Abstract
The epithelial growth factor receptor plays an important role in cell migration and cancer metastasis, but the underlying molecular mechanism is not fully understood. We show here that differential regulation of the rhodopsin-GTPase-activating (Rho-GAP) activity of deleted in liver cancer 1 (DLC1) by tensin3 and COOH-terminal tensin-like protein (cten) controls EGF-driven cell migration and transformation. Tensin3 binds DLC1 through its actin-binding domain, a region that is missing in cten, and thereby releases an autoinhibitory interaction between the sterile alpha motif and Rho-GAP domains of DLC1. Consequently, tensin3, but not cten, promotes the activation of DLC1, which, in turn, leads to inactivation of RhoA and decreased cell migration. Depletion of endogenous tensin3, but not cten, augmented the formation of actin stress fibers and focal adhesions and enhanced cell motility. These effects were, however, ablated by an inhibitor of the Rho-associated protein kinase. Importantly, activation of DLC1 by tensin3 or its actin-binding domain drastically reduced the anchorage-independent growth of transformed cells. Our study therefore links dynamic regulation of tensin family members by EGF to Rho-GAP through DLC1 and suggests that the tensin-DLC1-RhoA signaling axis plays an important role in tumorigenesis and cancer metastasis, and may be explored for cancer intervention.
Cell migration is a complex process involving dynamic changes in the actin cytoskeleton, focal adhesion, cell matrix, cell–cell adhesion, and extra- and intracellular signal transduction (1). The rhodopsin (Rho) family small GTPases play a critical role in extracellular signal-regulated cytoskeletal changes and cell migration (2). The family is composed of 20 mammalian members, with RhoA, Rac1, and Cdc42 being the most intensively studied and best characterized (3). A small GTPase cycle between the GTP-bound active and GDP-bound inactive states, with their interconversion catalyzed by guanine nucleotide exchange factors (or GEFs), which promotes GDP exchange for GTP, and by GTPase activating proteins (or GAPs), which speed up GTP hydrolysis.
Deleted in liver cancer 1 (DLC1) is a Rho-specific GAP that is recognized as a tumor suppressor and a regulator of cell proliferation and migration (4). Reduced expression of DLC1 is associated with most solid tumors (5, 6). Restoration of DLC1 expression in the liver, breast, or lung cancer cells inhibits cell growth (7–9). DLC1 knockdown was shown to promote hepatocellular carcinoma in mice and that reintroduction of DLC1 into hepatoma cells suppressed tumor growth in situ (10). DLC1 features a sterile alpha motif (SAM) domain (11), a catalytic Rho-GAP domain (12), and a steroidogenic acute regulatory protein-related lipid transfer (START) domain implicated in lipid binding (13). DLC1 functions as a negative regulator of the Rho small GTPase family and promotes the hydrolysis of GTP-bound Rho (in particular RhoA), and, to a lesser extent, Cdc42, but not Rac (7, 14). The full tumor suppressive function of DLC1 requires its Rho-GAP activity as well as its localization to focal adhesions controlled by tensins (14–16).
Tensins are a family of focal adhesion proteins that play important roles in signal transduction and cytoskeletal reorganization (17). There are four members in the family, namely tensin1, 2, 3, and 4 (or COOH-terminal tensin-like protein, cten), each containing an Src homology (SH) 2 and a phosphotyrosine-binding (PTB) domain that allows it to interact with the cytoplasmic tail of the β-integrins (14). Except for cten, the other tensins contain an actin-binding domain (ABD)/focal adhesion-binding region (17). Tensins have been shown to localize DLC1 to integrin-mediated focal adhesions through either or both of the PTB and SH2 domains (14–16). Although deregulation of tensin expression is frequently associated with cancer (18–20), the roles of the different tensins in tumorigenesis and metastasis remain largely undefined and, in some cases, controversial. Whereas all four family members are down-regulated in human kidney cancers (21), elevated cten expression was correlated with thymoma and lung cancer progression (18, 20). In invasive breast cancer, cten expression correlates with high epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 levels and metastasis to lymph nodes (22). Both tensin1 and tensin3 have been shown to regulate cell migration and invasive behavior (21, 23).
The EGFR plays a critical role in cell growth and migration, and its deregulation is associated with oncogenesis and cancer metastasis (24). A recent study identified EGF as an important regulator of dynamic tensin expression. EGF treatment of epithelial cells was shown to decrease tensin3 (or tns3), but increase cten expression (22). Although this finding links the dynamic expression of tensin family members to cell migration induced by EGF, the corresponding signaling pathway and molecular mechanism have not been elucidated. We show here that both tensin3 and cten signal through DLC1 to control RhoA activity and cell migration. They, however, exhibit distinct effects on DLC1. We identified a unique interaction between tensin3 and DLC1 that serves to release an autoinhibitory interaction in DLC1 and thereby increase its Rho-GAP activity. We further demonstrated that EGF-driven cell migration and cellular transformation is dependent on the inhibition of DLC1. Our work suggests that DLC1 is a therapeutic target and that future cancer treatment modalities should consider DLC1 together with the amplification of EGFR or a related receptor.
