Abstract
A deficit in early clearance of Pseudomonas aeruginosa (P. aeruginosa) is crucial in nosocomial pneumonia and in chronic lung infections. Few studies have addressed the role of Toll-like receptors (TLRs), which are early pathogen associated molecular pattern receptors, in pathogen uptake and clearance by alveolar macrophages (AMs). Here, we report that TLR5 engagement is crucial for bacterial clearance by AMs in vitro and in vivo because unflagellated P. aeruginosa or different mutants defective in TLR5 activation were resistant to AM phagocytosis and killing. In addition, the clearance of PAK (a wild-type P. aeruginosa strain) by primary AMs was causally associated with increased IL-1β release, which was dramatically reduced with PAK mutants or in WT PAK-infected primary TLR5−/− AMs, demonstrating the dependence of IL-1β production on TLR5. We showed that this IL-1β production was important in endosomal pH acidification and in inducing the killing of bacteria by AMs through asparagine endopeptidase (AEP), a key endosomal cysteine protease. In agreement, AMs from IL-1R1−/− and AEP−/− mice were unable to kill P. aeruginosa. Altogether, these findings demonstrate that TLR5 engagement plays a major role in P. aeruginosa internalization and in triggering IL-1β formation.
Keywords: flagellin, interleukin-1, lysosomal protease
The opportunist Gram-negative bacterium, Pseudomonas aeruginosa, is particularly important in nosocomial pneumonia and in chronic lung diseases such as cystic fibrosis (1). Alveolar macrophages (AMs) lie at the forefront of lung defense against pathogens such as P. aeruginosa. The main function of AMs is to clear pathogens (2), and a deficiency in early recognition of P. aeruginosa by AMs has been suspected in these pathologies (3, 4). Research has shown that pathogen-associated molecular patterns (PAMPs) are recognized by specific Toll-like receptors (TLRs) at the surface of phagocytes and mucosal epithelial cells. Surprisingly, although numerous studies have associated the ligand-induced TLR engagement to cytokine and chemokine production from phagocytes (5, 6), comparatively fewer studies have investigated the importance of TLRs in pathogen phagocytosis and killing. Furthermore, these studies have mostly used macrophages from bone marrow-differentiated cells (BMDMs), few have used live bacteria, and even fewer have used flagellated bacteria such as P. aeruginosa. Moreover most studies have used primed phagocytes (with LPS, zymosan) to boost pathogen uptake. Despite these caveats, the recruitment of membrane TLRs to phagosomes upon phagocytosis has been demonstrated (7–10), except for TLR5. TLR2, TLR4, and the adaptor molecule MyD88 have been shown to be important molecules in processing of heat-killed Escherichia coli and Staphylococcus aureus by BMDMs in late endosomes and lysosomes (9–11), suggesting that a blockade in phagosome maturation was occurring in phagocytes deleted for these molecules.
TLR5 is thought to be one of the key receptors implicated in the recognition of P. aeruginosa (5, 8), but no information is available about whether this molecule is important for bacterial phagocytosis. Here, we sought to investigate specifically the involvement of TLR5 in P. aeruginosa phagocytosis and killing by AMs. We describe a pathway linking P. aeruginosa engagement to TLR5 through MyD88, followed by IL-1β release and the involvement of a lysosomal cysteine protease (asparagine endopeptidase, AEP), all events concurring to AM-mediated P. aeruginosa phagocytosis and bacterial killing.
Results
TLR5 Engagement Is Required for P. aeruginosa Clearance.
