Abstract
The metabolic state of a cell is a key determinant in the decision to live and proliferate or to die. Consequently, balanced energy metabolism and the regulation of apoptosis are critical for the development and maintenance of differentiated organisms. Hypoxia occurs physiologically during development or exercise and pathologically in vascular disease, tumorigenesis, and inflammation, interfering with homeostatic metabolism. Here, we show that the hypoxia-inducible factor (HIF)-1–regulated glycolytic enzyme hexokinase II (HKII) acts as a molecular switch that determines cellular fate by regulating both cytoprotection and induction of apoptosis based on the metabolic state. We provide evidence for a direct molecular interactor of HKII and show that, together with phosphoprotein enriched in astrocytes (PEA15), HKII inhibits apoptosis after hypoxia. In contrast, HKII accelerates apoptosis in the absence of PEA15 and under glucose deprivation. HKII both protects cells from death during hypoxia and functions as a sensor of glucose availability during normoxia, inducing apoptosis in response to glucose depletion. Thus, HKII-mediated apoptosis may represent an evolutionarily conserved altruistic mechanism to eliminate cells during metabolic stress to the advantage of a multicellular organism.
Keywords: cell death, endogenous tolerance, fluorescence lifetime imaging microscopy-FRET, mitochondria, preconditioning
Balanced energy metabolism and regulation of apoptosis are of vital importance to all organisms (1, 2). Therefore, energy metabolism and regulation of apoptosis are interdependent (3). In cells, metabolism and apoptosis converge at mitochondria, thereby integrating pathways responsible for endogenous tolerance against substrate deprivation (4). All differentiated multicellular organisms have evolved strategies to promote survival when deprived of metabolic substrates, such as during hypoxia (5). Therefore, elucidating the underlying molecular mechanisms by which metabolism and apoptosis are coregulated may lead to novel therapeutic strategies for both acute and chronic diseases.
The transcription factor hypoxia-inducible factor (HIF)-1 is a key regulator in the adaptation to hypoxia and the resultant energy depletion, orchestrating the cellular response to hypoxic conditions (5–7). Induction of HIF-1 leads to the transcriptional regulation of a multitude of genes, ultimately resulting in a hypoxia-tolerant state of the cell (7). HIF-1 also links hypoxia and glycolysis (8) via complex and incompletely understood mechanisms. HIF-1 adapts cellular metabolism to hypoxic conditions during development (7) or exercise (9) and thereby prevents death of tumor cells and primary cells under various conditions of disease (5–7, 10). In addition, HIF-1 controls innate immunity by regulating glycolysis in cells of the immune system (11, 12). Finally, by controlling the expression of members of the glycolytic cascade, including hexokinase II (HKII) (7, 8), HIF-1 contributes to a proliferative metabolism (13).
Mitochondrial glycolytic hexokinase isoenzymes (HKI, HKII, or also HKIV) may mediate cytoprotection under various conditions (14–18). However, the molecular mechanisms have remained elusive. We therefore aimed to investigate the complex functional roles of mitochondrial HKs in the adaptive response to different states of metabolic deprivation during hypoxia and hypoglycemia.
Results
HIF-1–Dependent Activation of HKII in Primary Neurons and Hypoxia Tolerance.
First, we assessed whether mitochondrial hexokinases responded to a hypoxia-mimicking stimulus that activates HIF-1 transcriptional activity by interfering with degradation of HIF-1α (19). We treated cultured primary rat brain cortical neurons with the iron chelator deferoxamine (DFO), thereby mimicking hypoxia (19). DFO treatment resulted in marked protection from neuronal cell death after oxygen-glucose deprivation (OGD), an established model of cerebral ischemia (Fig. 1). At specified time points (Fig. 1A), we extracted RNA or protein from sister cultures to assess changes in gene expression for both HKI (Fig. 1B) and HKII (Fig. 1C). HKI is the predominating isoenzyme in the brain and HKII is typically expressed in insulin-sensitive tissues or in malignant tumors (20). However, messenger RNA (mRNA) expression of HKII was increased at 12 h after DFO treatment, whereas HKI expression did not change over 48 h. Immunoblotting of proteins from these cultures revealed a concordant increase in HKII protein content (Fig. 1D). Using isoenzyme-specific electrophoretic zymography, we further demonstrated a specific increase in HKII activity (factor of average increase = 2.28 ± 0.54), but not HKI activity in response to DFO treatment (Fig. 1 E and F). At 48 h after DFO treatment, neurons were protected from cell death, as indicated by decreased lactate dehydrogenase (LDH) release 24 h after OGD (Fig. 1G). We therefore hypothesized that up-regulation of HKII significantly contributes to hypoxia tolerance mediated by activation of HIF-1.
