Abstract
The yeast AGC kinase orthologs Ypk1 and Ypk2 control several important cellular processes, including actin polarization, endocytosis, and sphingolipid metabolism. Activation of Ypk1/2 requires phosphorylation by kinases localized at the plasma membrane (PM), including the 3-phosphoinositide-dependent kinase 1 orthologs Pkh1/Pkh2 and the target of rapamycin complex 2 (TORC2). Unlike their mammalian counterparts SGK and Akt, Ypk1 and Ypk2 lack an identifiable lipid-targeting motif; therefore, how these proteins are recruited to the PM has remained elusive. To explore Ypk1/2 function, we constructed ATP analog-sensitive alleles of both kinases and monitored global changes in gene expression following their inhibition, where we observed increased expression of stress-responsive target genes controlled by Ca2+-dependent phosphatase calcineurin. TORC2 has been shown previously to negatively regulate calcineurin in part by phosphorylating two related proteins, Slm1 and Slm2, which associate with the PM via plextrin homology domains. We therefore investigated the relationship between Slm1 and Ypk1 and discovered that these proteins interact physically and that Slm1 recruits Ypk1 to the PM for phosphorylation by TORC2. We observed further that these steps facilitate subsequent phosphorylation of Ypk1 by Pkh1/2. Remarkably, a requirement for Slm1, can be bypassed by fusing the plextrin homology domain of Slm1 alone onto Ypk1, demonstrating that the essential function of Slm1 is largely attributable to its role in Ypk1 activation. These findings both extend the scope of cellular processes regulated by Ypk1/2 to include negative regulation of calcineurin and broaden the repertoire of mechanisms for membrane recruitment and activation of a protein kinase.
The target of rapamycin (TOR) kinase is an evolutionarily conserved regulator of cell growth in eukaryotic organisms that functions within the context of two distinct protein complexes, termed TOR complexes 1 and 2 (TORC1 and TORC2) (1–5). These complexes function, in part, by phosphorylating distinct members of the AGC family of protein kinases (named for their similarity to mammalian protein kinases A, G, and C), specifically at positions referred to as turn and hydrophobic motifs (TM and HM, respectively) (6, 7). Thus, in budding yeast, TORC1 specifically recognizes Sch9, the likely ortholog of mammalian S6K1, and TORC2 specifically recognizes Ypk2, a likely ortholog of mammalian Akt and SGK (8, 9). Ypk2, as well as the closely related protein Ypk1, has been implicated in regulating several important cellular functions, including receptor-mediated endocytosis, actin polarization, and sphingolipid metabolism, processes that are also linked to TORC2 (9–14). Given the extreme sequence similarity (∼90%) between Ypk1 and Ypk2, as well as their overlapping functions, it has been assumed that Ypk1 is also a target of TORC2 (9).
As in mammalian cells, all three kinases, Sch9 and Ypk1/2, are phosphorylated within their activation loop (A-Loop) by the yeast 3-phosphoinositide-dependent kinase 1 orthologs Pkh1 and Pkh2 (15). Both TORC2 and Phk1/2 are localized at or adjacent to the plasma membrane (PM) in distinct punctate structures, and it has therefore been proposed that Ypk1/2 must associate, at least transiently, with these activators at the PM (16). However, unlike Akt and SGK, which can associate directly with the PM (17, 18), neither Ypk1 nor Ypk2 have an identifiable membrane-targeting domain and it is therefore unknown how PM recruitment might be achieved.
A distinct set of TORC2 targets that have been identified consists of two closely related PM-associated proteins, Slm1 and Slm2 (19). The genes encoding these proteins were isolated in a genetic screen for mutants that display a synthetic lethal phenotype in combination with a temperature-sensitive allele of MSS4, encoding the lipid kinase that produces PI(4,5)P2 at the PM. Indeed, localization of Slm1/2 at the PM is conferred in part through interactions between their plextrin homology (PH) domains and PI(4,5)P2 (19). Results of a number of additional studies have demonstrated that Slm1/2 associate physically and are phosphorylated directly by TORC2 (19–21). Moreover, loss of Slm1/2 activity has been correlated with changes in actin polarization and sphingolipid metabolism, suggesting a close correlation between the function of these proteins, TORC2, and Ypk1/2 (19, 22).
An important apparent exception to this correlation is that Slm1/2, but not Ypk1/2, have been identified as important downstream effectors of TORC2 that negatively regulate the expression of stress-responsive genes controlled by the transcription factor Crz1 (21). The activity of Crz1 is itself positively regulated by the Ca2+-dependent phosphatase calcineurin in the presence of cell stress (23). Thus, reciprocal antagonism between TORC2 and calcineurin, mediated by Slm1/2, is proposed to regulate the activity of Crz1. In support of this model is the finding that activation of calcineurin leads to Slm1/2 dephosphorylation (21). However, the mechanism by which Slm1/2 might inhibit calcineurin is completely unknown.
Here we demonstrate that Ypk1 is indeed a bona fide target of TORC2 and that Ypk1/2 play a previously unrecognized role in TORC2-dependent regulation of Crz1 activity mediated by calcineurin. These findings led us to examine closely the relationship between Ypk1 and Slm1/2, where our results demonstrate that an important function of Slm1/2 is to recruit Ypk1 to the PM for activation by TORC2.
Results and Discussion
Ypk1 Is a Target of TORC2.