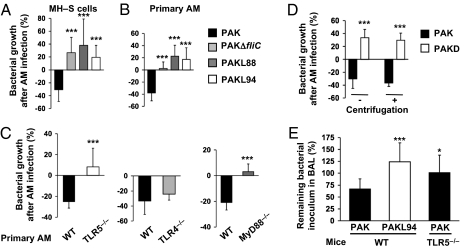
To determine whether the TLR5 ligand flagellin/flagella were involved in P. aeruginosa clearance by AMs, we infected murine alveolar MH–S cells with either WT P. aeruginosa strain PAK or PAK mutants deficient in the expression of flagellin, the primary flagellar subunit (the unflagellated PAKΔfliC), or expressing a flagellin monomer mutated in the TLR5-recognition site (PAKL88 and PAKL94; Table S1). FACS analysis indicated that MH–S cells expressed TLR5 (Fig. S1A). In the first part of our study, both AM supernatants and cell lysates were pooled for P. aeruginosa numeration (killing assays). In that context, MH–S cells significantly killed PAK, whereas no clearance of PAKΔfliC or flagellin-mutated PAKL88 or PAKL94 was observed, regardless of the duration of infection or the multiplicity of infection (MOI) used (Fig. 1A and Fig. S1B). These results were confirmed in primary WT AMs (Fig. 1B). In all of these experiments, the bacterial counts of all mutants continued to increase, in contrast to WT PAK. Moreover, AMs from TLR5−/− mice were unable to kill PAK, compared with WT AMs (Fig. 1C), whereas the bacterial clearance ability of AMs from TLR4−/− mice was not impaired. Importantly, MyD88−/− mice, the TLR5 mRNA levels of which were comparable to those of WT mice (Fig. S1C), were also unable to eliminate bacteria, underscoring the role of MyD88 in TLR5 signaling.
Fig. 1.
P. aeruginosa clearance by AMs is dependent on efficient TLR5-Flagella interaction. MH–S cells (A) or primary AMs (B) were infected for 4 h with WT PAK, PAKΔfliC, PAKL88, or PAKL94 mutants [MOI: 0.1 (B) or 10 (A)]. (C) Primary WT, TLR5−/−, TLR4−/−, or MyD88−/− AMs were infected for 4 h with WT PAK (MOI: 0.1). (D) Primary AMs were infected for 4 h with either WT PAK or PAKD (MOI: 0.1) with or without centrifugation. (A–D) CFU were quantified in AM supernatants pooled with cell lysates. Results are means ± SD of three experiments (**P < 0.01; ***P < 0.001) and are expressed as percentages as follows: (CFU counts recovered without AMs − CFU counts recovered after AM infection) × 100. (E) WT or TLR5−/− mice were infected intratracheally with 105 CFU of PAK or PAKL94. BALs were performed 2 h later and plated on LB agar plates to quantify total CFU counts. Results are means ± SD of three experiments (*P < 0.05; ***P < 0.001) and are expressed as percentage of surviving bacteria in BAL = (total CFU counts in BAL/ CFU counts of inoculum) × 100.
The resistance of unflagellated PAKΔfliC to AM killing clearly pointed to the P. aeruginosa flagellum as the organelle mainly implicated in AM stimulation (12). However, our data did not rule out that the flagellin monomer might be responsible for AM induction of bacterial killing. We, therefore, used an unflagellated PAK mutant, PAKD (Table S1), which, unlike PAKΔfliC, is still able to release high amounts of flagellin in the supernatant (Fig. S2A). Fig. 1D shows that PAKD, despite its ability to signal through TLR5 (TNFα output, 1201 ± 454 pg/mL vs. 2473 ± 487 pg/mL for WT PAK), was completely resistant to primary AM killing. Bacteria centrifuged onto cells to force bacteria–cell contact, did not increase PAKD killing by primary AMs, reinforcing the importance of specific functional flagellum–TLR5 interaction for P. aeruginosa clearance.
The implication of TLR5 recognition in P. aeruginosa clearance was further confirmed in vivo. Following intratracheal P. aeruginosa administration, we showed that 67% of the WT PAK inoculum was recovered in WT mice bronchoalveolar lavage (BAL) 2 h postinfection, whereas 101% of the same inoculum remained after mouse TLR5−/− infection (Fig. 1E). Moreover, all of the original PAKL94 inoculum remained and continued to proliferate after infection of WT mice (124%), suggesting a delay in bacterial clearance, compared with WT PAK. Importantly, these differences cannot be attributed to variations in the cellular composition of the airspaces (97−100% of AMs and 0−3% of neutrophils), and the total cell counts were not significantly different regardless of the bacteria strains used, thereby implicating solely the AMs in the events described.