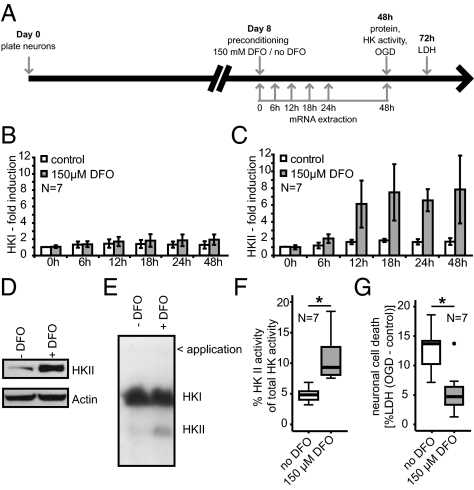
Fig. 1.
Hypoxia tolerance mediated by HIF-1–dependent activation of HKII in primary neurons. (A) Diagram of experimental paradigm. Analysis of HKI (B, E, and F) and HKII (C–F) expression in response to DFO treatment. (C) HKII expression was induced 12 h after hypoxia-mimicking treatment. After 48 h, (D) HKII protein and (E and F) enzyme activity were also increased. (B) mRNA expression and enzyme activity (E and F) of HKI remained unchanged. Representative immunoblot (D) and zymogram (E) 48 h after DFO treatment. (F) Summary of HKII activity using zymography after DFO treatment. *P = 0.007, unpaired two-tailed Student's t test. (G) DFO treatment reduced neuronal damage 24 h after OGD. *P = 0.006, unpaired two-tailed Student's t test. N indicates the number of independent experiments.
Overexpression of HKII Protects Primary Neurons from Hypoxic Cell Death.
To study the functional relevance of the increase in HKII expression and activity, we investigated the effect of overexpressed HKII in comparison with BclXL under hypoxic conditions. We chose BclXL as a positive control because of its strong antiapoptotic properties (21) and its role in preconditioning-induced neuroprotection (22). Transient transfection typically targets only a subset of cells (∼30% of neurons in a culture). Therefore, we visualized transfected cells using fluorescence after transfection of plasmids encoding fluorescent proteins (eGFP or mOrange). Cocultivation of transfected cells (Fig. 2 A and B, and Fig. S1) allowed us to examine the neuroprotective properties of transfected genes by counting green (overexpressing GFP alone or GFP together with either HKII or the positive control BclXL) and red (overexpressing mOrange only) fluorescent neurons before and after OGD under identical conditions. Overexpression of HKII resulted in marked protection from apoptosis in terminally differentiated neurons during cultivation (pre-OGD) (Fig. 2C) and mediated significant protection from cell death after exposure to OGD (post-OGD) (Fig. 2C). However, HKII mutants with diminished mitochondrial binding did not rescue neurons from apoptosis; instead, they promoted apoptosis in these cells (Fig. 2D). Both a mutant with a deletion of the N-terminal mitochondrial binding site (HKII∆N) and a phosphodeficient mutant (HKIIT473A), in which the Akt-kinase phosphorylation site was rendered nonphosphorylatable, exhibited a detrimental effect before and after OGD.
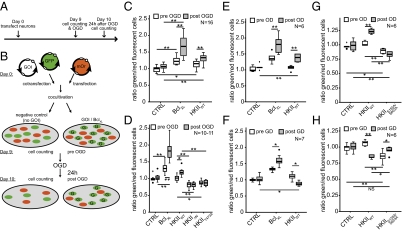
Fig. 2.
Mitochondrial HKII protects neurons from hypoxic cell death. We established an assay for investigating the functional impact of specific gene products (A and B). We cocultivated two independently transfected neuronal populations and used two different fluorescent protein (eGFP and mOrange) encoding plasmids to differentiate the two populations [cotransfected cells were eGFP + gene of interest (GOI), indicated by “G”]. We calculated ratios of the number of green and red fluorescent cells for each condition. A ratio of 1 means that equal numbers of green and red fluorescent cells survived; a ratio of 2 means that twice as many green as red fluorescent cells survived a damaging insult. Using this assay, we analyzed the effect of HKII on neuronal survival after OGD (C). The results demonstrated potent protection from cell death because of cultivation (pre-OGD) and in response to OGD (post-OGD). Mitochondrial binding (D) of HKII is essential for neuroprotection. Deletion of the mitochondrial binding site of HKII (HKII∆N) and nonphosphorylatable HKII (HKIIT473A) have a detrimental effect on survival. (E) HKIIWT protected neurons from OD, (F) but promoted cell death after GD. (G) Catalytically inactive HKIIS155A/S603A promoted cell death under OD, (H) but protected cells from detrimental GD. N indicates number of independent experiments. *P = 0.05, **P = 0.01, one-way ANOVA, Duncan post hoc.
HKII Senses the Metabolic State of the Cell and Regulates Cellular Fate Depending on its Catalytic Activity.
