Abstract
Riboswitches are motifs in the untranslated regions (UTRs) of RNA transcripts that sense metabolite levels and modulate the expression of the corresponding genes for metabolite import, export, synthesis, or degradation. All riboswitches contain an aptamer: an RNA structure that, upon binding ligand, folds to expose or sequester regulatory elements in the adjacent sequence through alternative nucleotide pairing. The coupling between ligand binding and aptamer folding is central to the regulatory mechanisms of thiamine pyrophosphate (TPP) riboswitches and has not been fully characterized. Here, we show that TPP aptamer folding can be decomposed into ligand-independent and -dependent steps that correspond to the formation of secondary and tertiary structures, respectively. We reconstructed the full energy landscape for folding of the wild-type (WT) aptamer and measured perturbations of this landscape arising from mutations or ligand binding. We show that TPP binding proceeds in two steps, from a weakly to a strongly bound state. Our data imply a hierarchical folding sequence, and provide a framework for understanding molecular mechanism throughout the TPP riboswitch family.
Keywords: optical trapping, optical tweezers, single molecule, single-molecule biophysics
Riboswitches that sense the essential coenzyme thiamine pyrophosphate (TPP) are found in all kingdoms of life, and regulate thiamine synthesis at the level of transcription, translation, or splicing (1–3). Members of the TPP riboswitch family share sequence elements, architectures, and modes of ligand binding (4–9). The TPP-binding aptamer in the 3′ UTR of the thiC gene from Arabidopsis thaliana possesses a “tuning-fork” architecture (10) comprising two sensor helix arms (P2/3 and P4/5) and a switch helix (P1), all stemming from a central junction (J2/4) (Fig. 1A). The aptamer is thought to bind its ligand as the sensor helix arms are brought together, and bulges (J2/3 and J4/5) in the arms join to form a bipartite binding pocket. Purine riboswitches also resemble tuning forks (11, 12), but in contrast have a single binding pocket comprised largely of nucleotides from the central junction.
Fig. 1.
Single-molecule observations of TPP riboswitch aptamer folding. (A) Sequence and secondary structure of the A. thaliana thiC TPP-binding aptamer, indicating mutations (red) and construct sequences measured in isolation (boxed). Short spacer sequences (lower case letters, gray) were used to connect all constructs to dsDNA handles. Nucleotides involved in the P3low and P3high transitions are indicated (dashed line). (B) Experimental geometry of the “dumbbell” optical-trapping assay with components labeled (not to scale). (C) Representative nonequilibrium FECs for the WT aptamer in the absence of ligand, obtained by increasing (red) or decreasing (black) the distance between traps (100 cycles). WLC curve-fits (blue) to the data below or above all transitions are shown. Inset, histograms of the unfolding and refolding work (5 molecules, 1,291 cycles). (D) Left, equilibrium traces of extension acquired at constant force showing individual folding transitions; the corresponding transitions in the FECs are indicated (black arrows). Right, histograms of extension (red) and Gaussian fits (black). The fully folded (F) and unfolded (U) states, as well as the pairs of peaks corresponding to specific transitions (P1, P2, P3high, P4, P5) are labeled.
It has been proposed that riboswitches may be sorted into two functional types (13): Type I and Type II, of which the purine and TPP riboswitches are prototypic examples, respectively [as more riboswitch sequences and structures have been determined, additional classification schemes have been discussed (1, 14)]. The two types are distinguished by binding pocket architecture and the scale of the structural rearrangement accompanying ligand binding, with Type I and Type II undergoing local and long-distance rearrangements, respectively. An earlier single-molecule study (15) of the Type I pbuE aptamer from Bacillus subtilis, which binds adenine, revealed that secondary and tertiary structure formation were interleaved during folding, in that a competent binding site (constituting a tertiary element) was formed prior to the closure of the base of the switch helix, P1 (a secondary element). Here, we have extended the single-molecule approaches used previously to investigate the folding and energetics of the TPP aptamer, which is significantly larger than the adenine aptamer (111 nt vs. 62 nt). By measuring the energy landscape of the TPP aptamer and its sensitivity to ligand binding and mutations, we explored the hierarchy of folding in this Type II aptamer and obtained evidence supporting the Type I/Type II dichotomy.