TLR5/MyD88 Signaling Is Important for P. aeruginosa Phagocytosis.
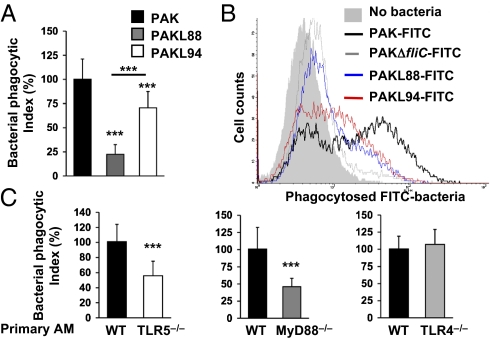
Because bacterial clearance could not be explained either by bactericidal activity of AM-infected supernatants (Table S2) or by AM cytotoxicity, we assessed whether the differential killing between WT and PAK mutants was attributable to different AM uptake. MH–S cells were incubated with bacteria for 1 h, followed by antibiotic treatment, and then the amount of phagocytosed bacteria was determined in cell lysates only. Fig. 2A shows a deficit in phagocytosis for PAKL88 and PAKL94 compared with WT PAK, whereas mutant CFU counts increased in supernatants. Using FITC-labeled bacteria (Fig. 2B), FACS analysis showed a near complete absence of labeling in MH–S cells with PAKΔfliC-FITC, i.e., an almost complete deficit of phagocytosis, whereas PAKL88-FITC and PAKL94-FITC presented an intermediate phenotype of AM uptake, with PAKL88-FITC being less phagocytosed than PAKL94-FITC. To confirm that the P. aeruginosa uptake is dependent upon flagellum–TLR5 (and not flagellin–TLR5) interaction, we performed phagocytosis assays with PAKD. In Fig. S2B, PAKD (despite high levels of secreted flagellin; Fig. S2A) was less phagocytosed than WT P. aeruginosa, even when bacteria–cell contact was increased by centrifugation, demonstrating that bacterial internalization is not attributable to flagellin/TLR5 engagement. Immunoblotting of intracellular flagellin also reflected differences in bacterial uptake (Fig. S2A). Phagocytosis was shown to be TLR5 receptor-specific, because centrifugation did not significantly abolish differences in AM uptake between WT PAK and mutants (Fig. S2 B–D). Moreover, competition experiments with P. aeruginosa flagellin significantly reduced PAK binding (Fig. S2E). Finally, such a deficit in P. aeruginosa phagocytosis was shown in TLR5−/− and MyD88−/− primary AMs (Fig. 2C). In contrast, TLR4 was shown to play no role in P. aeruginosa internalization by primary AMs. Taken together, these observations established the importance of flagellum/TLR5–MyD88 engagement for P. aeruginosa phagocytosis of primary AMs.
Fig. 2.
Phagocytosis of P. aeruginosa by AMs is dependent on TLR5-MyD88 signaling. (A and C) MH–S cells were infected for 1 h with WT PAK, PAKL88, or PAKL94 mutants (A), or primary WT, TLR5−/−, MyD88−/−, or TLR4−/− AMs were infected with WT PAK (MOI: 10) for 1 h (C). After stimulation, cells were washed and treated with tobramycin and the number of ingested bacteria was determined by counting CFU from AM lysates on LB agar plates. Results are means ± SD of three experiments (***P < 0.001) and are expressed as percentages of relative phagocytosis index = (CFU counts in mutant PAK-treated cells/CFU counts in WT PAK-treated cells) × 100. (B) FACS analysis of MH–S cells following 1 h infection with FITC-labeled WT or FITC-mutant P. aeruginosa strains. Results are representative of three independent experiments.