We next investigated whether HKII would protect only from hypoxia or also other from types of metabolic deprivation, such as glucose deprivation (GD). Indeed, overexpression of HKII resulted in protection from cell death induced by oxygen deprivation (OD) in neurons (Fig. 2E). However, when neurons were starved of glucose (GD) (Fig. 2F), HKII promoted cell death. Furthermore, overexpression of HKIIS155A/S603A, a mutant of wild-type HKII (HKIIWT) that is largely deficient of its catalytic activity (23), exhibited the opposite effect: it promoted cell death under baseline conditions (pre-OD/GD) (Fig. 2 G and H) and after OD (Fig. 2G), but offered a relative protection from cell death after GD (Fig. 2H), thereby blunting the detrimental effect of HKIIWT after GD. Taken together, these data suggest that the catalytic activity is required for HKII to sense the metabolic state of the cell, thereby protecting cells from hypoxic death but inducing cell death upon glucose starvation.
HKII Regulates Apoptotic Signaling After Metabolic Disturbance but Not After Genotoxic Injury.
Next, we investigated whether HKII would protect cells from apoptosis in general or only when cells were stressed by metabolic disturbances (e.g., hypoxia). Transfected HeLa cells were submitted to hypoxic conditions for 6 h (Fig. 3A) with 24 h of reoxygenation or for 21 h (Fig. 3B). Alternatively, apoptosis was induced by treatment with 100 nM actinomycin D (Fig. 3C) or 75 μM etoposide (Fig. 3D). Cell death was analyzed in transfected cells using flow cytometry. Although HKII rescued cells from apoptosis after hypoxia irrespective of severity (Fig. 3 A and B), it did not rescue cells after actinomycin D or etoposide treatment (Fig. 3 C and D). BclXL, used as positive control, mediated significant protection from cell death in both models. These data demonstrate that HKII regulates apoptosis after metabolic disturbances but not after genotoxic injury.
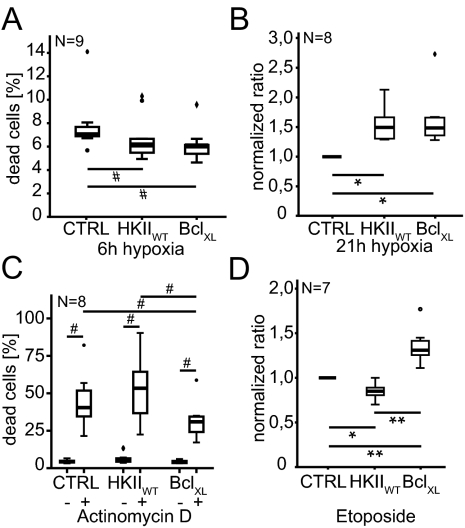
Fig. 3.
HKII protects cancer cells from death after hypoxia. HKIIWT rescued cancer cells (HeLa) from cell death after (A) short and (B) long periods of hypoxia similar to BclXL (positive control). HKIIWT did not rescue HeLa cells after induction of apoptosis by genotoxic treatment with (C) 100 nM actinomycin D or (D) 75 μM etoposide. *P = 0.05, **P = 0.01, one way ANOVA, Duncan post hoc; for A, #P = 0.004, Friedman test; for C, #P < 0.0001, Friedman test.
Identification of Phosphoprotein Enriched in Astrocytes as Direct Interactor of HKII.
To investigate the molecular mechanism that governs HKII-dependent regulation of apoptosis, we screened a mouse brain cDNA library for putative interactors of HKII using a membrane-based split-ubiquitin yeast two-hybrid system (Fig. 4A). Using this approach, we identified phosphoprotein enriched in astrocytes (PEA15) as a candidate for interaction with HKII. To investigate the regulation of PEA15, we first measured its expression after hypoxia-mimicking activation of HIF-1. In contrast to HKII (Fig. 1), PEA15 mRNA was not regulated under these conditions (Fig. S2). However, consistent with the interaction of HKII with PEA15 contributing to the antiapoptotic function of HKII at mitochondria, immunoblot analysis of cytosolic and mitochondrial fractions from primary cortical neurons demonstrated that PEA15 was located both in the cytosol and at mitochondria (Fig. 4B). Coimmunoprecipitation of HKII from primary cortical neurons revealed an interaction of endogenous HKII with PEA15 in neurons under physiological conditions (Fig. 4C). To determine whether HKII and PEA15 also interact in live cells, we used fluorescence lifetime imaging microscopy (FLIM) to quantify fluorescence resonance energy transfer (FRET) between monomeric mCerulean (donor) fused to HKII and the monomeric yellow