Results
An EGF-Induced Tensin3/Cten Switch Controls RhoA Activation and Trafficking.
To elucidate the mechanism of EGF-induced mammary cell migration, we first confirmed that the mRNA levels for tensin3 and cten indeed change with EGF treatment in MCF10A cells (22). Using real-time PCR, we showed that EGF (10 ng/mL) stimulation led to a decrease in the tensin3 and a concomitant increase in the cten transcript (Fig. S1). Because DLC1 is a Rho-specific GAP capable of binding tensins (14, 15), we suspected that DLC1 might be a target of EGF or the tensin3/cten switch. The former possibility was eliminated because EGF did not induce a significant change in the dlc1 transcript (Fig. S1). To test the latter possibility, we examined the interaction of DLC1 with tensin3 and cten in MCF10A cells where all three proteins are expressed (Fig. S2). Robust interactions between DLC1 and both tensins were observed (Fig. 1A).
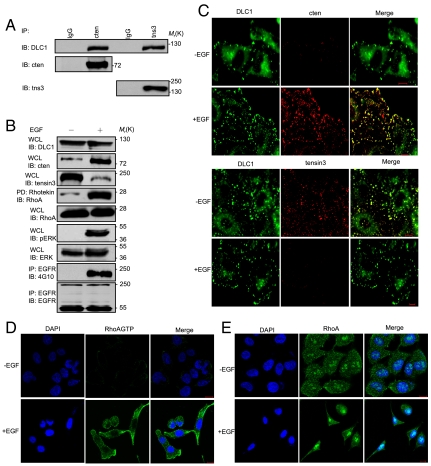
Fig. 1.
EGF induces a tensin3/cten switch that leads to RhoA activation via DLC1. (A) Endogenous DLC1 binds to tensin3 and cten in MCF10A cells. Tensin3 and cten were respectively immunoprecipitated from cell lysate and immunoblotted for DLC1 (Top). IgG, nonspecific IgG control. The blot was stripped and reprobed for cten (Middle) or tensin3 (Bottom). (B) The effect of EGF treatment on the protein levels of DLC1, cten, and tensin3 and on the activation of RhoA, ERK, and EGFR. Serum-starved MCF10A cells were incubated for 12 h with EGF (10 ng/mL). Immunoblots (IB) of the whole cell lysates (WCL) were used to assess the protein levels. Rhotekin-conjugated beads were used to pull down activated RhoA, followed by IB with anti-RhoA. ERK activation was examined using an antibody specific for phosphorylated ERK (p-ERK). EGFR activation was assessed by IB with 4G10 (anti-pTyr). (C) Colocalization of DLC1 with tensin3 or cten in MCF10A cells before or after EGF treatment. Confocal images were taken for serum-starved MCF10A cells before (-EGF) or after 24 h (+EGF) of EGF treatment. (D and E) Confocal immunofluorescence analysis of RhoA-GTP (D) and total RhoA (E) in serum-starved MCF10A cells before or after EGF treatment. (Scale bars: 10 μm.) Images shown are representative of at least three independent experiments.
Because DLC1 is capable of binding to either tensin3 or cten, we reasoned that the EGF-induced tensin3/cten switch might regulate RhoA activity through DLC1. To test this hypothesis, we examined whether EGF treatment would affect RhoA activation. Indeed, EGF induced a drastic change in RhoA-GTP, but not the total RhoA level (Fig. 1B). The marked increase in RhoA activation was not caused by differential expression of DLC1 because neither its mRNA nor protein level was altered by EGF (Fig. 1B and Fig. S1). Instead, it could be due to the dynamic changes in the tensin3 and cten levels (Fig. 1B). As expected, EGF treatment led to robust EGFR and ERK phosphorylation (22) (Fig. 1B).
In keeping with the biochemical data, confocal microscopy showed DLC1 colocalization with tensin3 in untreated cells, but with cten in EGF-treated cells (Fig. 1C). To further characterize the EGF-induced RhoA activation, we immunostained activated RhoA and total RhoA, respectively, using specific antibodies. Robust RhoA activation was seen in the EGF-treated MCF10A cells, but not in the untreated cells (Fig. 1D). Moreover, RhoA-GTP was found dispersed in the periphery of the cytosol or concentrated in areas of protrusions in cells that were apparently undergoing randomized migration (Fig. 1D, Lower), which is in stark contrast to the localization pattern of RhoA (Fig. 1E). A specific antibody detected RhoA in both the cytosol and the nuclei (as puncta) regardless of EGF treatment (Fig. 1E). Presumably the nuclear form of RhoA was GDP bound because it was undetectable by the RhoA-GTP-specific antibody (Fig. 1D). Therefore, EGF not only activates RhoA but mobilizes it from the nucleus to the cytosol or the plasma membrane.
Tensin3 and Cten Oppositely Regulate RhoA Activation via DLC1.