IL-1β Production Is Dependent on TLR5 Signaling.
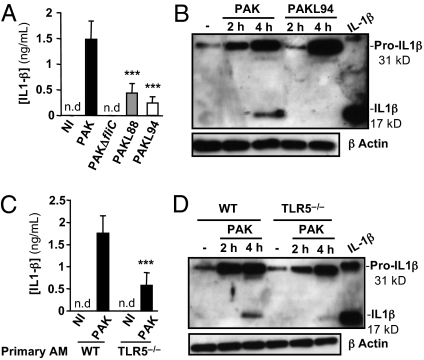
Although PAKL94 was not killed, it was shown to be better phagocytosed than PAKL88, suggesting that other intracellular PAMP receptors might be important in the detection and the killing of P. aeruginosa by AMs. Because the intracellular PAMP receptor ICE protease-activating factor (IPAF) participates in the processing of pro-IL-1β to IL-1β through caspase-1/inflammasome-dependent activation in P. aeruginosa-infected macrophages (13, 14), we measured mature secreted IL-1β levels in primary AM supernatants as a potential index of inflammasome involvement. We observed that high levels of IL-1β were associated with AM-mediated PAK killing and low IL-1β levels were detected with PAKL94 infection (Fig. 3A). The reduction of IL-1β by PAKL94-treated AMs was associated with a delay and a reduction in pro-IL-1β (31 kDa) production, leading to an important inhibition in mature IL-1β conversion (17 kDa) 4 h postinfection (Fig. 3B). We have demonstrated that purified flagellin was able to induce the pro-IL-1β synthesis but not the caspase-1 p10 and, therefore, was not involved in the mature IL-1β generation (Fig. S3 A–C). The dependence of IL-1β production on TLR5 was further demonstrated by using PAK-infected primary TLR5−/− AMs. Indeed, for these cells, a significant reduction of IL-1β secretion was observed, compared with WT AMs, caused by a delay in pro-IL-1β production 2 h postinfection and a decrease in mature IL-1β conversion (Fig. 3 C and D).
Fig. 3.
IL-1β production in primary AMs is dependent on TLR5. A total of 105 primary AMs from WT (A and B) or TLR5−/− (C and D) mice were infected with WT PAK, PAKL88, or PAKL94 (MOI: 1). IL-1β secretion was measured in supernatants 4 h postinfection (A and C). Results are means ± SD of three experiments (***P < 0.001). NI, noninfected cells; nd, not detected. Lysates obtained from primary WT (B) or TLR5−/− (D) AMs infected with bacteria were analyzed by immunoblotting for pro-IL-1β processing. Equal loading was controlled for by β-actin detection. Results are representative of three independent experiments.
Importantly, the deficiency in mature IL-1β conversion observed in PAKL94-infected WT AMs was correlated with a reduction in mature caspase-1 p10 production (Fig. S4A), whereas this was not the case with PAK-infected TLR5−/− AMs, compared with WT AMs (Fig. S4B).
IL-1β Is Required for P. aeruginosa Bacterial Killing.
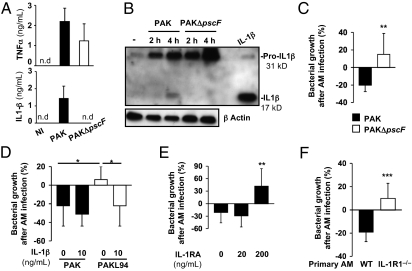
To examine the connection between IL-1β production and bacterial killing, we studied AM infection with PAKΔpscF, a PAK mutant deficient in the type III secretion system (T3SS) (14), which has been described to block the caspase-1-dependent processing of IL-1β (15). Whereas TNFα secretion was similar between WT PAK- and PAKΔpscF-infected AMs, released IL-1β levels were undetectable in supernatants after infection of primary AMs with this mutant (Fig. 4A). Western blot analysis showed that, although PAKΔpscF induced high levels of pro-IL-1β, mature IL-1β was not produced (Fig. 4B). This is likely attributable to its inability to activate caspase-1, as evidenced by the absence of the caspase-1 p10 subunit in PAKΔpscF-infected AMs 4 h postinfection (Fig. S4C). Importantly, although PAKΔpscF was as efficiently phagocytosed by AMs (125% ± 28%) as WT PAK (100% ± 25%), this nonmature IL-1β producer was not killed by primary AMs (Fig. 4C), validating a link between defective killing and a major reduction of IL-1β secretion.