fluorescent protein (YFP) mVenus (acceptor) fused to PEA15 (Fig. 4 D and E). We transfected human breast cancer (MCF-7) cells using plasmids encoding these fusion proteins. We then measured the fluorescence lifetime of the mCerulean FRET donor on a cell-by-cell basis using time-correlated single-photon counting (TCSPC) 24 h after transfection. The average lifetime of HKII-mCerulean in the presence of cytoplasmic (noninteracting) mVenus was 2.54 ns ± 0.15, which was significantly decreased to 2.31 ns ± 0.17 in the presence of PEA15-Venus, corresponding to a FRET efficiency of 9.1% (Fig. 4D). As controls, we also measured the average lifetimes of mCerulean expressed alone (2.58 ns ± 0.16) and HKII-Cerulean and mCerulean coexpressed with PEA15-Venus (2.54 ns ± 0.11 and 2.57 ns ± 0.12, respectively). These whole-cell measurements confirmed that the fluorescence lifetime of mCerulean was unchanged by the experimental conditions used (Fig. 4E) and that the lifetime reduction in the presence of PEA15-Venus was because of FRET. Determining the average fluorescence lifetime for whole-cell FRET analysis is unbiased and compensates for the low spatial resolution in the lifetime images (Fig. 4E, Id and IId) compared with the intensity images (Fig. 4E, Ib and Ic, and IIb and IIc). However, whole-cell measurements underestimate FRET efficiency because the interaction is expected to be confined to specific subcellular compartments (i.e., mitochondria), resulting in localized sites of low and normal lifetimes in each cell. Analysis of the data of the fluorescence lifetime images as corresponding histograms provided a more accurate demonstration of the interaction between HKII and PEA15 in the double-transfected cells. As expected, the fluorescence lifetime distributions overlapped in the analysis of single cells (Fig. 4E, histograms) and the entire cell population (Fig. 4D and Fig. S3). Nevertheless, coexpression of HKII-mCerulean and PEA15-Venus significantly reduced the global fluorescence lifetime of HKII-mCerulean compared with all control conditions, confirming that HKII and PEA15 interact in live cells. Furthermore, we investigated whether HKIIT473A, which cannot bind to mitochondria, interacts with PEA15. The average lifetime of HKIIT473A-mCerulean coexpressed with PEA15-Venus was 2.23 ns ± 0.15, corresponding to a FRET efficiency of 12.2% (Fig. S4), indicating that this mutant HKII interacts with PEA15 to a similar extent as HKIIWT. In summary, with our findings in the cell-survival assay (Fig. 2D), these data suggest that the complex of HKII and PEA15 has to be anchored at mitochondria to prevent cell death.
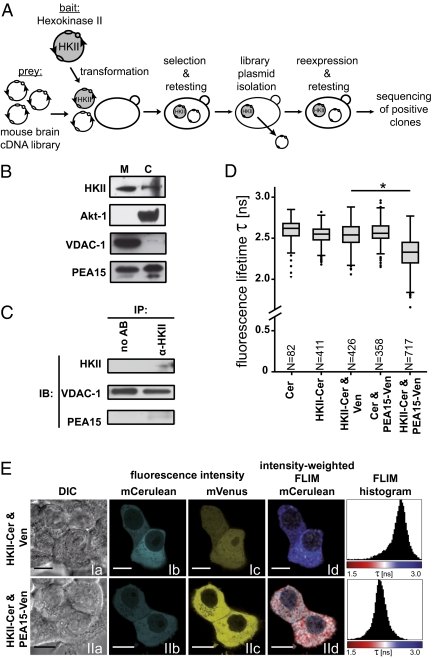
Fig. 4.
Identification and characterization of PEA15 as a molecular interactor of HKII. (A) Using HKII as bait, we screened it against a mouse brain cDNA library as prey in a membrane-based yeast two-hybrid screen. In this way, we identified PEA15 as a putative interactor of HKII. (B) HKII and PEA15 are both present in mitochondrial fractions from primary neurons. (C) HKII coimmunoprecipitation after cell lysis under mild conditions demonstrated the interaction of endogenous HKII and PEA15 in primary neurons. (D and E) Whole-cell FLIM-FRET analysis in MCF-7 cells demonstrated the interaction of HKII and PEA15 in live cells. (D) Fluorescence lifetime (τ) in the interacting condition (HKII-Cer and PEA15-Venus coexpression) was significantly reduced compared with all control conditions, corresponding to a FRET efficiency (E%) of 9.1%. (E) (a) DIC; (b and c) fluorescence intensity; (d) intensity-weighted fluorescence lifetime image and fluorescence lifetime histogram across entire cell. N represents number of cells imaged. (Scale bar: 10 μm.) *P < 0.0001, one-way ANOVA, Tukey-HSD post hoc.
PEA15 Is Essential for HKII-Mediated Protection.