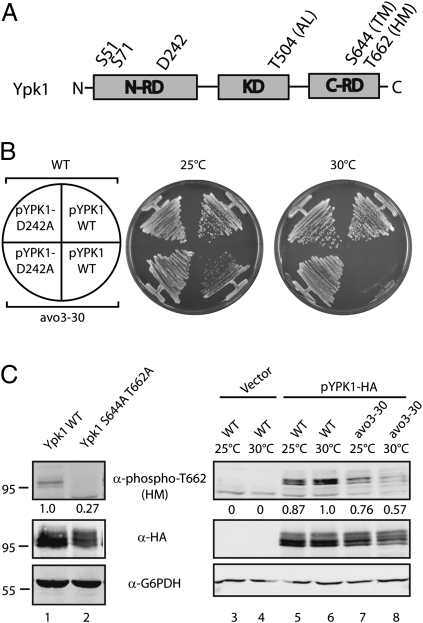
Ypk2 was first linked to TORC2 by genetic studies, where a single-point mutation (D239A) within a presumptive N-terminal autoinhibitory domain was identified that bypasses the need for TORC2-dependent phosphorylation of the TM and HM sites (9). To determine whether Ypk1 also functions downstream of TORC2, we introduced the corresponding mutation (D242A) into Ypk1 (Fig. 1A). This allele was placed under control of the strong MET promoter on a centromeric plasmid and introduced into a strain that possesses a temperature-sensitive variant, termed avo3-30, of the essential TORC2 component Avo3 (3). This strain is viable at 25 °C but displays a significant growth defect at 30 °C (13). We observed that expression of Ypk1D242A, but not wild-type Ypk1, rescued the growth defect of avo3-30 cells at the nonpermissive temperature (Fig. 1B). We observed further that the D242A mutation suppressed the lethality of mutations in the TM and HM sites in Ypk1, consistent with these sites being important specifically for TORC2-dependent activation (Fig. S1).
Fig. 1.
Ypk1 functions downstream from and is a target of TORC2. (A) Schematic diagram of Ypk1 indicating phosphorylation sites and functional domains: AL, activation loop (A-Loop); C-RD, C-terminal regulatory domain; HM, hydrophobic motif; KD, kinase domain; N-RD, N-terminal regulatory domain; TM, turn motif. (B) Plasmids expressing wild-type Ypk1 (pPL215) or Ypk1D242A (pPL240) were introduced into wild-type (W303α) or avo3-30 (PLY1134) strains. Cells were streaked out onto SCD minus uracil solid agar plates and grown at 25 °C or 30 °C for ∼2 d. (C) Plasmids expressing wild-type Ypk1 (pPL215), Ypk1S644A/T662A (pPL534), or control vector (pPL187) were introduced into wild-type W303α (lanes 1–6) or avo3-30 (PLY1134) (lanes 7 and 8), as indicated. Cells were grown in SCD minus uracil at 25 °C O/N, then shifted to 30 °C for 3 h, lysed, and the resulting extracts were resolved by SDS/PAGE and immunoblotted with α-phospho-T504, α-HA, and α-G6PDH antibodies. Quantification below the respective blot describes the difference relative to wild-type after normalizing to the α-HA signal.
To determine if Ypk1 is phosphorylated by TORC2 in vivo, we monitored phosphorylation of the predicted HM target site residue T662 using a phospho-specific antibody directed against this position (Methods). A control experiment demonstrated that this antibody recognized efficiently plasmid-expressed, HA epitope-tagged wild-type Ypk1 but not Ypk1S644A/T662A in extracts prepared from wild-type cells (Fig. 1C, compare lanes 1 and 2). We examined T662 phosphorylation in extracts prepared from avo3-30 cells, where a significant reduction in phosphorylation was observed when cells were temperature shifted to 30 °C, and to a lesser extent at 25 °C, compared with wild-type (Fig. 1C, lanes 5–8). We also determined that recombinant Ypk1, like Ypk2, was efficiently phosphorylated in vitro by TORC2 immunopurified from yeast, confirming that it a direct target for TORC2 (Fig. S2). Finally, we determined that loss of phosphorylation of Ypk1 by TORC2 resulted in significant reduction in A-Loop phosphorylation by Pkh1/2 (Fig. S3). This latter result suggests TORC2-dependent phosphorylation of Ypk1 is required for subsequent activation by Pkh1/2, as has been observed for other AGC kinases (6, 17, 24, 25).
Ypk1/2 Links TORC2 to Calcineurin-Dependent Gene Expression.