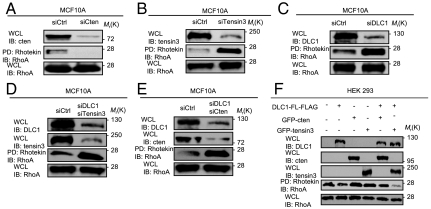
The correlation between the tensin3/cten switch and RhoA activation suggests that the two tensin family members may have distinct effects on RhoA activity. To interrogate this possibility, we deleted tensin3, cten, and DLC1, either individually or in combination, by siRNA-mediated knockdown in MCF10A cells or by overexpression (OE) in HEK293 cells deficient in these proteins (Fig. S2). Whereas the depletion of cten led to a marked reduction in RhoA activation, knockdown of tensin3 resulted in a drastic increase in the RhoA-GTP level in MCF10A cells (Fig. 2 A and B). The opposing effects of tensin3 and cten on RhoA activation are dependent on DLC1 because knockdown of DLC1 together with either had the same effect on RhoA activation as DLC1 knockdown alone (Fig. 2 C–E). These data indicate that the tensin3/cten switch impinges on DLC1 to control RhoA activation. This assertion was corroborated by data obtained from HEK293 cells. Whereas overexpression of DLC1 alone had a weak effect on RhoA inactivation, overexpression of either tensin3 or cten had little effect. Coexpression of cten and DLC1 also showed a negligible effect on RhoA activity. Intriguingly, coexpression of tensin3 and DLC1 led to a marked decrease in the RhoA-GTP level, indicating that tensin3 is an activator of the DLC1 GAP function (Fig. 2F).
Fig. 2.
Effects of cten, tensin3, and DLC1 knockdown or overexpression on RhoA activation. (A–E) Effects of siRNA-mediated depletion of cten, tensin3, and DLC1, individually or in combination, on RhoA activation in MCF10A cells. The efficiency of depletion was confirmed by the corresponding immunoblots (IB). RhoA activation was assessed by Rhotekin affinity pull-down followed by an anti-RhoA IB. Scrambled siRNA control, siCtrl. (F) Effects of overexpressing cten, tensin3, and DLC1, individually or in combination, on RhoA activity in HEK293 cells. HEK293 cells were transfected with indicated constructs and the corresponding whole cell lysates (WCL) were analyzed for RhoA activation. Data shown are representative of three independent experiments.
Tensin3 Activates DLC1 Rho-GAP Function by Releasing an Autoinhibitory Interaction.
It was recently shown that the GAP activity of DLC1 is controlled by its SAM domain that functions as an autoinhibitory switch (25). It is therefore likely that the binding of tensin3 to DLC1 releases this autoinhibition to enhance GAP function. To interrogate this possibility, we generated expression constructs that encoded, respectively, the full-length DLC1 (DLC1-FL), the SAM domain (DLC1-SAM), or a truncated version in which the SAM domain was deleted (i.e., DLC1∆SAM) (Fig. S3) and transfected each into HEK293 cells. Rhotekin pull-down assays were performed (14) in cells expressing the different proteins. Compared to DLC1-FL, which had a weak effect, the DLC1∆SAM exhibited a strong inhibitory effect on RhoA activation (Fig. 3A). Intriguingly, coexpression of the SAM domain with the DLC1∆SAM mutant completely reversed the effect of the latter on RhoA, indicating that the DLC1 Rho-GAP activity is inhibited by an intramolecular interaction involving its SAM domain (25) and that loss of this inhibition leads to enhanced GAP activity (Fig. 3A).
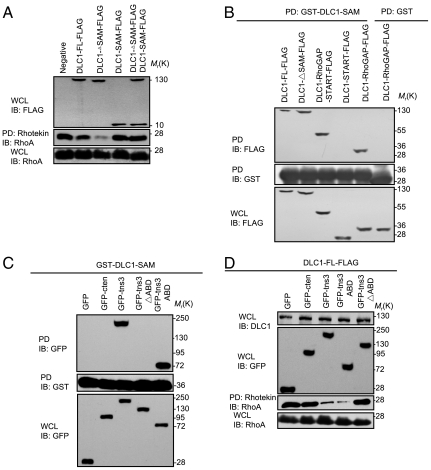
Fig. 3.
The tensin3 ABD binds to the SAM domain of DLC1 to activate its Rho-GAP function. (A) Effects of the FL and different segments of DLC1 on RhoA activation, respectively. HEK293 cells were transfected with indicated constructs and the corresponding whole cell lysates (WCL) were analyzed for RhoA activation. An aliquot of the WCL was subjected to immunoblot (IB) analysis with anti-FLAG and anti-RhoA to show the levels of the FLAG-tagged proteins and the total RhoA. (B) The SAM domain binds to the Rho-GAP domain in DLC1. HEK293 cells transfected with the indicated constructs were subjected to pull-down with glutathione beads coupled to GST-DLC1-SAM or GST alone, and the bound proteins were separated by SDS-PAGE and detected by IB with anti-FLAG (Top). The GST proteins were verified by probing the membrane with anti-GST antibody (Middle). The expression levels of the different constructs were assayed by an anti-FLAG blot (Bottom). (C) The DLC1 SAM domain binds to the tensin3 ABD domain. HEK293 cells transfected with the indicated constructs were subjected to pull-down with GST-DLC1-SAM. The bound proteins were detected in an anti-GFP blot. (D) The ABD domain of tensin3 is sufficient for activating DLC1. The FL or a truncated version of tensin3 (as GFP fusion) was coexpressed with DLC1-FLAG in HEK293 cells and the effect on RhoA activity was subsequently assessed. Similar levels of GFP proteins and total RhoA were confirmed by the corresponding immunoblots. All scanned films are representative of three independent experiments.
