56:965–971. doi: 10.1016/0024-3205(94)00035-q. [DOI] [PubMed] [Google Scholar]
- 11.Ekstrom J, Malmberg L. Development of supersensitivity to methacholine in the rat detrusor following either parasympathetic denervation or decentralization. Acta Physiol Scand. 1984;122:175–179. doi: 10.1111/j.1748-1716.1984.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 12.Ekstrom J, Malmberg L. Disuse as cause of supersensitivity in the rat urinary bladder. Acta Physiol Scand. 1986;126:429–432. doi: 10.1111/j.1748-1716.1986.tb07837.x. [DOI] [PubMed] [Google Scholar]
- 13.Ekstrom J, Malmberg L, Wallin A. Transient supersensitivity in the hypertrophied rat urinary bladder. Acta Physiol Scand. 1986;126:365–370. doi: 10.1111/j.1748-1716.1986.tb07828.x. [DOI] [PubMed] [Google Scholar]
- 14.Gabella G, Uvelius B. Urinary bladder of rat: fine structure of normal and hypertrophic musculature. Cell Tissue Res. 1990;262:67–79. doi: 10.1007/BF00327747. [DOI] [PubMed] [Google Scholar]
- 15.Gillberg PG, Sundquist S, Nilvebrant L. Comparison of the in vitro and in vivo profiles of tolterodine with those of subtype-selective muscarinic receptor antagonists. Eur J Pharmacol. 1998;349:285–292. doi: 10.1016/s0014-2999(98)00214-3. [DOI] [PubMed] [Google Scholar]
- 16.Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishizuka O, Gu BJ, Yang ZX, Nishizawa O, Andersson KE. Functional role of central muscarinic receptors for micturition in normal conscious rats. J Urol. 2002;168:2258–2262. doi: 10.1016/S0022-5347(05)64367-4. [DOI] [PubMed] [Google Scholar]
- 18.Krichevsky VP, Pagala MK, Vaydovsky I, Damer V, Wise GJ. Function of M3 muscarinic receptors in the rat urinary bladder following partial outlet obstruction. J Urol. 1999;161:1644–1650. [PubMed] [Google Scholar]
- 19.Luthin GR, Harkness J, Artymyshyn RP, Wolfe BB. Antibodies to a synthetic peptide can be used to distinguish between muscarinic acetylcholine receptor binding sites in brain and heart. Mol Pharmacol. 1988;34:327–333. [PubMed] [Google Scholar]
- 20.Nilvebrant L, Ekstrom J, Malmberg L. Muscarinic receptor density in the rat urinary bladder after denervation, hypertrophy and urinary diversion. Acta Pharm Toxicol. 1986;59:306–314. doi: 10.1111/j.1600-0773.1986.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 21.Ogasawara M, Shimoda N, Sato K, Kato T, Sugaya K. The effects of the antagonists of muscarinic acetylcholine receptor subtypes in rat brain on urinary bladder contraction. Jpn J Urol. 2002;93:427–434. doi: 10.5980/jpnjurol1989.93.427. [DOI] [PubMed] [Google Scholar]
- 22.Sawyer GW, Ehlert FJ. Contractile roles of the M2 and M3 muscarinic receptors in the guinea pig colon. J Pharmacol Exp Ther. 1998;284:269–277. [PubMed] [Google Scholar]
- 23.Sawyer GW, Ehlert FJ. Muscarinic M3 receptor inactivation reveals a pertussis toxin-sensitive contractile response in the guinea pig colon: evidence for M2/M3 receptor interactions. J Pharmacol Exp Ther. 1999;289:464–476. [PubMed] [Google Scholar]
- 24.Sohn UD, Chiu TT, Bitar KN, Hillemeier C, Behar J, Biancani P. Calcium requirements for acetylcholine-induced contraction of cat esophageal circular muscle cells. Am J Physiol Gastrointest Liver Physiol. 1994;266:G330–G338. doi: 10.1152/ajpgi.1994.266.2.G330. [DOI] [PubMed] [Google Scholar]
- 25.Sohn UD, Harnett KM, Cao W, Rich H, Kim N, Behar J, Biancani P. Acute experimental esophagitis activates a second signal transduction pathway in cat smooth muscle from the lower esophageal sphincter. J Pharmacol Exp Ther. 1997;283:1293–1304. [PubMed] [Google Scholar]
- 26.Somogyi GT, de Groat WC. Evidence for inhibitory nicotinic and facilitatory muscarinic receptors in cholinergic nerve terminals of the rat urinary bladder. J Auton Nerv Syst. 1992;37:89–97. doi: 10.1016/0165-1838(92)90237-b. [DOI] [PubMed] [Google Scholar]
- 27.Somogyi GT, de Groat WC. Function, signal transduction mechanisms and plasticity of presynaptic muscarinic receptors in the urinary bladder. Life Sci. 1999;64:411–418. doi: 10.1016/s0024-3205(98)00580-3. [DOI] [PubMed] [Google Scholar]
- 28.Somogyi GT, Tanowitz M, de Groat WC. M1 muscarinic receptor-mediated facilitation of acetylcholine release in the rat urinary bladder. J Physiol. 1994;480:81–89. doi: 10.1113/jphysiol.1994.sp020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas EA, Ehlert FJ. Involvement of the M2 muscarinic receptor in contractions of the guinea pig trachea, guinea pig esophagus, and rat fundus. Biochem Pharmacol. 1996;51:779–788. doi: 10.1016/0006-2952(95)02396-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]