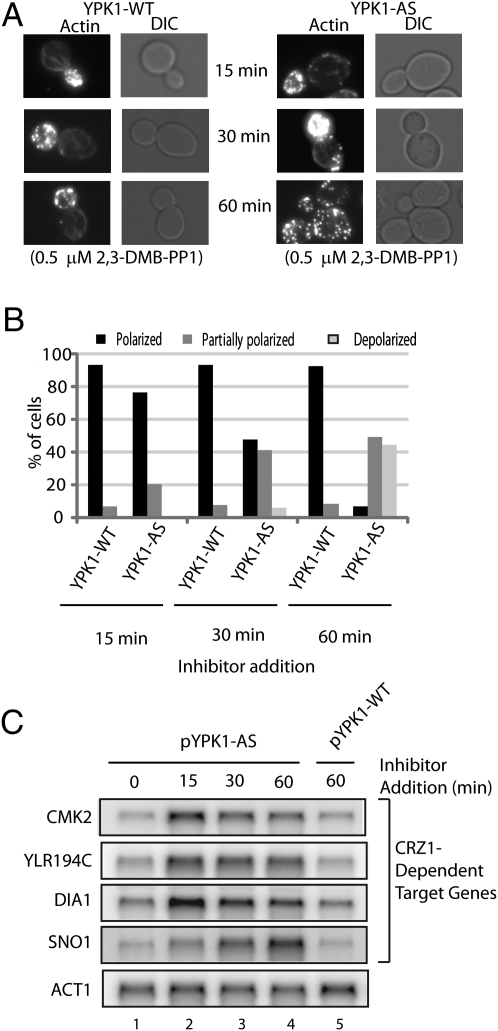
TORC2 negatively regulates a concise set of stress-responsive genes controlled by the transcription factor Crz1, which is also positively regulated by the Ca2+-dependent phosphatase calcineurin (21). Given our results above, we sought to (i) test whether this regulatory circuit also involves Ypk1/2 and (ii) determine the scope of genes potentially regulated by these kinases. For this process we developed ATP analog-sensitive (AS) alleles for Ypk1/2 to specifically and rapidly inhibit their activity within cells (26, 27) (Fig. S4). To demonstrate the efficacy of this system, we observed that treating ypk1Δypk2Δ cells that expressed an AS allele of Ypk1 (Ypk1-AS) with inhibitor resulted in significant actin depolarization within 30 min of drug treatment, whereas no effect was observed on cells expressing wild-type Ypk1 (Fig. 2 A and B).
Fig. 2.
Inhibition of an AS allele of Ypk1 perturbes actin polarization and negatively regulates calcineurin-dependent transcription. (A) Ypk1-WT (PLY1154) and Ypk1-AS (PLY1155) strains grown to OD600 = 0.4, then treated for the indicated times in 0.5 μM 2,3-DMB-PPI, fixed, and stained for actin with rhodamine-phalloidin. (B) Quantification of data in C, where at least 100 cells were counted for each sample. (C) Northern blot of RNA from Ypk1-WT and Ypk1-AS strains, treated as described above, where indicated Crz1-dependend target gene transcripts were probed, using ACT1 as a reference gene.
We next examined global transcriptional changes that resulted from loss of Ypk1 or Ypk2 activity. To this end, DNA microarrays were probed with fluorescently labeled cDNAs prepared from inhibitor-treated ypk1Δypk2Δ cells that expressed either wild-type or AS alleles of Ypk1/2. We observed that after 30–60 min of drug treatment, a concise set of ∼70 genes displayed a significant (≥fourfold) increase in expression in cells expressing Ypk1/2 AS alleles versus wild-type Ypk1/2 (Fig. S4 and Table S1). Further inspection of these genes revealed remarkable overlap in comparison with genes induced transcriptionally following inhibition of TORC2 activity (21). Importantly, a significant proportion of genes that were induced following Ypk1/2 inhibition correspond to stress-responsive genes regulated by the calcineurin-dependent transcription factor Crz1 (23, 28).
We used Northern blot analysis to confirm these results, where we probed for individual transcripts corresponding to representative Crz1 target genes in inhibitor-treated cells expressing either wild-type Ypk1 or Ypk1-AS. Here we observed induction of Crz1 target genes in cells expressing the Ypk1-AS allele as early as 15 min following drug treatment, compared with no effect in cells expressing wild-type Ypk1 (Fig. 2C). Importantly, the kinetics of this response were considerably faster compared with the previous study that used temperature-sensitive alleles of essential TORC2 components, where several hours were required to observe Crz1 target gene induction (21). Deletion of CNB1, which encodes the calcineurin regulatory subunit B, largely abolished Crz1 target gene induction when cells expressing the Ypk1-AS allele where treated with inhibitor (Fig. S4). Taken together, we conclude from these experiments that Ypk1 and -2 provide an important link between TORC2 and inhibition of calcineurin-regulated Crz1-dependent gene expression.
Ypk1 Activation by TORC2 Requires Slm1/2.