Our observation that exogenous SAM could reestablish the inhibition in the DLC1ΔSAM mutant suggests that the former may directly bind to the Rho-GAP domain. To test this possibility, we used purified GST-DLC1-SAM to pull down either DLC1 or a truncated version of the protein (ie., DLC1∆SAM, DLC1-Rho-GAP-START, DLC1-Rho-GAP, and DLC1-START) (Fig. S3). A specific interaction between the SAM and Rho-GAP domains was observed (Fig. 3B). Therefore, the DLC1 SAM domain binds directly to the Rho-GAP domain to inhibit its activity.
Because tensin3, but not cten, activates DLC1-Rho-GAP, and because the two tensins differ only in the N-terminal region (Fig. S3), we predicted that the ABD of the former would interact with the SAM domain to release the autoinhibitory interaction in DLC1. To test this prediction, we used purified GST-DLC1-SAM to pull down GFP-fused cten, tensin3, a tensin3 mutant with the ABD deleted (tns3∆ABD), or the ABD alone expressed in HEK293 cells. As seen in Fig. 3C, the SAM domain bound to FL and the ABD segment, but not to cten or the tns3∆ABD mutant, indicating that the tensin3 ABD is capable of binding to the DLC1 SAM domain. To examine whether this interaction is responsible for releasing the autoinhibition in DLC1, DLC1-FLAG was coexpressed, respectively, with cten, tensin3, or the tensin3-ABD or the tensin3∆ABD mutant. The resulting cell lysates were then subjected to pull-down by Rhotekin. We found that the effect of tensin3 on DLC1 activation, and subsequently, RhoA inactivation, was completely abolished by the deletion of the ABD (as in the tns3∆ABD mutant). In contrast, ABD alone exhibited the same effect as the FL tensin3 in activating DLC1 (Fig. 3D). Based on these data, we conclude that the ABD-SAM interaction competes for the intramolecular SAM-Rho-GAP interaction to enhance the GAP activity of DLC1, resulting in and decreasing the RhoA-GTP cellular level.
The Tensin3/Cten-DLC1 Interactions Differentially Regulate the Actin Cytoskeleton and Focal Adhesion.
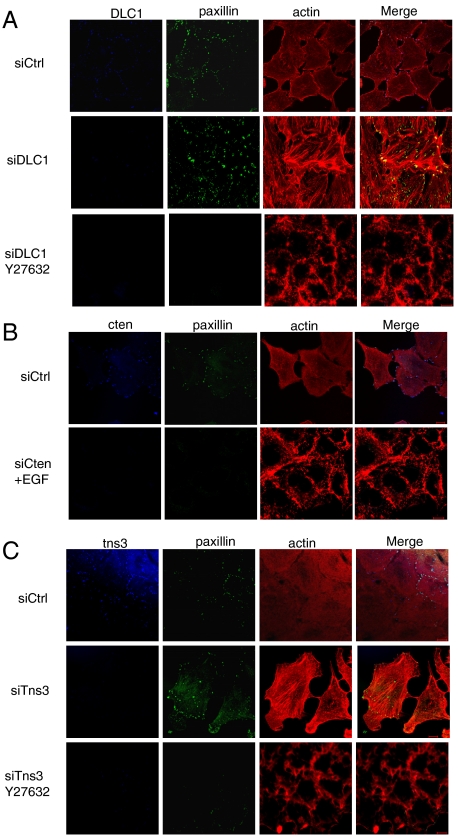
As DLC1 is a Rho-specific GAP, we anticipated that a change in its cellular level would affect both focal adhesion and stress fiber formation. Indeed, when DLC1 is depleted from MCF10A cells, a marked increase in focal adhesions (identified by paxillin staining) and actin stress fibers (visualized by phalloidin staining) were observed, compared to cells transfected with the control siRNA (Fig. 4A). Because Rho-associated protein kinase (ROCK) functions downstream of RhoA (26), we investigated whether its inhibition would alter focal adhesion and actin stress fiber formation. Indeed, a ROCK-specific inhibitor, Y27632, was able to abolish the effect of DLC1 depletion. In the presence of the inhibitor, both focal adhesion and actin staining were drastically weakened. The ROCK inhibitor also blocked the formation of actin stress fibers induced by EGF (Fig. S4), reinforcing the notion that EGF signals to RhoA to control reorganization of the cytoskeleton.
Fig. 4.