We characterized the thermodynamics and kinetics of folding for the TPP aptamer using a single-molecule optical-trapping assay in which a controlled force was applied via the 5′ and 3′ ends of the RNA (Fig. 1B). In the absence of ligand, the aptamer unfolded through a series of transitions observed in both nonequilibrium (Fig. 1C) and equilibrium (Fig. 1D) measurements. Similar transitions were observed in RNA constructs containing only portions of the aptamer sequence (Fig. 1A, Fig. S1, Table S1). Constant-force measurements carried out at equilibrium supplied data for the energy, rate, and distance associated with each folding transition. The data from the constructs were first used to assign each transition to a given helix, and then to reconstruct the energy landscape for secondary structure formation (Fig. 2, red). To study aspects of tertiary structure formation, we compared aptamer folding for wild-type and mutant sequences in the presence and absence of ligands.
Fig. 2.
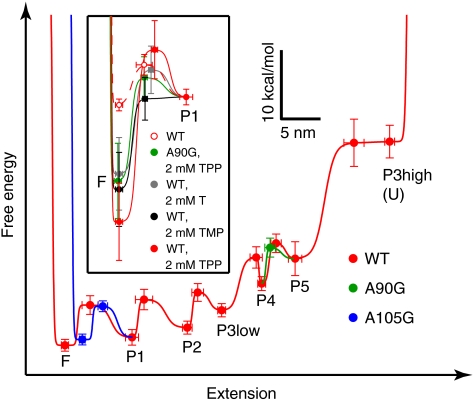
Reconstructed energy landscapes for thiC aptamers. The experimentally determined free energies for folded, unfolded, and intermediate states are plotted vs. extension, along with the transition energy barriers separating these for the WT, A90G, and A105G aptamers (mean ± s.e.; see SI Methods). States are labeled in the order that unfolding transitions occurred as the extension was raised (e.g., P1 is unfolded in the state marked “P1”); smooth curves (red, blue, green) connect the points. The landscapes for the WT (red filled circles) and mutant aptamers (blue and green filled circles) were indistinguishable except where indicated. Landscapes are shown tilted by the work done by 8 pN applied force. Inset, view of the F-to-P1 transition illustrating the stabilization of the F state by ligand binding under the indicated conditions (legend).
Results and Discussion
We observed and measured individual folding transitions within single WT aptamer molecules by holding each molecule at equilibrium, and gradually lowering the applied force while recording the molecular end-to-end extension (Fig. 1D). Our measurements allowed us to identify each transition and calculate its respective contribution to the folding energy landscape (Fig. 2). In the absence of ligand, the energetic stabilities of the helices decreased systematically, going from the distal ends of the sensor helix arms toward the 5′ and 3′ termini of the RNA. Previous models have suggested that aptamer folding, in concert with TPP binding, proceeds with a similar “outside-in” directionality: TPP binding juxtaposes the sensor helices, promoting formation of the central junction and thereby stabilizing the switch helix (10, 16). Our data demonstrate that TPP binding is not required to fold P1 (the least stable helix), but is not inconsistent with mechanisms where this switch helix, in the context of a complete riboswitch, remains unfolded in the absence of TPP due to alternative pairing with the expression platform (17–19).