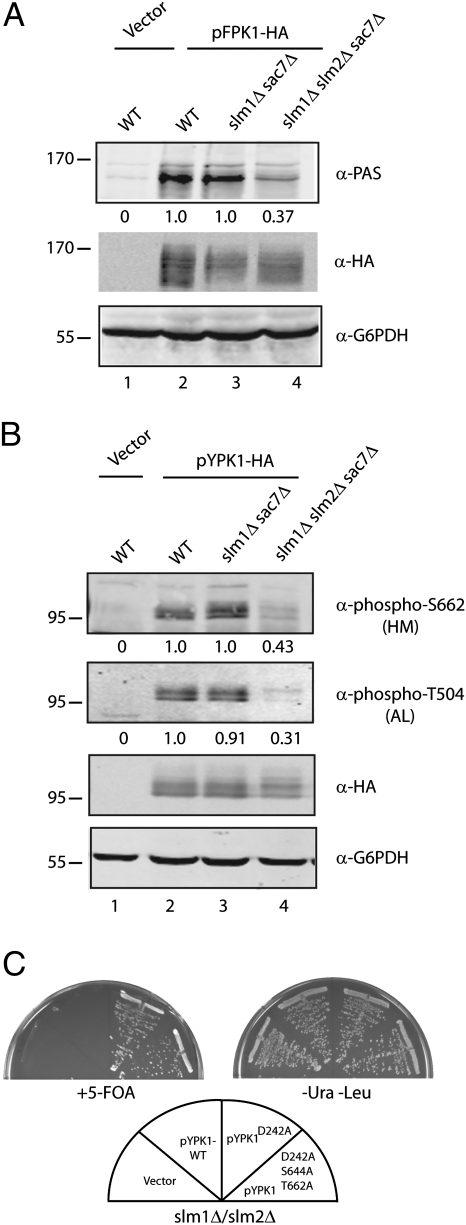
Given that regulation of calcineurin and Crz1 activity has been linked previously to TORC2 via Slm1/2 (21), we sought to understand the functional relationship between Ypk1/2 and Slm1/2. We focused on Ypk1 and asked whether the activity of this protein was altered in the absence of Slm1/2. Here we first examined the phosphorylation state of Fpk1, a recently identified substrate for Ypk1, using an antibody that recognizes the Ypk1-target site consensus sequence RXRXX(S/T), of which there are three in Fpk1 (29). Because loss of both Slm1 and Slm2 is lethal, we constructed a strain that was additionally deleted for SAC7, encoding the Rho1 GTPase activating protein (30), which suppresses the lethality of slm1Δ slm2Δ cells (19). Here we observed a significant reduction in Fpk1 phosphorylation in slm1Δ slm2Δ sac7Δ cells, compared with either wild-type or slm1Δ sac7Δ cells, suggesting Ypk1 activity is indeed impaired in the absence of Slm1/2 (Fig. 3A).
Fig. 3.
Ypk1 activation by TORC2 requires Slm1/2. (A) Wild-type (SEY6210) and slm1Δslm2Δsac7Δ (PLY1447) strains expressing empty vector (pPL187) or Fpk1 (pPL428) were grown at 30 °C, lysed, and the resulting extracts were resolved by SDS/PAGE and immunoblotted with α-PAS, α-HA, and α-G6PDH antibodies. (B) Wild-type (SEY6210), slm1Δsac7Δ (PLY1446), and slm1Δslm2Δsac7Δ (PLY1447) strains expressing empty vector (pPL187) or Ypk1 (pPL215) were processed for Western blot analysis, probing with α-phospho-T662, α-phospho-T504, α-HA, and α-G6PDH antibodies. Quantification below the respective blot describes the difference relative to wild-type after normalizing to the α-HA signal. (C) Strain PLY1357 (slm1Δslm2Δ pPL421) was transformed with a control vector (pPL420) or the indicated Ypk1 plasmids (pPL433, pPL434, and pPL522). The resulting transformants were streaked onto SCD minus uracil and leucine or onto 5-Fluoroorotic acid (5-FOA) solid agar plates and grown at 30 °C for ∼2 d.
To determine whether loss of Fpk1 phosphorylation correlates directly with impaired activation of Ypk1, we monitored the phosphorylation state of the A-Loop and HM sites in Ypk1 using phospho-specific antibodies, as described above (Fig. 2 and Fig. S3). Here we observed significant reduction in phosphorylation at both positions in slm1Δ slm2Δ sac7Δ cells, demonstrating that activation of Ypk1 indeed depends on the function of Slm1/2 (Fig. 3B, compare lanes 2 and 4). To test whether Slm1/2 activity is required specifically for TORC2-dependent activation of Ypk1, we used a plasmid shuffle approach to determine if expression of the TORC2-independent Ypk1D239A allele suppressed the lethality of slm1Δ slm2Δ cells. Indeed, we observed that plasmid-borne Ypk1D239A, but not wild-type Ypk1, could support growth of slm1Δ slm2Δ cells, consistent with a model wherein Slm1/2 is required for TORC2-dependent phosphorylation of Ypk1 (Fig. 3C). In agreement with this interpretation, we observed that a Ypk1D239A/S644A/T662A triple mutant was also able to support growth of slm1Δ slm2Δ cells, demonstrating that a requirement for both TORC2 and Slm1/2 can be bypassed in the presence of the TORC2-independent allele of Ypk1 (Fig. 3C).
Stable Association of Ypk1 at the PM Requires Slm1/2.
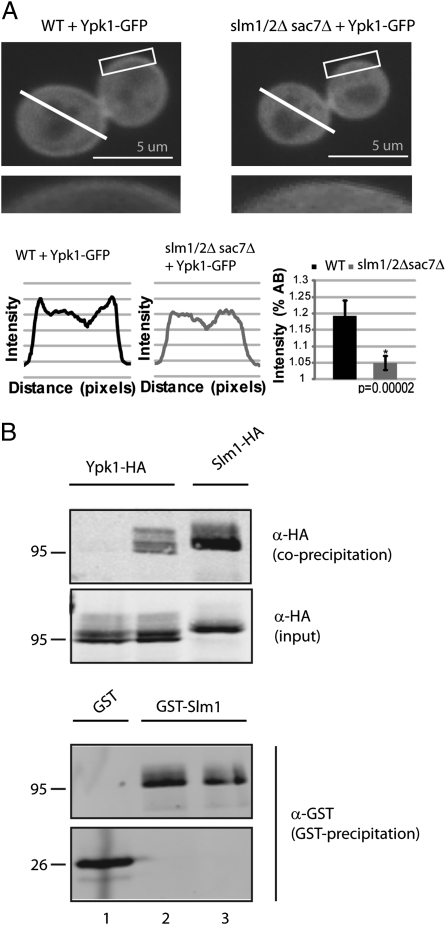
Previous studies have demonstrated that Ypk1 localizes both within the cytoplasm as well as at the PM (10, 31). To explore the mechanism by which Slm1/2 might mediate Ypk1 activation, we first asked whether the steady-state distribution of Ypk1 was altered in the absence of Slm1/2 activity. Toward this end we used fluorescence microscopy to monitor the localization of GFP-tagged Ypk1 in both wild-type and slm1Δ slm2Δ sac7Δ cells. In agreement with previous studies, we observed that Ypk1-GFP was localized throughout the cell, yet was also distinctly enriched at the PM (Fig. 4A). In slm1Δ slm2Δ sac7Δ cells, however, Ypk1 was localized within the cytoplasm exclusively and PM enrichment was essentially no longer detected (Fig. 4A). This difference was not because of loss of Sac7 in the triple mutant, because the pattern of Ypk1-GFP localization in sac7Δ cells was indistinguishable from wild-type (Fig. S5). We conclude from these results that in the absence of Slm1/2 activity, Ypk1 fails to localize stably at the PM.