Effects of cten, tensin3, and DLC1 depletion on the formation of ROCK-mediated actin stress fibers and focal adhesions in MCF10A cells. (A) Depletion of DLC1 by siRNA resulted in a marked increase of actin stress fibers and focal adhesions, an effect that was eliminated by the addition of Y27632, a ROCK inhibitor (10 μM for 1 h). (B) Depletion of cten in EGF-treated (10 ng/mL) MCF10A cells led to decreases in stress fibers and focal adhesions. (C) Depletion of tensin3 in resting MCF10A cells led to enhanced levels of stress fibers and focal adhesions. The effect, however, was blocked by Y27632. Confocal microscopy images shown are representative of at least three independent experiments. (Scale bars: 10 μm.) Scrambled siRNA control, siCtrl.
Because tensin3 and cten exhibit distinct effects on DLC1 activity, we wondered whether the loss or gain of either tensin protein would alter focal adhesions and/or actin stress fibers. Depletion of cten abolished the focal adhesions even in cells treated with EGF, implying that cten is necessary for the formation of focal adhesions. Actin staining in these cells was restricted to the cell cortex (Fig. 4B). In contrast, knockdown of tensin3 led to a marked increase in focal adhesions and actin stress fibers—an effect that was abolished by Y27632 (Fig. 4C). To test whether ectopic expression of cten or tensin3 is sufficient to alter actin stress fiber formation, we transfected MCF10A cells with plasmids encoding GFP, GFP-tensin3 (FL), GFP-cten (FL), GFP-tensin3∆ABD, or GFP-tensin3-ABD. Although GFP alone did not show an apparent effect on the actin cytoskeleton structure, ectopic expression of tensin3 or tensin3-ABD led to shrinking and rounding of the cell, and the formation of thick cortical actin bundles (Fig. S5). In contrast, cells that expressed GFP-cten or GFP-tensin3∆ABD were characterized with an elongated morphology and more actin stress fibers formed across the cell body (Fig. S5). All these results demonstrate that tensin3 and cten differentially regulate focal adhesion and the actin cytoskeleton via DLC1.
The Tensin3/Cten-DLC1 Interactions Play Distinct Roles in Cell Migration.
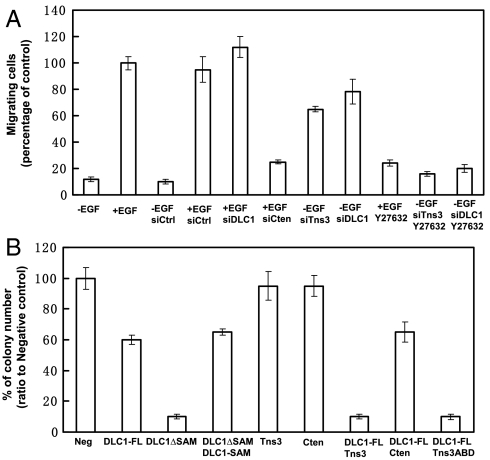
We next examined the motility, by the Boyden chamber cell migration assay (22), of MCF10A cells in which the cellular level of tensin3, cten, or DLC1 was altered. As expected, the rate of migration for the control cells was greatly enhanced by EGF treatment. However, EGF-induced cell migration was largely abolished with the knockdown of cten (Fig. 5A). In contrast, depletion of either tensin3 or DLC1 resulted in dramatic increases in cell migration even in the absence of EGF. These changes were, however, abolished by Y27632 (Fig. 5A). Together these data demonstrate that EGF promotes cell migration by controlling the cellular levels of tensin3 and cten which, in turn, control DLC1 Rho-GAP activity. The dependence of EGF-induced cell migration on the tensin3/cten-DLC1 pathway was confirmed in A549 cells that lack DLC1 (Fig. S2). Depletion of cten or tensin3 had negligible effect on the motility of these cells (Fig. S6).
Fig. 5.
The tensin3/cten-DLC1 signaling pathway regulates cell migration and anchorage-independent cell growth. (A) Tensin3 and cten regulates cell migration via DLC1. MCF10A cells were transfected with the indicated siRNA oligos to deplete the corresponding proteins and plated in transwell chambers containing full medium without or with EGF (10 ng/mL) and Y27632 (10 μM). Percentage of migrating cells relative to cells with EGF stimulation was presented. Data shown are mean ± SD, n = 9. (B) DLC1 plays a key role in cellular transformation. HEK293 cells were transfected with the indicated constructs and incubated for 21 d in soft agar. The number of colonies formed for cells transfected with a specific construct was compared to that of the negative control cells, and the resulting percentage number was presented, with error bars representing standard deviation (SD, n = 3). Scrambled siRNA control, siCtrl.
The Effect of the Tensin3/Cten-DLC1 Interactions on Cellular Transformation.