Fig. 4.
IL-1β secretion is required for AMs to kill P. aeruginosa. (A) Primary WT AMs (105) were infected with WT PAK or PAKΔpscF mutant (MOI: 1) for 4 h. TNFα or IL-1β was measured in supernatants. Results are means ± SD of three experiments. NI, noninfected cells; nd, not detected. (B) Lysates from infected AMs were assessed for pro-IL-1β processing. Equal loading was controlled by β-actin detection. Results are representative of three independent experiments. (C) Primary WT AMs were infected with WT PAK or PAKΔpscF (MOI: 0.1) for 4 h. (D and E) MH–S cells were infected with WT PAK or PAKL94 mutant (MOI: 0.1) for 4 h in the presence or not of IL-1β or IL-1 receptor antagonist (IL-1RA). (F) Primary WT or IL-1R1−/− AMs were infected for 4 h with WT PAK (MOI: 0.1). (C–F) CFU were quantified in supernatants pooled with cell lysates. Results are means ± SD of three experiments (*P < 0.05; **P < 0.01) and are expressed as in Fig 1.
Importantly, Fig. 4D showed that exogenously added IL-1β restored PAKL94 killing to levels equivalent to that of WT PAK. We also demonstrated that IL-1 receptor antagonist (IL-1RA), an inhibitor of IL-1β signaling, was able to inhibit MH–S cell killing of WT PAK (Fig. 4E), without affecting IL-1β release (3867 ± 649 pg/mL with IL-1RA vs. 3393 ± 309 pg/mL without IL1-RA; 5 × 105 AMs). To reinforce that point, we performed additional experiments on primary AMs from IL-1R1−/− mice. Fig. 4F clearly demonstrated that primary IL-1R1−/− AMs were unable to kill WT P. aeruginosa, compared with WT AMs. Furthermore, we measured the same amount of IL-1β in AMs from IL-1R1−/− (333 ± 144 pg/mL) and WT (271 ± 34 pg/mL) mice, demonstrating the autocrine feedback of IL-1β on P. aeruginosa clearance by primary AMs.
IL-1β-Induced Bacterial Killing Is Dependent on AEP.
Because bacterial fate is ultimately determined in acidic phagolysosome compartments, we then set out to determine the mechanisms responsible for PAK killing in AMs. We showed that blocking phagolysosome acidification by bafilomycin A (BafA) treatment (Fig. S5A), an inhibitor of vacuolar type H+-ATPase, inhibited significantly PAK killing in primary AMs (Fig. S5B), whereas IL-1β release was unaffected (Fig. S5C). Using specific probes to measure the pH in endolysosomes, we showed that exogenous administration of IL-1β significantly reduced the pH in MH–S cell endosomes (Fig. 5A), suggesting that IL-1β-mediated acidification of AM phagolysosomes may be a key element in the control of bacterial killing.
Fig. 5.