Fig. 4.
Ypk1 recruitment to the PM requires Slm1/2. (A) Wild-type (SEY6210) and slm1Δ/slm2Δ/sac7Δ (PLY1447) strains expressing Ypk1-GFP (pPL481) were imaged by confocal microscopy. Representative midsections are shown, with insets indicating higher magnification of the boxed areas. The profile plot shows intensity over distance (in pixels) for the area indicated by the line. Bar graph displays the intensity of the cell boundary as a percentage above background intracellular fluorescence. Average represents six to eight cells per strain, and is presented with means ± SEM. P values were calculated using the Student t test (Microsoft Excel). (B) Wild-type (SEY6210) strains expressing Ypk1-HA (pPL215) and coexpressing with either GST alone (pPL446) or GST-Slm1 (pPL447) were grown in SCD minus uracil and leucine. Fifty micrograms of total yeast protein was loaded for “input” lanes, whereas 3 mg of yeast protein extract was used for GST coprecipitation samples. Proteins were resolved by SDS/PAGE, and immunoblotted with α-HA and α-GST antibodies.
As described in the introduction to this article, Slm1/2 are also localized at the PM (16, 32). Given our results above, we wanted to determine whether Ypk1 and Slm1 also interact physically in vivo. Toward this end, we performed coprecipitation experiments using strains that expressed GST alone or GST fused to Slm1, in addition to HA-tagged Ypk1. As a positive control, we also constructed a strain that expressed both GST- and HA-tagged versions of Slm1, to examine Slm1 self-association that has been described previously (33). Extracts were prepared from these strains and GST-tagged proteins were precipitated using glutathione-linked agarose, followed by Western blot analysis to detect coprecipitated HA-tagged proteins. We observed that Ypk1-HA coprecipitated with GST-Slm1 but not with GST alone (Fig. 4B, compare lanes 1 and 2). Quantification of these data revealed that only a fraction (∼1.2%) of total Ypk1-HA was present in coprecipitated samples, indicating that only a portion of Ypk1 associates with Slm1 under these conditions. Nevertheless, the extent of this interaction was comparable to what was observed between Slm1-HA and GST-Slm1 (Fig. 4B, compare lanes 2 and 3). We note that these findings are consistent with results of a previous global affinity-capture study that detected an interaction between Slm1 and Ypk1 (34).
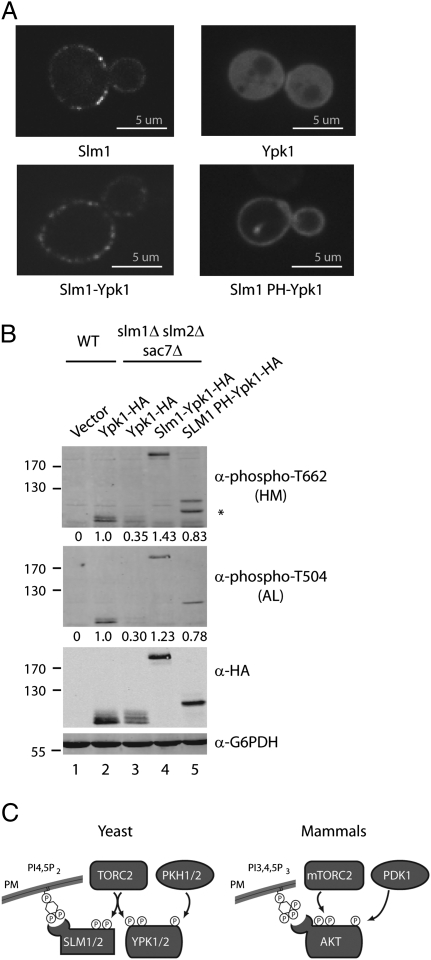
Taken together, the above results suggest that interactions with Slm1 are required for PM targeting of Ypk1 and subsequent activation by TORC2 and Pkh1/2. A prediction of this model is that targeting Ypk1 independently to the PM may be sufficient to restore TORC2 and Pkh1/2-dependent phosphorylation in the absence of endogenous Slm1/2 activity. To test this theory, we fused the PH domain alone of Slm1 to GFP- and HA-tagged versions of Ypk1 and examined the behavior of these fusion proteins in slm1Δ slm2Δ sac7Δ cells. We observed that GFP-tagged Slm1 PH-Ypk1 was localized essentially uniformly at the PM (Fig. 5A). Importantly, HA-tagged Slm1 PH-Ypk1 was phosphorylated at both the HM and A-loop sites (Fig. 5B) and, moreover, was completely functional, based on its ability to replace wild-type Ypk1 in a plasmid-shuffling assay (Fig. S6). Additionally, these results demonstrate that TORC2 activity is not inherently dependent upon the presence of Slm1/2. Interestingly, we observed that fusing all of Slm1 to Ypk1 resulted in a fusion protein that adopted a punctate pattern at the PM that was very similar to native Slm1 (Fig. 5A). This fusion was also fully active, based on its phosphorylation state and its ability to replace wild-type Ypk1 in a plasmid-shuffling assay (Fig. 5B and Fig. S6). We conclude from these results that association with the PM represents a crucial step in activation of Ypk1 and represents an important function of Slm1. An important corollary to these findings is that tethering Ypk1 artificially at the PM, either uniformly or in Slm1-like puncta, does not interfere with its function, suggesting Ypk1 retains access to essential targets under these conditions.