DLC1 is a tumor suppressor, but the role of tensin3 or cten in tumorigenesis is less well defined (18–23). To address whether the tensin3/cten-DLC1 complexes play a role in tumorigenesis, we examined ability of colony formation in soft agar by HEK293 cells that were made to express full-length or a truncated versions of DLC1, tensin3, or cten, individually or in various combination. Whereas the expression of DLC1 led to a significant reduction of the number of colonies formed, DLC1∆SAM essentially ablated colony formation in soft agar. As expected, coexpression of the SAM domain with DLC1∆SAM had the same effect as DLC1 itself, suggesting that activation of DLC1 is conducive to inhibition of tumor formation. Of note, although the expression of either tensin3 or cten alone had no effect, coexpression of tensin3 with DLC1 strongly inhibited colony formation. Importantly, coexpression of the ABD with DLC1 had the same inhibitory effect on anchorage-independent cell growth as coexpression of DLC1 and tensin3, which is in contrast to cten which showed no added effect on colony formation when it was coexpressed with DLC1 (Fig. 5B and Fig. S7). These results indicate that the tumor suppressive function of tensin3 is dependent on its ability to promote DLC1 Rho-GAP activity via the ABD domain. It is likely that the overall RhoA activity controlled by DLC1-tensin3/cten signaling axis underlie the transformation ability of HEK293 cells that overexpress these proteins.
Discussion
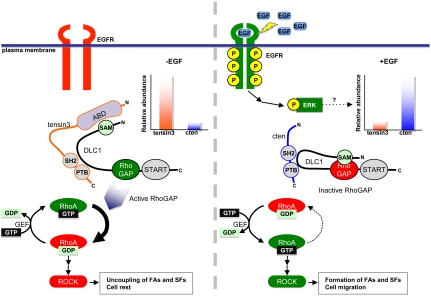
We show here that EGF-driven cell migration involves the dynamic expression of tensin3 and cten that differentially regulate DLC1 Rho-GAP activity (Fig. 6). Unstimulated cells are characterized with a higher level of tensin3 than cten (22). Whereas cten has no apparent effect, tensin3 activates the Rho-GAP activity of DLC1 through binding of its ABD to the SAM domain of the latter, thereby releasing an autoinhibitory interaction of DLC1. Activation of DLC1 results in rapid hydrolysis of RhoA-GTP to RhoA-GDP and the down-regulaiton of ROCK activity, ultimately leading to reduced stress fibers and the dissociation of focal adhesions, which are characteristic of resting cells. In the presence of EGF, however, the dynamics is reversed. Activation of the EGFR leads to enhanced phosphorylation and nuclear translocation of ERK. Phospho-ERK may function as the transcription factor to up-regulate the transcription of cten, but down-regulate tensin3. Cten subsequently replaces tensin3 to recruit DLC1 to the sites of focal adhesions but without activating its Rho-GAP function, allowing the accumulation of RhoA-GTP that is generated by a yet-to-be-identified GEF. Activated RhoA stimulates ROCK activity to drive the formation of actin stress fibers and focal adhesions, leading to cell migration (Fig. 6) (26). Although the current work focuses on the role of RhoA in EGF-induced cell migration, it should be pointed out that other signaling pathways such as the PI3K-Akt pathway may contribute to this process via regulating the activation of Rac and Cdc42 (27).
Fig. 6.
A model for the tensin3/cten-DLC1-RhoA signaling axis in regulating EGF-driven cell migration. Prior to EGF stimulation, mammary cells (such as MCF10A) express more tensin3 than cten. The Rho-GAP activity of DLC1 is inhibited by an intramolecular interaction with its SAM domain. However, binding of tensin3 ABD to the SAM domain releases this inhibition, resulting in an increase in DLC1 Rho-GAP activity, which, in turn, leads to a decrease in the RhoA-GTP level and destabilization of focal adhesions (FA) and stress fibers (SF). EGF treatment activates EGFR and ultimately, ERK. Phosphorylated ERK down-regulates tensin3, but concomitantly up-regulates cten expression. Without an ABD, cten is incapable of releasing the autoinhibition of DLC1 Rho-GAP activity, leading to increased RhoA activity and enhanced formation of focal adhesions and stress fibers, which together contributes to cell migration. The effect of the tensin3/cten- DLC1-RhoA signaling axis on cell migration depends on ROCK.
One of the most intriguing findings of our work is that EGF-induced tensin3/cten switch impinges on DLC1 to regulate mammary cell migration. Depletion of DLC1 by siRNA renders MCF10A cells irresponsive to EGF stimulated cell migration and accordingly, knockdown of tensin3 or cten has no effect on the migration of A549 cells that lack DLC1. Our study therefore identifies DLC1 as an important marker for metastatic cancer. Because DLC1 is a tumor suppressor frequently deleted in cancers (4), we suggest that deletion of DLC1 or dynamic expression of tensins with DLC1 predicts cancer outcome. For cancers in which both the EGFR family and the DLC1 are expressed, one would expect overexpression of cten and reduced expression of tensin1, 2, and 3 because they contain an ABD domain (Fig. S3). We found this expectation to be indeed the case for a cohort of human colorectal adenomas (28) in which normal levels of DLC1 and EGFR family receptors, but elevated cten and reduced tensin1 and tensin3 levels, are observed (Table S1). Similarly, we noticed elevated DLC1, ErbB2, and cten expression, but reduced tensin1, 2, and 3 expression, in a cohort of breast cancer stroma samples compared to the controls (29) (Table S2).