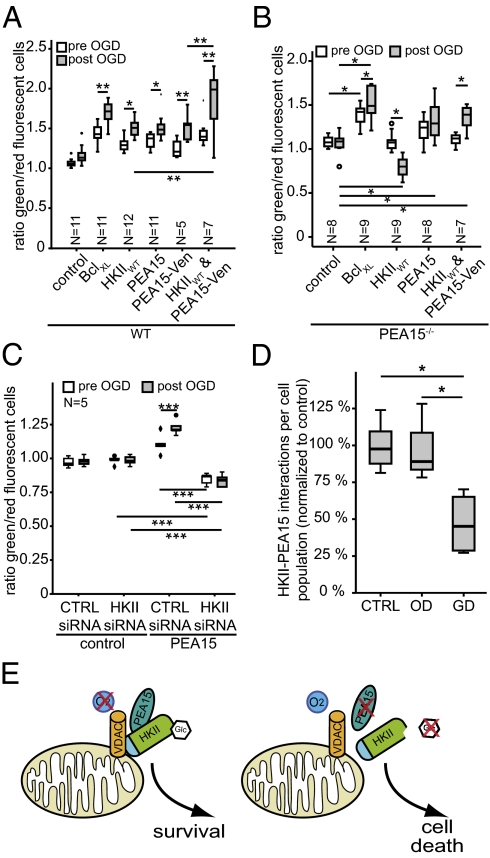
To investigate the functional implication of the HKII and PEA15 interaction, we transfected primary cortical neurons using plasmids encoding both proteins. Similar to HKII, PEA15 and PEA15-Venus were equally potent in mediating neuroprotection from a damaging OGD insult (Fig. 5A). However, consistent with our data showing a direct interaction between HKII and PEA15, coexpression of the proteins resulted in a synergistic protective effect on neuronal survival. The beneficial effect on survival was potentiated after neurons were exposed to OGD and allowed to recover for 24 h (Fig. 5A), indicating the functional relevance of this interaction for protection from hypoxic cell death. However, in primary cortical neurons from PEA15 knockout (PEA15−/−) mice, overexpression of HKII did not result in protection from cell death (Fig. 5B). In contrast to neurons from wild-type mice, HKII promoted neuronal cell death upon OGD. Exogenous expression of PEA15 protected PEA15−/− neurons and overexpression of HKII together with PEA15 reconstituted the protective effect of HKII in PEA15−/− neurons after OGD. Furthermore, knockdown of HKII using siRNA oligonucleotides abolished the protective effect of PEA15 and promoted neuronal cell death (Fig. 5C). Knockdown was specific for HKII and was confirmed by quantitative RT-PCR (Fig. S5). Taken together, these data demonstrate that PEA15 is essential for the antiapoptotic properties of HKII and in turn, that PEA15 cannot prevent cell death without HKII.
Fig. 5.
PEA15 is indispensable for HKII-mediated protection. (A) Overexpression of PEA15 protected wild-type neurons to a similar extent as HKII after OGD. Coexpression of HKII and PEA15-Venus resulted in a synergistic protective effect on neuronal survival in wild-type cells. (B) HKII overexpression induced cell death after OGD in PEA15−/− primary cortical neurons (HKIIWT). Following the reconstitution of PEA15 (HKIIWT and PEA15-Venus), overexpression of HKII again mediated significant protection from hypoxic cell death. PEA15 also mediated protection from OGD when overexpressed in PEA15−/− neurons. (C) Knockdown of endogenous HKII abolished protective effect of PEA15 overexpression in wild-type neurons. (D) HKII/PEA15 interaction is disturbed under GD but not under OD. Number of cells per group are indicated in Fig. S7. (E) HKII acts as a molecular switch controlling cellular survival depending on the metabolic state of the cell by sensing intracellular glucose levels. Under hypoxic conditions, mitochondrial HKII promotes cellular survival when PEA15 is present. Under normoxic conditions but in the absence of PEA15 or glucose, HKII induces cell death. Presumably, this interaction is important for antiapoptotic signaling under various physiological and pathophysiological conditions. N indicates number of independent experiments. *P = 0.05, **P = 0.01, ***P = 0.001, one-way ANOVA, Duncan post hoc.
Glucose Deprivation Decreases Interaction of HKII and PEA15.
Next, we investigated the mechanism of the regulation of cell death by HKII and PEA15. To determine the effect of OD or GD on the interaction of HKII and PEA15, we used proximity ligation assays (PLA) with minimal unspecific background (Fig. S6). We exposed HKII and PEA15 cotransfected neurons to sublethal OD, GD, or control conditions and determined the relative number of interactions on a cell-by-cell basis across all cells imaged using a software algorithm. This approach underestimates the effect of changes in transfected cells, as we did not differentiate transfected and nontransfected cells. We found that GD significantly decreased the interaction of HKII and PEA15 (Fig. 5D and Fig. S7). In particular, the population of cells with a high number of PLA signals per cell was decreased under GD (Fig. S7C). OD, however, did not alter the interaction between the two proteins (Fig. 5D and Fig. S7B). These data are consistent with the detrimental effect of GD on HKII-transfected neurons, suggesting that dissociation of HKII from mitochondria under GD (24) induces cell death alongside a disturbed interaction of HKII and PEA15.