Fig. 5.
Artificial targeting of Ypk1 to the PM bypasses a requirement for Slm1/2. (A) Wild-type (SEY6210) strains expressing Ypk1-GFP (pPL481), Slm1-Ypk1-GFP (pPL502), Slm1 PH-Ypk1-GFP (pPL500), as well as a Slm1-GFP expressing strain (AAY1622), were imaged by confocal microscopy. Representative midsections are shown. (B) A control plasmid (pPL420) as well as plasmids expressing Ypk1 (pPL433), Slm1-Ypk1 (pPL501), Slm1 PH-Ypk1 (pPL495) were introduced into wild-type (SEY6210) and slm1Δslm2Δsac7Δ (PLY1447) strains. Cells were grown in SCD minus leucine, lysed, and the resulting extracts were resolved by SDS/PAGE and immunoblotted with α-phospho-T662, α-phospho-T504, α-HA, and α-G6PDH antibodies. Asterisk denotes a nonspecific band. (C) Model comparing activation of AGC kinases Ypk1/2 and AKT in yeast and mammalian cells, respectively. See text for details.
Conclusions
We have demonstrated that Slm1/2 play an essential role in the activation of the AGC kinase Ypk1 and, by extension, its close ortholog Ypk2, in budding yeast. Our results support a model wherein Slm1 interacts directly with Ypk1 to facilitate its localization at the PM, allowing for phosphorylation of TM and HM sites within Ypk1 by TORC2. We conclude that these events are required for subsequent A-Loop site phosphorylation by Pkh1/2. Whereas in mammalian cells Akt associates directly with the PM through interactions between its PH domain and PIP3 (17), we propose that in yeast the Slm1/2 proteins act as adaptors that mediate association between Ypk1 and the PM (Fig. 5C).
Because Slm1/2 are also themselves targets of TORC2, where their phosphorylation state is responsive to a variety of signals, including PIP2 levels, sphingolipid metabolism, and cell stress (19, 22), interactions between Slm1/2 and Ypk1/2 may have evolved to provide an added level of regulation to TORC2-dependent events, particularly in response to signals generated at the PM. This finding raises the question as to the relationship between Slm1/2 phosphorylation and Ypk1/2 activation and the extent to which their interactions are regulated. Thus, although we conclude that association with Slm1 is required for Ypk1 PM localization and activation, these proteins have very different steady-state distributions (Fig. 5A), suggesting their interaction is likely to be transient or dynamic.
Our findings also demonstrate the close correspondence between events governed by TORC2 via both Slm1/2 and Ypk1/2, including the discovery of the link between Ypk1/2 and negative regulation of calcineurin-dependent gene expression. Consistent with the conserved nature of TOR signaling, recent studies suggest that in mammalian cells insulin signaling also involves functional interactions between Akt and calcineurin (35). Understanding how Ypk1/2 contributes to the regulation of calcineurin in yeast thus represents an important next step for investigation.
Methods
Strains, Media, and Plasmids.
Yeast strains and plasmids used in this study are listed in Tables S2 and S3, respectively. Culture medium used was synthetic complete dextrose (SCD) (0.8% yeast nitrogen base without amino acids, pH 5.5, 2% dextrose) supplemented with amino acids, as described previously (36). All yeast transformations were conducted using a lithium acetate procedure (37). Construction of deletion strains by replacement of complete ORFs with a selectable marker was performed as described previously (38). SLM1-YPK1 fusions were created by splicing together PCR amplified SLM1 and YPK1 by PCR SOEing, and inserted into PL420 at the Xba1 and Sal1 sites. To create pPL420, the Met25 promoter-MCS with C-terminal HA3 tag-Tcyc terminator cassette from pPL187 was PCR-amplified and inserted into pRS315 at the Sac1 and Xma1 sites.
Actin-Staining and Fluorescence Microscopy.
Actin-staining and detection in yeast cells was performed as described previously (13). Fluorescence microscopy was performed using a Nikon E600 fluorescent microscope and an Orca ER charge-coupled device camera (Hamamatsu) controlled by SimplePCI software. All images are z-series projections. GFP images were captured from strains expressing GFP-tagged plasmids grown in SCD minus uracil media to midlog phase. Cells were imaged using the spinning disk module of a Marianas SDC Real Time 3D Confocal-TIRF microscope (Intelligent Imaging Innovations) fit with a 100×, 1.46 NA objective and EMCCD camera. The z stacks were collected using a step size of 0.4 μm. Image capture and processing was done using SlideBook5 software (Intelligent Imaging Innovations), and Photoshop (Adobe). Intensity plot values were collected using ImageJ software.