Because of the strict dependence of EGF-dependent mammary cell migration on DLC1, our study provides an alternative strategy for cancer intervention targeting DLC1 to conventional methods that aims to inhibit EGFR signaling (30). We show that DLC1 alone is not an effective tumor suppressor because it only moderately reduced colony formation in soft agar (Fig. 5B). However, expression of an active form of DLC1 (i.e., DLC1ΔSAM) or coexpression of an activator such as tensin3 essentially ablated anchorage-independent cell growth. Importantly, the ABD fragment of tensin3 had the same effect in inhibiting cellular transformation as the full-length protein. Aberrant activation of RhoA has been implicated in oncogenesis, and loss of DLC1 may lead to hyperactivation of RhoA, resulting in enhanced cell migration (31). Our study raises the possibility that the ABD or a smaller fragment that is capable of activating DLC1 may be harnessed to treat cancers associated with aberrant Rho activity (32, 33).
Experimental Procedures
RhoA Activation Assay.
RhoA activities were measured using the Rho Activation Assay Biochem Kit (Cytoskeleton). Cells at 40–60% confluency were treated with EGF (10 ng/mL) or transfected with the indicated siRNA or plasmids. After culturing for 24 h, the cells were washed with ice-cold PBS and lysed. Equal amounts of whole cell lysates were incubated with 20 μg GST-Rho-binding domain of Rhotekin beads for 1 h at 4 °C. The beads were washed three times with Washing Buffer, and the bound Rho proteins were analyzed by Western blots using an anti-Rho antibody (Cytoskeleton).
Confocal Microscopy.
MCF10A cells grown in 35-mm glass bottom dishes (P35G-1.0-14-C; MatTek) were transfected with appropriate expression constructs or siRNA oligos and incubated for 24 h at 37 °C in 5% CO2. Cells were then fixed as described previously (14). After rinsing with PBS, cells were incubated with anti-DLC1 rabbit or goat polyclonal (Santa Cruz, 1∶100), anti-tns3 rabbit (Sigma-Aldrich, 1∶200), or goat (Santa Cruz, 1∶100) polyclonal, anti-cten mouse monoclonal (Sigma-Aldrich, 1∶200), anti-paxillin rabbit polyclonal (Santa Cruz, 1∶100), anti-RhoA rabbit polyclonal (Santa Cruz, 1∶100) or antiactive RhoA-GTP mouse monoclonal antibodies (NewEast, 1∶200) for 2 h at room temperature. Samples were then incubated with the corresponding Alexa Fluor -405, -488, -546, or -633 conjugated secondary antibodies (Invitrogen-Molecular Probes, 1∶1,000) for 1 h followed by incubation with rhodamine phalloidin (Invitrogen, 1∶50) or DAPI (Calbiochem, 1∶1,000) for 30 min. Samples were visualized with the LSM 510 META or LSM 510 META/Confocor2 confocal microscope (Carl Zeiss MicroImaging).
Transwell Cell Migration Assay.
Transwell assay was performed as described previously (22). Photographs of three different fields of stained cells were captured using Motic AE30/31 Inverted Microscope (Motic Incorporation, Ltd.) with Infinity Capture Imaging system (Lumenera Corporation).
Colony Formation in Soft Agar.
HEK293 cells were transfected with indicated constructs by polyethylenimine and grown overnight. On the following day, cells were trypsinized and plated at a density of 1 × 104 cells in 0.25% agarose in DMEM (10% FBS), on top of 0.5% agarose in DMEM (10% FBS) in 60-mm dishes in triplicate. Cells were maintained at 37 °C in 5% CO2 for 21 d, and then stained overnight with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide. Colonies were counted and the numbers averaged.
Supplementary Material
Acknowledgments.
This work was supported by grants (S.S.-C.L.) from the Cancer Research Society, Inc. and the Canadian Cancer Society. S.S.-C.L. holds a Canada Research Chair in Functional Genomics and Cellular Proteomics.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114368109/-/DCSupplemental.