The significant energy differences between most folding transitions (Fig. 2) may serve to prevent misfolding of the aptamer, which we never observed, and the relatively high stabilities of the P3 and P5 helices likely ensure correct nucleation of the TPP binding sites at J2/3 and J4/5. In particular, P3 is the first helix of the aptamer to be transcribed, and the ∼32 nt at its distal end, which are not required for TPP binding or L5-P3 docking (10, 20), participated in the highest-force folding transition in the aptamer (P3high). The P3high transition occurred separately from that involving the ∼12 nt at the base of the helix (P3low) (Table S1). The size of the P3 element varies greatly among species (6, 9), and its distal portion may have evolved as a “thermodynamic anchor” for the aptamer, ensuring correct folding by suppressing competing RNA structures incompatible with the riboswitch mechanism.
We determined the free energy change for complete unfolding of the bare aptamer (61 ± 3 kcal/mol) by measuring distributions of the work done to unfold and refold the aptamer under nonequilibrium conditions (Fig. 1C, inset). This value closely matched the sum of energies to unfold the individual helices (66 ± 3 kcal/mol), suggesting that no strong tertiary interactions are present in the absence of TPP. Both these experimental values are somewhat greater than that estimated by the mfold program (∼53 kcal/mol) (21). The difference is likely attributable to Mg2+, which is not accounted for by mfold, but was present at 4 mM here. Previous structural studies using small-angle X-ray scattering (SAXS) have found that under similar conditions, the sensor helix arms are splayed apart (20), but the aptamer becomes partially compacted as magnesium levels are increased (20, 22). In light of our findings, this compactness may be due to divalent cations stabilizing these helices without participating in energetically significant contacts between them.
Whether the TPP aptamer is “preorganized,” that is, whether it contains secondary or tertiary structure to facilitate ligand binding, has garnered controversy. Upon solving the crystal structure of the TPP-bound aptamer from A. thaliana, Thore, et al. conjectured that J2/4 and P1 do not form until TPP binds and bridges the two sensor helices (10). Lang, et al. presented kinetic data in support of this conjecture for the shorter Escherichia coli thiM aptamer, but could not rule out the possibility that the P1 helix was paired prior to TPP binding (16). In a calorimetric study, Kulshina, et al. observed diminishing enthalpic gains and entropic penalties for TPP binding to the same aptamer as the magnesium concentration was raised, and therefore suggested that magnesium induces preorganization (5). However, the authors did not demonstrate that this preorganization included any tertiary structure. Most recently, Steen, et al. chemically modified the thiM RNA in the absence of ligand and observed a pattern of protections indicative of fully formed secondary structure, but no tertiary structure (23). Our data are consistent with all the data (but not necessarily all the interpretations) in these prior studies, as well as with the SAXS studies mentioned above, and argue that any preorganization of the A. thaliana thiC aptamer involves the formation of helices P1-P5 and the juxtaposition of those helices due to stacking or primary connectivity.
In the presence of saturating TPP, larger, abrupt transitions (“rips”) were observed in force-extension curves (FECs) as the aptamer was unfolded (Fig. 3A). Worm-like chain (WLC) functions fitted to the appropriate portions of each curve revealed that 109 ± 6 nt are released in these rips, which equals the number measured upon unfolding all helices in the absence of TPP (Fig. 1C, Table S2) and the total length of the aptamer. This equivalence suggests that the aptamer unfolds monolithically upon TPP dissociation when force is increased slowly. As the aptamer was gradually refolded near equilibrium in the presence of TPP, the folding behaviors of the P3, P4, and P5 helices were unperturbed (Table S1), but the free, reversible folding of the P2 and P1 helices was interrupted by an event, presumed to be TPP binding, that locked the aptamer into its fully folded state (Fig. 3A). Refolding FECs appeared identical regardless of whether TPP was present (Fig. 1C, Fig. 3A). All together, these observations indicate that TPP binds concomitantly with P1 folding. Analyses of the rip-force distributions were carried out using the methods developed by Dudko (24) and Maitra (25), which supplied the heights and locations of the transition energy barriers associated with the rips, and placed the barrier for TPP dissociation at a distance of 12 ± 1 nt from the fully folded state of the aptamer (Table S2). This distance corresponds closely to the length of the P1 helix.