Antibody Production.
Antiphospho-Ypk1 (T662) polyclonal antibody was raised by immunizing rabbits with a phospho-peptide sequence from Ypk2 [peptide sequence CKQFGGW-pT-YIGDE, coupled to keyhole limpet hemocyanin (KLH) at the N-terminal cysteine] surrounding the proposed hydrophobic motif. This sequence has very high similarity to the Ypk1 hydrophobic motif sequence KQFGGWTYVGNE (differences in bold and underlined).
Western and Northern Blotting.
Protein extracts were prepared using the NaOH cell lysis method (38), and loaded onto SDS/PAGE gels and transferred to nitrocellulose membrane. Membranes were probed with α-HA (12CA5, 1:5,000; Covance), α-GST (1:1,000; Millipore), α-PKC (pan) zeta T410 (1:1,000; Cell Signaling Technology) for phospho-Ypk1(S504), α-phospho-Ypk1 (T662) (1:20,000; described above), α-phospho-AKT substrate (PAS, 1:1,000; Cell Signaling Technology) for phospho-Fpk1, and α-G6PDH (1:100,000; Sigma-Aldrich) primary antibodies, and visualized using the appropriate secondary antibodies conjugated to IRDye (1:5,000; LI-COR Biosciences) on the Odyssey Infrared Imaging System (LI-COR Biosciences). Images were quantified using ImageQuant software (GE Healthcare). Northern blot analysis was performed as described (39).
GST Coprecipitation Assays.
Yeast strains expressing Ypk1- HA3 and either Slm1-GST or GST alone were grown at 30 °C to 0.5 OD600/mL in SCD without uracil and leucine. Cells were pelleted, washed in H2O, then in yeast extract buffer (YEB; 50 mM Hepes-KOH, pH 7.1, 100 mM β-glycerolphosphate, 50 mM NaF, 5 mM EGTA, 5 mM EDTA, 10% (vol/vol) glycerol, 0.25% Tween 20, and 150 mM KCl). Pellets were resuspended 1:1 (w/vol) in YEB containing protease inhibitors (mixture tablet; Roche Diagnostics), 2 mM DTT, and 2 mM phenylmethylsulfonyl fluoride; cell lysates were frozen into pellets by dripping into liquid nitrogen. These pellets were then beat in a freezer mill (6750 SPEX Sample Prep) three times for 1 min. Upon thawing, the lysate was spun two times for 20 min at 14,000 × g at 4 °C. Three milligrams total protein was added to YEB-washed Glutathione-Agarose beads, and rotated for 2 h at 4 °C. After four washes in 1 mL YEB, bound beads were resuspended in SDS-sample buffer and boiled to remove bound protein. “Input” samples of 50 μg total yeast protein and “precipitation” samples were separated by 10% SDS/PAGE, followed by Western blotting using α-GST and α-HA antibodies.
Supplementary Material
Acknowledgments
We thank to C. Zhang and K. Shokat for advice and reagents in developing analog-sensitive alleles of Ypk1/2; J. Derisi for DNA microarrays; S. Emr for yeast strains; K. Kaplan for help with fluorescence microscopy; and J. Nunnari, R. Loewith, M. Hall, and members of the T.P. laboratory for helpful discussions. This work was supported by National Institutes of Health Grant GM086387 (to T.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE33185).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117563109/-/DCSupplemental.
References
- 1.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Rohde JR, Bastidas R, Puria R, Cardenas ME. Nutritional control via Tor signaling in Saccharomyces cerevisiae. Curr Opin Microbiol. 2008;11:153–160. doi: 10.1016/j.mib.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loewith R, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 4.Wedaman KP, et al. Tor kinases are in distinct membrane-associated protein complexes in Saccharomyces cerevisiae. Mol Biol Cell. 2003;14:1204–1220. doi: 10.1091/mbc.E02-09-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinke A, et al. TOR complex 1 includes a novel component, Tco89p (YPL180w), and cooperates with Ssd1p to maintain cellular integrity in Saccharomyces cerevisiae. J Biol Chem. 2004;279:14752–14762. doi: 10.1074/jbc.M313062200. [DOI] [PubMed] [Google Scholar]
- 6.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 7.Hara K, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 8.Urban J, et al. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell. 2007;26:663–674. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Kamada Y, et al. Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol Cell Biol. 2005;25:7239–7248. doi: 10.1128/MCB.25.16.7239-7248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roelants FM, Torrance PD, Bezman N, Thorner J. Pkh1 and Pkh2 differentially phosphorylate and activate Ypk1 and Ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol Biol Cell. 2002;13:3005–3028. doi: 10.1091/mbc.E02-04-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deHart AK, Schnell JD, Allen DA, Hicke L. The conserved Pkh-Ypk kinase cascade is required for endocytosis in yeast. J Cell Biol. 2002;156:241–248. doi: 10.1083/jcb.200107135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanoue D, et al. The requirement for the hydrophobic motif phosphorylation of Ypk1 in yeast differs depending on the downstream events, including endocytosis, cell growth, and resistance to a sphingolipid biosynthesis inhibitor, ISP-1. Arch Biochem Biophys. 2005;437:29–41. doi: 10.1016/j.abb.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Aronova S, et al. Regulation of ceramide biosynthesis by TOR complex 2. Cell Metab. 