Discussion
We found that the glycolytic enzyme HKII acts as a molecular switch, determining the fate of the cell depending on its metabolic state, requiring PEA15 to mediate protective signaling (Fig. 5E). A hypoxia-mimicking stimulus activated HKII and protected primary neurons from hypoxic damage. In the absence of PEA15, HKII accelerated apoptosis. This effect is reminiscent of the role of HKII under GD and normoxia, in which HKII induces cell death and the interaction between HKII and PEA15 is disturbed. Thus, the functional impact of the interaction between HKII and PEA15 depends on the availability of glucose. Furthermore, HKII relies on its capacity to phosphorylate glucose to regulate cell death in response to changes in the metabolic environment of the cell. Although apoptosis is crucial for development (2), postmitotic cells, such as neurons and cancer cells, have evolved mechanisms to overcome cell death because of substrate deprivation (1, 5, 13) and other apoptotic stimuli (25). Both HKII and PEA15 have functional roles in glucose metabolism (20, 26) and are involved in antiapoptotic signaling under multiple but not all (e.g., genotoxic) conditions.
We propose that the interaction of HKII and PEA15 has to occur at mitochondria and possibly within a larger multiprotein complex, to promote antiapoptotic signaling under various physiological and pathophysiological conditions. Our data suggest that its catalytic activity enables HKII to sense intracellular glucose levels and to initiate a program to kill cells depleted of glucose. In summary, we propose that mitochondrial binding of HKII, its capacity to phosphorylate glucose, and the degree of interaction with PEA15 are important features of HKII-dependent regulation of cell death upon metabolic disturbances. Hence, HKII is a crucial element in the decision between life and death, linking glucose metabolism to the regulation of cell death and cellular proliferation (1).
Glycolytic enzymes are crucial for cellular survival because they are essential to the energy supply of the cell (1, 27). However, additional functions of these enzymes, including roles for mitochondrial HKs and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), in controlling apoptosis have emerged (15, 16, 18, 27, 28). In the HK family, glucokinase (i.e., HKIV) binds to mitochondria by interacting with BCL-2 antagonist of cell death (BAD) in a multiprotein complex, enabling modulation of apoptosis in liver and pancreatic β-cells by BAD in response to changes in glucose levels (15). Our data demonstrate a critical role for the more ubiquitously expressed (17, 20), albeit regulated (17), isoenzyme HKII in mediating cytoprotection. HKII is also up-regulated in a variety of cancers (17) in which metabolism and cellular proliferation are dysregulated (29).
Our results are consistent with previous observations in cardiomyocytes and Rat1a fibroblasts that demonstrated the importance of mitochondrial binding of HKII for protection from apoptosis (16, 30, 31). Remarkably, postmitotic neurons and cancer cells share not only a metabolic reliance on oxygen and glucose, but also similarity in key mechanisms to constrain apoptosis (25). Thus, the mechanism by which HKII regulates cell death is likely to be similar in many cell types, including primary neurons and cancer cells.
We therefore conclude that HKII functions as a sentinel to control two different conditions: (i) hypoxia, in which adaptation of cellular metabolism and increased glycolysis provide a benefit for survival (7, 25); and (ii) aglycemia, in which apoptosis is rapidly induced in response to impeded metabolic flux to prevent nutrient depletion in the remaining tissue or organism (3, 32). HKII is well suited to function as a molecular sensor of the intracellular metabolic state given its high affinity (low Km) for glucose and potent inhibition by its product glucose-6-phosphate (20). Our data accord with previous evidence suggesting that for HKII to prevent apoptosis, it must localize to mitochondria by binding to the outer mitochondrial membrane voltage-dependent anion channel (VDAC) (33) after Akt-kinase–dependent phosphorylation (18, 30). Furthermore, forced displacement of HKII from VDAC by a peptide corresponding to its N terminus (34) and dissociation of HKII from mitochondria under glucose deprivation (24) increase cellular susceptibility to apoptosis (16, 35). Astoundingly, enteroinvasive and possibly other bacteria have conquered the hexokinase-VDAC binding for their own good and use stabilization of this interaction to delay host-cell apoptosis (36), thereby prototyping a pathway for exploitation of this interaction in infection therapy. Presumably, HKII is also up-regulated in inflamed tissue in a HIF-1–dependent fashion to enhance glycolysis-dependent mechanisms of host-defense (12).