Fig. 3.
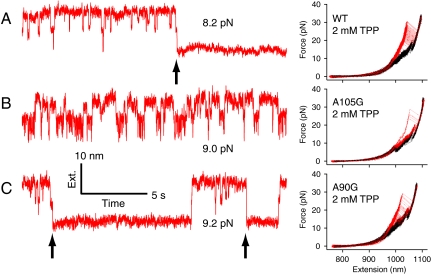
Effects of mutations on TPP binding. Left, records of extension vs. time for P1 and P2 folding under constant forces (as indicated) for aptamers in the presence of TPP, showing presumed TPP binding events (black arrows): (A) WT plus 2 μM TPP, (B) A105G plus 2 mM TPP, and (C) A90G plus 2 mM TPP. Right, FECs of WT, A105G, and A90G aptamers (150 cycles each) in the presence of 2 mM TPP, showing transitions (red, unfolding; black, refolding).
We determined the additional energetic stability conferred by TPP binding, including any tertiary contacts formed, by comparing the free energies for aptamer unfolding in the presence and absence of TPP, computed from the work distributions (Fig. 1C, Table S2, Fig. S2). The difference, 17 ± 5 kcal/mol, is comparable to the value of 11 ± 1 kcal/mol measured calorimetrically for the thiM aptamer from E. coli (5). Because ligand binding is closely correlated with the folding of the P1 helix in our experiment, we interpreted this difference to correspond to the additional stabilization imparted to the fully folded state of the aptamer (Fig. 2, inset).
This additional stabilization could be abolished by mutating the conserved adenine residue A105 in the central junction to guanine, corroborating findings from previous studies (4, 18). When present at saturating concentrations, TPP bound the A105G aptamer in just 2% of repeated nonequilibrium folding cycles (Fig. 3B), compared with 98% for the WT. Moreover, the folding behavior of the A105G-P2 and P1 helices at equilibrium was unaffected by TPP levels (Table S1). However, P1 folded faster in the A105G aptamer than WT in the absence of TPP, and involved 6 ± 2 fewer nucleotides, consistent with the P1 helix fraying, due to a shift in the registration of the 5′ and 3′ strands (Fig. S3). Such a misregistration is, in fact, predicted by mfold, and serves to explain the lower affinity of the mutant for TPP. In the WT aptamer, the A105 residue is unpaired and participates in several tertiary contacts across J2/4 (10). These contacts would be broken were this residue to pair in a misregistered P1 helix, disfavoring the orientation of the sensor helices required to bind TPP.
In addition to tertiary contacts formed in J2/4, tertiary contacts form between the L5 loop and P3 helix when TPP is bound (10). Mutation of certain L5 residues prevents TPP-induced compaction in the E. coli aptamer (5). Here, we observed that mutating A90 to G reduced the TPP-induced stabilization of the fully folded state by ∼30%, to 12 ± 5 kcal/mol (Fig. 2, inset; Fig. 3C). The reconstructed energy landscapes for the WT and A90G aptamers did not differ significantly in the absence of TPP (the sum of A90G helix energies is 66 ± 3 kcal/mol; Fig. S4), but in the presence of TPP and near the equilibrium folding forces for the P2 and P1 helices, ligand bound to the A90G aptamer only transiently (Fig. 3C), dissociating on a time scale of seconds, rather than hours.
We also acquired FECs (Fig. S2) and constant-force data (Fig. S5) for the WT aptamer binding to the substrates thiamine (T) and thiamine monophosphate (TMP). These alternative ligands carry fewer phosphates than TPP and are unable to bind the P4/5 arm via native, magnesium-mediated contacts (19, 26–28). Nevertheless, the WT aptamer displayed similar energetic stabilization by either T or TMP, when present at saturating concentrations, as did the A90G aptamer bound to TPP (Fig. 2, inset; Table S2). In addition, the WT aptamer also bound T or TMP transiently as P1 folded at equilibrium (Fig. S5). These characteristics support the existence of a common, weak-binding state for the aptamer that either lacks full coordination of TPP at its binding sites, or lacks productive L5-P3 docking around TPP.