References
- 1.Sahai E. Mechanisms of cancer cell invasion. Curr Opin Genet Dev. 2005;15:87–96. doi: 10.1016/j.gde.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 3.Wennerberg K, Der CJ. Rho-family GTPases: It’s not only Rac and Rho (and I like it) J Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 4.Durkin ME, et al. DLC-1: A Rho GTPase-activating protein and tumour suppressor. J Cell Mol Med. 2007;11:1185–1207. doi: 10.1111/j.1582-4934.2007.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan BZ, Durkin ME, Popescu NC. Promoter hypermethylation of DLC-1, a candidate tumor suppressor gene, in several common human cancers. Cancer Genet Cytogenet. 2003;140:113–117. doi: 10.1016/s0165-4608(02)00674-x. [DOI] [PubMed] [Google Scholar]
- 6.Wong CM, Lee JM, Ching YP, Jin DY, Ng IO. Genetic and epigenetic alterations of DLC-1 gene in hepatocellular carcinoma. Cancer Res. 2003;63:7646–7651. [PubMed] [Google Scholar]
- 7.Goodison S, et al. The RhoGAP protein DLC-1 functions as a metastasis suppressor in breast cancer cells. Cancer Res. 2005;65:6042–6053. doi: 10.1158/0008-5472.CAN-04-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Thorgeirsson SS, Popescu NC. Restoration of DLC-1 gene expression induces apoptosis and inhibits both cell growth and tumorigenicity in human hepatocellular carcinoma cells. Oncogene. 2004;23:1308–1313. doi: 10.1038/sj.onc.1207246. [DOI] [PubMed] [Google Scholar]
- 9.Healy KD, et al. DLC-1 suppresses non-small cell lung cancer growth and invasion by RhoGAP-dependent and independent mechanisms. Mol Carcinog. 2008;47:326–337. doi: 10.1002/mc.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue W, et al. DLC1 is a chromosome 8p tumor suppressor whose loss promotes hepatocellular carcinoma. Genes Dev. 2008;22:1439–1444. doi: 10.1101/gad.1672608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao F, Bowie JU. The many faces of SAM. Sci STKE. 2005;2005:re7. doi: 10.1126/stke.2862005re7. [DOI] [PubMed] [Google Scholar]
- 12.Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003;13:13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 13.Ponting CP, Aravind L. START: A lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem Sci. 1999;24:130–132. doi: 10.1016/s0968-0004(99)01362-6. [DOI] [PubMed] [Google Scholar]
- 14.Qian X, et al. Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities. Proc Natl Acad Sci USA. 2007;104:9012–9017. doi: 10.1073/pnas.0703033104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao YC, Si L, deVere White RW, Lo SH. The phosphotyrosine-independent interaction of DLC-1 and the SH2 domain of cten regulates focal adhesion localization and growth suppression activity of DLC-1. J Cell Biol. 2007;176:43–49. doi: 10.1083/jcb.200608015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yam JW, Ko FC, Chan CY, Jin DY, Ng IO. Interaction of deleted in liver cancer 1 with tensin2 in caveolae and implications in tumor suppression. Cancer Res. 2006;66:8367–8372. doi: 10.1158/0008-5472.CAN-05-2850. [DOI] [PubMed] [Google Scholar]
- 17.Lo SH. Tensin. Int J Biochem Cell Biol. 2004;36:31–34. doi: 10.1016/s1357-2725(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki H, et al. Cten mRNA expression was correlated with tumor progression in lung cancers. Lung Cancer. 2003;40:151–155. doi: 10.1016/s0169-5002(03)00037-0. [DOI] [PubMed] [Google Scholar]
- 19.Lo SH, Lo TB. Cten, a COOH-terminal tensin-like protein with prostate restricted expression, is down-regulated in prostate cancer. Cancer Res. 2002;62:4217–4221. [PubMed] [Google Scholar]
- 20.Sasaki H, Yukiue H, Kobayashi Y, Fukai I, Fujii Y. Cten mRNA expression is correlated with tumor progression in thymoma. Tumour Biol. 2003;24:271–274. doi: 10.1159/000076141. [DOI] [PubMed] [Google Scholar]
- 21.Martuszewska D, et al. Tensin3 is a negative regulator of cell migration and all four Tensin family members are downregulated in human kidney cancer. PLoS One. 2009;4:e4350. doi: 10.1371/journal.pone.0004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz M, et al. A reciprocal tensin-3-cten switch mediates EGF-driven mammary cell migration. Nat Cell Biol. 2007;9:961–969. doi: 10.1038/ncb1622. [DOI] [PubMed] [Google Scholar]
- 23.Hall EH, Daugherty AE, Choi CK, Horwitz AF, Brautigan DL. Tensin1 requires protein phosphatase-1alpha in addition to RhoGAP DLC-1 to control cell polarization, migration, and invasion. J Biol Chem. 2009;284:34713–34722. doi: 10.1074/jbc.M109.059592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Kim TY, et al. Effects of structure of Rho GTPase-activating protein DLC-1 on cell morphology and migration. J Biol Chem. 2008;283:32762–32770. doi: 10.1074/jbc.M800617200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong CC, et al. Deleted in liver cancer 1 (DLC1) negatively regulates Rho/ROCK/MLC pathway in hepatocellular carcinoma. PLoS One. 2008;3:e2779. doi: 10.1371/journal.pone.0002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian Y, et al. ILK mediates actin filament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling. Oncogene. 2005;24:3154–3165. doi: 10.1038/sj.onc.1208525. [DOI] [PubMed] [Google Scholar]
- 28.Sabates-Bellver J, et al. Transcriptome profile of human colorectal adenomas. Mol Cancer Res. 2007;5:1263–1275. doi: 10.1158/1541-7786.MCR-07-0267. [DOI] [PubMed] [Google Scholar]
- 29.Finak G, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 30.Xia W, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 31.Kim TY, Vigil D, Der CJ, Juliano RL. Role of DLC-1, a tumor suppressor protein with RhoGAP activity, in regulation of the cytoskeleton and cell motility. Cancer Metastasis Rev. 2009;28:77–83. doi: 10.1007/s10555-008-9167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 33.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.