Although originally discovered as a substrate of PKC in astrocytes (37), PEA15 is a multifunctional protein widely expressed in a variety of tissues. PEA15 is involved in the regulation of apoptosis, glucose metabolism, cellular proliferation, and oncogenesis (26, 37–41), and it has been suggested to contribute to insulin resistance in type 2 diabetes (26). Similar to HKII (17), up-regulation of PEA15 is an important feature of multiple malignant tumors (39). The mitochondrial localization of PEA15 reported above was unexpected because it has previously been described as a cytosolic protein, interacting with Fas-associated protein with death Domain (FADD) and FADD-like IL-1β–converting enzyme (FLICE) through its death effector domain (DED), thereby blocking the formation of the death-inducing signaling complex (DISC) (41). However, PEA15 mediates sensitivity to Bcl2-induced survival in cells undergoing mitochondria-dependent type-II apoptosis (42) and also interacts with the mitochondrial serine protease Omi/HtrA2 (43).
Although HKII and PEA15 fulfill distinct roles in glucose metabolism and apoptosis, we provide mechanistic insight into the interdependence of these two major pathways governing cellular fate. Our data help answer unresolved questions of how and why metabolic pathways, such as glycolysis and pathways governing cellular fate, communicate (1). The inhibition of apoptosis because of metabolic deprivation by the interaction of HKII and PEA15 and the initiation of apoptosis by HKII under glucose depletion or in the absence of PEA15 in neurons and cancer cells, two markedly different cell types, points to the ubiquitous relevance of balanced metabolism for survival. Hence, postmitotic and mitotic cells can use similar mechanisms to adapt to substrate deprivation and promote survival (25). Consequently, the interaction of HKII and PEA15 in a mitochondrial complex may represent a prototypic molecular decision maker that senses glucose availability and promotes cell survival during hypoxia but induces apoptosis under normoxia when glucose is not present.
Methods
Detailed methods are provided in SI Methods.
Cell Culture.
Primary neuronal cultures of cerebral cortex from rat embryos (embryonic day 18) or from PEA15−/− mouse embryos (embryonic day 15) were dissected and cultured for up to 9 d to ensure maturation of neurons as described (19). All animal procedures were performed in accordance with the standards for animal care of our institution, and permission was obtained from the Landesamt für Gesundheit und Soziales Berlin according to the national regulations.
Isoenzyme-Specific HK-Zymography.
To distinguish hexokinase isoenzymes, electrophoresis of extracts from preconditioned neurons was performed under native conditions.
Analysis of Neuronal Survival by Cotransfection/Cocultivation.
OGD was performed for 2.5–3 h, OD and GD for 5–6 h (Fig. 2). For analysis of neuronal survival, green and red fluorescent neurons were counted immediately before and 24 h after OGD, OD, or GD, survival of transfected neurons was assessed. Ratios of green and red fluorescent cells were calculated.
Preparation of Mitochondria.
Mitochondria from neurons were prepared by differential centrifugation.
Coimmunoprecipitation.
Neurons were washed, harvested, and lysed. Immunoprecipitation was performed using an antibody against HKII (Santa Cruz).
Fluorescence Resonance Energy Transfer.
Fluorescence resonance energy transfer (FRET) was measured by time-correlated single-photon counting (TCSPC) fluorescence lifetime imaging microscopy (FLIM-FRET). TCSPC raw data were analyzed with SPCimage (Becker and Hickl) and images further analyzed with ImageJ.
Statistical Evaluation.
Data are shown as mean ± SD. Statistical testing was performed in PASW Statistics 18.0.
Supplementary Material
Acknowledgments
We thank R. Gusinda, C. Muselmann, and M. Thielke for technical support; J. McNicol for experimental assistance; M. Grohmann and H. Chneiweiss for discussion and reagents; C. Harms and R. Tsien for plasmids; F. Fernández-Klett for discussion; S. Sigrist for microscope access; and the staff at Charité-Forschungseinrichtung für Experimentelle Medizin for animal breeding and handling. PEA15−/− mice were a kind gift from H. Chneiweiss. This work was supported by the European Union's Seventh Framework Programme (FP7/2008-2013) under Grant Agreements 201024 and 202213 (European Stroke Network); the Deutsche Forschungsgemeinschaft (NeuroCure Cluster of Excellence, Exc 257); the Bundesministerium für Bildung und Forschung (Center for Stroke Research Berlin, 01 EO 08 01); the Helmholtz Gemeinschaft (SO-022NG); grants from Sanofi and the Felgenhauer Foundation (to P.M.); and by Canadian Institutes of Health Grant FRN12517. D.W.A. holds the Canada Research Chair in Membrane Biogenesis. Support for the McMaster Biophotonics Facility was provided by grants from the Canada Foundation for Innovation and the Ontario Innovation Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.R.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108225109/-/DCSupplemental.