We obtained corroborating evidence for such a state by probing the kinetics of TPP binding to the WT aptamer at subsaturating ligand concentrations. By unfolding each molecule under nonequilibrium conditions and then relaxing the force rapidly, we measured the probability that refolding and subsequent TPP binding would occur after a given interval between unfolding cycles, signaled by a TPP-dependent rip. We observed two populations of rip forces (Fig. 4 A and B) with high and low mean values, comparable with those measured for dissociating TPP from the WT and A90G aptamers, respectively, at saturating ligand concentrations (Fig. 3 A and C). The probabilities for observing a TPP-dependent rip at any force (Fig. 4C) and for observing the rip at high force (Fig. 4D) both increased with the refolding time and TPP concentration. These probabilities were globally fit by a minimal, four-state kinetic model (Fig. 4D) that collapses all helix-folding transitions into a single irreversible, fast step and incorporates two TPP-bound states, representing weak, initial binding followed by strong, final binding. A ligand dissociation constant of 7 ± 1 × 10-8 M was calculated from the model, which is consistent with previous estimates (5, 18, 19). We propose that progression from weak to strong binding, which generally occurred when the WT aptamer was paired with TPP, but not when a mutant RNA sequence or alternative ligand was substituted, may serve as a final step in the folding process to ensure selectivity and the fidelity of riboswitch activation.
Fig. 4.
Kinetics of TPP binding. (A) Representative FECs (N = 38) showing TPP-dependent rips at high force (red) or low force (green), or lacking either kind of rip (blue). (B) Histograms (mean ± s.e.) of TPP-dependent rip forces determined after 1- or 10-s refolding times (10 μM TPP; N = 100 rips per refolding time). (C) Probability (solid circles, mean ± binomial error) that TPP is bound to the WT aptamer (indicated by a TPP-dependent rip) as a function of the refolding time for the TPP concentration indicated (color legend). (D) Probability (solid circles, mean ± binomial error) that TPP binding is strong (indicated by a rip at high force) as a function of the refolding time, and a minimal (four-state) kinetic model for binding, with both weak (F′) and strong (F′′) binding states (bottom). The legend (inset) shows the best-fit rate constants; datasets in (C) and (D) were globally fitted (solid lines; colored by TPP concentration as in (C)).
Taken all together, our single-molecule data suggest that secondary structure forms quickly in the TPP riboswitch aptamer, and that it does so independent of ligand binding, whereas tertiary structure forms comparatively slowly, and does so in concert with ligand binding. We note that we could only indirectly observe ligand-dependent juxtaposition of the P2/3 and P4/5 helix arms, and L5-P3 docking, because these tertiary folding events lack a projection along our reaction coordinate, the molecular end-to-end extension (Figs. 1, 2). Therefore, we cannot strictly rule out the possibility that some minor amount of tertiary structure may form in the absence of ligand. Nevertheless, the hierarchical sequence of folding events that we observed stands in stark contrast to that of the adenine riboswitch aptamer (15), and it highlights key differences between folding in Type I (e.g., adenine) and Type II (e.g., TPP) riboswitches (13). In our experiments, productive TPP binding generally required P1 helix folding, but under some conditions TPP was observed briefly to remain strongly bound if P1 (and in rare cases, P2) became unpaired (Fig. S6) during unfolding. Such unpairing will weaken or disrupt J2/4, and therefore indicates that most of the energy for binding ligand arises from the interactions between the sensor helix arms, which TPP brings closer together.