2008;7:148–158. doi: 10.1016/j.cmet.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmelzle T, Helliwell SB, Hall MN. Yeast protein kinases and the RHO1 exchange factor TUS1 are novel components of the cell integrity pathway in yeast. Mol Cell Biol. 2002;22:1329–1339. doi: 10.1128/mcb.22.5.1329-1339.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powers T. TOR signaling and S6 kinase 1: Yeast catches up. Cell Metab. 2007;6:1–2. doi: 10.1016/j.cmet.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Berchtold D, Walther TC. TORC2 plasma membrane localization is essential for cell viability and restricted to a distinct domain. Mol Biol Cell. 2009;20:1565–1575. doi: 10.1091/mbc.E08-10-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22:6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pao AC, et al. NH2 terminus of serum and glucocorticoid-regulated kinase 1 binds to phosphoinositides and is essential for isoform-specific physiological functions. Am J Physiol Renal Physiol. 2007;292:F1741–F1750. doi: 10.1152/ajprenal.00027.2007. [DOI] [PubMed] [Google Scholar]
- 19.Audhya A, et al. Genome-wide lethality screen identifies new PI4,5P2 effectors that regulate the actin cytoskeleton. EMBO J. 2004;23:3747–3757. doi: 10.1038/sj.emboj.7600384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadri M, Daquinag A, Wang S, Xue T, Kunz J. The pleckstrin homology domain proteins Slm1 and Slm2 are required for actin cytoskeleton organization in yeast and bind phosphatidylinositol-4,5-bisphosphate and TORC2. Mol Biol Cell. 2005;16:1883–1900. doi: 10.1091/mbc.E04-07-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulet JM, Martin DE, Loewith R, Hall MN. Mutual antagonism of target of rapamycin and calcineurin signaling. J Biol Chem. 2006;281:33000–33007. doi: 10.1074/jbc.M604244200. [DOI] [PubMed] [Google Scholar]
- 22.Tabuchi M, Audhya A, Parsons AB, Boone C, Emr SD. The phosphatidylinositol 4,5-biphosphate and TORC2 binding proteins Slm1 and Slm2 function in sphingolipid regulation. Mol Cell Biol. 2006;26:5861–5875. doi: 10.1128/MCB.02403-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stathopoulos AM, Cyert MS. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 1997;11:3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biondi RM, Kieloch A, Currie RA, Deak M, Alessi DR. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 2001;20:4380–4390. doi: 10.1093/emboj/20.16.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Shah K, Yang F, Witucki L, Shokat KM. Engineering Src family protein kinases with unnatural nucleotide specificity. Chem Biol. 1998;5:91–101. doi: 10.1016/s1074-5521(98)90143-0. [DOI] [PubMed] [Google Scholar]
- 27.Shokat K, Velleca M. Novel chemical genetic approaches to the discovery of signal transduction inhibitors. Drug Discov Today. 2002;7:872–879. doi: 10.1016/s1359-6446(02)02391-7. [DOI] [PubMed] [Google Scholar]
- 28.Matheos DP, Kingsbury TJ, Ahsan US, Cunningham KW. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 1997;11:3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roelants FM, Baltz AG, Trott AE, Fereres S, Thorner J. A protein kinase network regulates the function of aminophospholipid flippases. Proc Natl Acad Sci USA. 2010;107:34–39. doi: 10.1073/pnas.0912497106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt A, Schmelzle T, Hall MN. The RHO1-GAPs SAC7, BEM2 and BAG7 control distinct RHO1 functions in Saccharomyces cerevisiae. Mol Microbiol. 2002;45:1433–1441. doi: 10.1046/j.1365-2958.2002.03110.x. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, et al. Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Mol Cell Biol. 2000;20:4411–4419. doi: 10.1128/mcb.20.12.4411-4419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamble C, Jain S, Murphy E, Kim K. Requirements of Slm proteins for proper eisosome organization, endocytic trafficking and recycling in the yeast Saccharomyces cerevisiae. J Biosci. 2011;36:79–96. doi: 10.1007/s12038-011-9018-0. [DOI] [PubMed] [Google Scholar]
- 33.Yu H, et al. High-quality binary protein interaction map of the yeast interactome network. Science. 2008;322:104–110. doi: 10.1126/science.1158684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krogan NJ, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- 35.Ni YG, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci USA. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 37.Geitz RD, Woods RA. Transformation of yeast by the lithium acetate/single-stranded carrier DNA/PEG method. In: Brown AJP, Tuite MF, editors. Methods in Microbiology. Vol 26. New York: Academic Press; 1998. [Google Scholar]
- 38.Dilova I, Aronova S, Chen JC, Powers T. Tor signaling and nutrient-based signals converge on Mks1p phosphorylation to regulate expression of Rtg1.Rtg3p-dependent target genes. J Biol Chem. 2004;279:46527–46535. doi: 10.1074/jbc.M409012200. [DOI] [PubMed] [Google Scholar]
- 39.Powers T, Walter P. Regulation of ribosome biogenesis by the rapamycin-sensitive TOR-signaling pathway in Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:987–1000. doi: 10.1091/mbc.10.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.