References
- 1.Buchakjian MR, Kornbluth S. The engine driving the ship: Metabolic steering of cell proliferation and death. Nat Rev Mol Cell Biol. 2010;11:715–727. doi: 10.1038/nrm2972. [DOI] [PubMed] [Google Scholar]
- 2.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 3.Plas DR, Thompson CB. Cell metabolism in the regulation of programmed cell death. Trends Endocrinol Metab. 2002;13:75–78. doi: 10.1016/s1043-2760(01)00528-8. [DOI] [PubMed] [Google Scholar]
- 4.Dirnagl U, Meisel A. Endogenous neuroprotection: Mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 6.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 8.Iyer NV, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Formenti F, et al. Regulation of human metabolism by hypoxia-inducible factor. Proc Natl Acad Sci USA. 2010;107:12722–12727. doi: 10.1073/pnas.1002339107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mergenthaler P, Dirnagl U. Protective conditioning of the brain: Expressway or roadblock? J Physiol. 2011;589:4147–4155. doi: 10.1113/jphysiol.2011.209718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer T, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Rathmell JC, et al. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danial NN, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 16.Majewski N, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 18.Gottlob K, et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prass K, et al. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro. J Cereb Blood Flow Metab. 2002;22:520–525. doi: 10.1097/00004647-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JE. Isozymes of mammalian hexokinase: Structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 21.Leber B, Lin J, Andrews DW. Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene. 2010;29:5221–5230. doi: 10.1038/onc.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyawaki T, et al. Ischemic preconditioning blocks BAD translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc Natl Acad Sci USA. 2008;105:4892–4897. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai HJ, Wilson JE. Functional organization of mammalian hexokinases: Both N- and C-terminal halves of the rat type II isozyme possess catalytic sites. Arch Biochem Biophys. 1996;329:17–23. doi: 10.1006/abbi.1996.0186. [DOI] [PubMed] [Google Scholar]
- 24.John S, Weiss JN, Ribalet B. Subcellular localization of hexokinases I and II directs the metabolic fate of glucose. PLoS ONE. 2011;6:e17674. doi: 10.1371/journal.pone.0017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10:1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Condorelli G, et al. PED/PEA-15 gene controls glucose transport and is overexpressed in type 2 diabetes mellitus. EMBO J. 1998;17:3858–3866. doi: 10.1093/emboj/17.14.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30:142–150. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Colell A, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 29.DeBerardinis RJ, et al. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- 31.Sun L, Shukair S, Naik TJ, Moazed F, Ardehali H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol Cell Biol. 2008;28:1007–1017. doi: 10.1128/MCB.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vander Heiden MG, et al. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40:171–182. doi: 10.1007/s10863-008-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 35.Majewski N, Nogueira V, Robey RB, Hay N. Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Mol Cell Biol. 2004;24:730–740. doi: 10.1128/MCB.24.2.730-740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sukumaran SK, et al. A soluble form of the pilus protein FimA targets the VDAC-hexokinase complex at mitochondria to suppress host cell apoptosis. Mol Cell. 2010;37:768–783. doi: 10.1016/j.molcel.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Danziger N, et al. Cellular expression, developmental regulation, and phylogenic conservation of PEA-15, the astrocytic major phosphoprotein and protein kinase C substrate. J Neurochem. 1995;64:1016–1025. doi: 10.1046/j.1471-4159.1995.64031016.x. [DOI] [PubMed] [Google Scholar]
- 38.Eckert A, et al. The PEA-15/PED protein protects glioblastoma cells from glucose deprivation-induced apoptosis via the ERK/MAP kinase pathway. Oncogene. 2008;27:1155–1166. doi: 10.1038/sj.onc.1210732. [DOI] [PubMed] [Google Scholar]
- 39.Fiory F, Formisano P, Perruolo G, Beguinot F. Frontiers: PED/PEA-15, a multifunctional protein controlling cell survival and glucose metabolism. Am J Physiol Endocrinol Metab. 2009;297:E592–E601. doi: 10.1152/ajpendo.00228.2009. [DOI] [PubMed] [Google Scholar]
- 40.Kitsberg D, et al. Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNFalpha-induced apoptosis. J Neurosci. 1999;19:8244–8251. doi: 10.1523/JNEUROSCI.19-19-08244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaidyanathan H, et al. ERK MAP kinase is targeted to RSK2 by the phosphoprotein PEA-15. Proc Natl Acad Sci USA. 2007;104:19837–19842. doi: 10.1073/pnas.0704514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peacock JW, et al. PTEN loss promotes mitochondrially dependent type II Fas-induced apoptosis via PEA-15. Mol Cell Biol. 2009;29:1222–1234. doi: 10.1128/MCB.01660-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trencia A, et al. Omi/HtrA2 promotes cell death by binding and degrading the anti-apoptotic protein ped/pea-15. J Biol Chem. 2004;279:46566–46572. doi: 10.1074/jbc.M406317200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.