In A. thaliana, TPP binding to the riboswitch aptamer promotes the formation of a longer, less stable thiC RNA, by causing a transcript processing site to be spliced out of the 3′ UTR (4, 7) (Fig. S7A). Ligand binding is thought to occur cotranscriptionally, and acts to prevent base-pairing between the 5′ splice site (located ∼150 nt upstream of the aptamer) and the P4/5 helix arm of the aptamer (7). Our data suggest that ligand binding cannot occur until the entire aptamer has been transcribed and the final elements of secondary structure (J2/4, P1) have formed. This arrangement would allow the 5′ strand of P4/5 to pair with, and sequester, the 5′ splice site immediately after transcription, thereby preventing splicing even at high TPP concentrations (Fig. S7B). Because splicing-incompetent configurations of the nascent 3′ UTR likely do not contain functional aptamers, they may represent kinetic traps, escape from which requires reconfiguration of base-pairing in the 3′ UTR, coupled with TPP binding. The 3′ splice site is situated within the aptamer, and its accessibility may change when TPP binds, as has been suggested (4). Based on our findings, this change is likely to occur as TPP binding progresses from weak to strong.
We anticipate that our study will serve as a starting point for a more complete biophysical picture of aptamer folding coupled to TPP binding and mRNA splicing in A. thaliana, and of TPP riboswitch function in other organisms. By extending the single-molecule methods applied previously to the folding of the adenine-binding pbuE aptamer (15), we directly measured the energetics, kinetics, and hierarchy of folding for an aptamer that is nearly twice as large. Several new approaches were employed to make these measurements, including: a “divide-and-conquer” strategy to assign individual folding events to the overall aptamer structure, the use of analytical methods from nonequilibrium thermodynamics to quantify the impact of ligand binding on the folding energy landscape, and use of point mutations and alternative ligands to perturb and elucidate folding. We expect single-molecule methods to be broadly applicable in future studies of additional riboswitches and structured RNAs.
Materials and Methods
Sample Preparation.
RNA constructs were transcribed in vitro and annealed to ∼kb-long dsDNA handles, each having a single-stranded overhang at one end and a biotin or digoxigenin modification at the other (see SI Methods). Dumbbells (29) were assembled by incubating the resulting construct with avidin- and anti-digoxigenin-coated polystyrene beads, and measured in 50 mM Hepes buffer containing (unless otherwise noted) 130 mM KCl and 4 mM Mg2+, plus trace components and ligand.
Instrument.
All data were acquired on a dual-beam optical-trapping microscope (30, 31), in which the trapping beams were steered using acousto-optical deflectors. Bead positions were detected using duolateral position-sensitive detectors.
Measurement of Nonequilibrium FECs with Hysteresis.
Global aptamer unfolding and refolding events were induced by repeatedly moving the traps apart and together, respectively, at rates of ∼60–200 nm/s. Rips were identified as local maxima in the unfolding FECs. Parameters describing the free energy barriers for folding transitions or ligand binding (Δx‡, ΔG‡, koff) were extracted from nonequilibrium distributions of rip forces (24, 25). The work corresponding to unfolding or refolding was calculated by integrating each FEC and subtracting the work of stretching the handles and fully unfolded RNA. The total free energy of unfolding (ΔG) was calculated from an analysis of work histograms (32).
Folding Energy Landscapes.
Equilibrium measurements conducted at constant force were carried out for the individual folding transitions in the full-length and truncated aptamer constructs. Each folding transition was analyzed as a two-state system, and the associated parameters describing the energetics and kinetics of folding (Δx, Δx‡, F1/2, and k1/2) were extracted (31). The set of folding transitions present in each construct, and values of the parameters describing each transition in this set, were compared among the constructs and correlated with the underlying sequence in order to assign transitions to specific helices. These parameters were then used as described (15) to reconstruct landscapes representing aptamer folding in the absence of ligand. Perturbations of the energy landscapes by ligand binding near the fully folded state were characterized using the parameters obtained from the analysis of nonequilibrium FECs.
TPP Binding Kinetics.
The frequency and strength of TPP binding to the WT aptamer were determined from FECs measured while moving the traps apart at ∼400 nm/s to induce unfolding. The TPP concentration and time between cycles were varied. TPP-dependent rips were identified by eye and classified as either low-force or high-force using the P3high transition as a threshold.
Supplementary Material
Acknowledgments.
We thank M. Ali, A. Sim, V. Chu, V. Pande, D. Herschlag, S. Doniach, and members of the Block lab for helpful comments. This work was supported by a National Science Foundation Graduate Research Fellowship (to P.C.A.) and a grant from the National Institute of General Medical Sciences (to S.M.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115045109/-/DCSupplemental.
References
- 1.Roth A, Breaker RR. The structural and functional diversity of metabolite-binding riboswitches. Annu Rev Biochem. 2009;78:305–334. doi: 10.1146/annurev.biochem.78.070507.135656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miranda-Rios J. The THI-box riboswitch, or how RNA binds thiamin pyrophosphate. Structure. 2007;15:259–265. doi: 10.1016/j.str.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Bocobza SE, Aharoni A. Switching the light on plant riboswitches. Trends Plant Sci. 2008;13:526–533. doi: 10.1016/j.tplants.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Bocobza S, et al. Riboswitch-dependent gene regulation and its evolution in the plant kingdom. Genes Dev. 2007;21:2874–2879. doi: 10.1101/gad.443907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulshina N, Edwards TE, Ferre-D’Amare AR. Thermodynamic analysis of ligand binding and ligand binding-induced tertiary structure formation by the thiamine pyrophosphate riboswitch. RNA. 2010;16:186–196. doi: 10.1261/rna.1847310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudarsan N, Barrick JE, Breaker RR. Metabolite-binding RNA domains are present in the genes of eukaryotes. RNA. 2003;9:644–647. doi: 10.1261/rna.5090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wachter A, et al. Riboswitch control of gene expression in plants by splicing and alternative 3′ end processing of mRNAs. Plant Cell. 2007;19:3437–3450. doi: 10.1105/tpc.107.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrick JE, Breaker RR. The distributions, mechanisms, and structures of metabolite-binding riboswitches. Genome Biol. 2007;8:R239. doi: 10.1186/gb-2007-8-11-r239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubodera T, et al. Thiamine-regulated gene expression of Aspergillus oryzae thiA requires splicing of the intron containing a riboswitch-like domain in the 5′-UTR. FEBS Lett. 2003;555:516–520. doi: 10.1016/s0014-5793(03)01335-8. [DOI] [PubMed] [Google Scholar]
- 10.Thore S, Leibundgut M, Ban N. Structure of the eukaryotic thiamine pyrophosphate riboswitch with its regulatory ligand. Science. 2006;312:1208–1211. doi: 10.1126/science.1128451. [DOI] [PubMed] [Google Scholar]
- 11.Batey RT, Gilbert SD, Montange RK. Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature. 2004;432:411–415. doi: 10.1038/nature03037. [DOI] [PubMed] [Google Scholar]
- 12.Serganov A, et al. Structural basis for discriminative regulation of gene expression by adenine- and guanine-sensing mRNAs. Chem Biol. 2004;11:1729–1741. doi: 10.1016/j.chembiol.2004.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montange RK, Batey RT. Riboswitches: emerging themes in RNA structure and function. Annu Rev Biophys Bio. 2008;37:117–133. doi: 10.1146/annurev.biophys.37.032807.130000. [DOI] [PubMed] [Google Scholar]
- 14.Garst AD, Batey RT, Edwards AL. Riboswitches: structures and mechanisms. Cold Spring Harb Perspect Biol. 2011;3:a003533. doi: 10.1101/cshperspect.a003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenleaf WJ, Frieda KL, Foster DA, Woodside MT, Block SM. Direct observation of hierarchical folding in single riboswitch aptamers. Science. 2008;319:630–633. doi: 10.1126/science.1151298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang K, Rieder R, Micura R. Ligand-induced folding of the thiM TPP riboswitch investigated by a structure-based fluorescence spectroscopic approach. Nucleic Acids Res. 2007;35:5370–5378. doi: 10.1093/nar/gkm580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croft MT, Moulin M, Webb ME, Smith AG. Thiamine biosynthesis in algae is regulated by riboswitches. Proc Natl Acad Sci USA. 2007;104:20770–20775. doi: 10.1073/pnas.0705786105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudarsan N, Cohen-Chalamish S, Nakamura S, Emilsson GM, Breaker RR. Thiamine pyrophosphate riboswitches are targets for the antimicrobial compound pyrithiamine. Chem Biol. 2005;12:1325–1335. doi: 10.1016/j.chembiol.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Winkler W, Nahvi A, Breaker RR. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature. 2002;419:952–956. doi: 10.1038/nature01145. [DOI] [PubMed] [Google Scholar]
- 20.Ali M, Lipfert J, Seifert S, Herschlag D, Doniach S. The ligand-free state of the TPP riboswitch: a partially folded RNA structure. J Mol Biol. 2010;396:153–165. doi: 10.1016/j.jmb.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baird NJ, Ferre-D’Amare AR. Idiosyncratically tuned switching behavior of riboswitch aptamer domains revealed by comparative small-angle X-ray scattering analysis. RNA. 2010;16:598–609. doi: 10.1261/rna.1852310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steen KA, Malhotra A, Weeks KM. Selective 2′-hydroxyl acylation analyzed by protection from exoribonuclease. J Am Chem Soc. 2010;132:9940–9943. doi: 10.1021/ja103781u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudko OK, Hummer G, Szabo A. Intrinsic rates and activation free energies from single-molecule pulling experiments. Phys Rev Lett. 2006;96:108101. doi: 10.1103/PhysRevLett.96.108101. [DOI] [PubMed] [Google Scholar]
- 25.Maitra A, Arya G. Influence of pulling handles and device stiffness in single-molecule force spectroscopy. Phys Chem Chem Phys. 2011;13:1836–1842. doi: 10.1039/c0cp01528h. [DOI] [PubMed] [Google Scholar]
- 26.Edwards TE, Ferre-D’Amare AR. Crystal structures of the thi-box riboswitch bound to thiamine pyrophosphate analogs reveal adaptive RNA-small molecule recognition. Structure. 2006;14:1459–1468. doi: 10.1016/j.str.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Noeske J, Richter C, Stirnal E, Schwalbe H, Wohnert J. Phosphate-group recognition by the aptamer domain of the thiamine pyrophosphate sensing riboswitch. Chembiochem. 2006;7:1451–1456. doi: 10.1002/cbic.200600151. [DOI] [PubMed] [Google Scholar]
- 28.Thore S, Frick C, Ban N. Structural basis of thiamine pyrophosphate analogues binding to the eukaryotic riboswitch. J Am Chem Soc. 2008;130:8116–8117. doi: 10.1021/ja801708e. [DOI] [PubMed] [Google Scholar]
- 29.Woodside MT, et al. Direct measurement of the full, sequence-dependent folding landscape of a nucleic acid. Science. 2006;314:1001–1004. doi: 10.1126/science.1133601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuman KC, Block SM. Optical trapping. Rev Sci Instrum. 2004;75:2787–2809. doi: 10.1063/1.1785844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodside MT, et al. Nanomechanical measurements of the sequence-dependent folding landscapes of single nucleic acid hairpins. Proc Natl Acad Sci USA. 2006;103:6190–6195. doi: 10.1073/pnas.0511048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirts MR, Bair E, Hooker G, Pande VS. Equilibrium free energies from nonequilibrium measurements using maximum-likelihood methods. Phys Rev Lett. 2003;91:140601. doi: 10.1103/PhysRevLett.91.140601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.