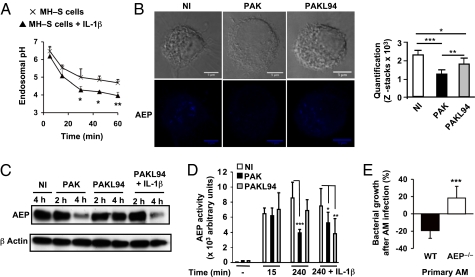
AEP protease is essential for P. aeruginosa killing by primary AMs. (A) Kinetics of endolysosomal pH in MH–S cells ± IL-1β (10 ng/mL) was measured. Results are means ± SD of three experiments. (B) Confocal microscopy of AEP was measured in primary WT AMs infected or not for 3 h with WT PAK or PAKL94. Quantification was performed using Image J software (n = 5). (C) Immunoblotting AEP expression in lysates from WT PAK- or PAKL94-infected primary WT AMs (MOI: 1) with or without IL-1β. Equal loading was controlled by β-actin detection. Results are representative of three independent experiments. (D) AEP activity was measured in lysates from WT PAK- or PAKL94-infected MH–S cells (MOI: 10) with a fluorometer by assessing the hydrolysis of the specific substrate for AEP. (A–D) Unpaired t test (*P < 0.01; **P < 0.005; ***P < 0.0001). NI, noninfected cells. (E) Primary WT or AEP−/− AMs were infected with WT PAK (MOI: 0.1) for 4 h. CFUs were quantified in supernatants pooled with cell lysates. Results are means ± SD of three experiments (***P < 0.001) and are expressed as in Fig 1.
Because most of the lysosomal cysteine proteases are dependent on a low pH for their activities, we chose to study the role of the protease AEP in PAK clearance. Using confocal microscopy, AEP staining was detected in endosomal EEA1+ vesicles in noninfected primary AMs. In contrast, AEP labeling was significantly decreased upon PAK challenge, whereas a reduction of staining was much less pronounced in PAKL94-infected AMs (Fig. 5B). Immunoblotting (Fig. 5C) and enzymatic activity assays using AEP-specific fluorogenic substrate (Fig. 5D) confirmed a significant AEP consumption in WT PAK-infected AMs 4 h postinfection, whereas this was not the case with PAKL94-infected primary AMs. However, exogenously added IL-1β restored AEP consumption in PAKL94-infected AMs 3 h postinfection (Fig. 5 C and D). AEP consumption was associated with mature IL-1β secretion but was independent of bacterial uptake, because PAKΔpscF, which is efficiently phagocytosed but unable to produce IL-1β, did not affect AEP expression during infection (Fig. S6A).
Furthermore we showed that recombinant AEP can exert a dose-dependent direct lytic activity on P. aeruginosa at pH 6 (Fig. S6B). Finally, the importance of AEP activity in AM-mediated bacterial killing of P. aeruginosa was confirmed in primary AEP−/− AMs. Although PAK was phagocytosed at comparable levels in primary AEP−/− AMs and WT AMs (Fig. S6C), we observed that primary AEP−/− AMs were unable to kill PAK, compared with WT AMs (Fig. 5E). The absence of killing by primary AEP−/− AMs was not caused by a deficiency in pro-IL-1β processing, because these cells did not secrete less mature IL-1β compared with WT AMs (Fig. S6 D and E).
Discussion
Using nonopsonic conditions, thus mimicking resting and naïve conditions in the alveolar space, we show that TLR5 and MyD88 (but not TLR4) are of paramount importance for P. aeruginosa phagocytosis and killing. Indeed, P. aeruginosa mutants for the TLR5-recognition site of flagellin monomer domain D1 [PAKL88 or PAKL94 (16, 17)] were resistant to AM killing, and primary TLR5−/− AMs were unable to clear P. aeruginosa. Importantly, in these unprimed conditions, bacterial flagellum was shown to be key for AM–P. aeruginosa interaction because unflagellated mutants, PAKΔfliC or PAKD (the latter secreting flagellin in the supernatant), were equally resistant to AM killing. Crucially, the importance of flagellum/TLR5 interaction on bacterial killing was confirmed by using primary TLR5−/−, TLR4−/−, or MyD88−/−AMs, as well as in vivo, where we demonstrated that 2 h postinfection, TLR5 expression on AMs was the most important factor in clearing bacteria and in containing pulmonary infection.
Flagellum/TLR5-mediated killing was not related to AM-secreted antimicrobial activity, but was associated with TLR5-dependent intracellular entry, because PAK mutants for the TLR5-recognition sites L88 and L94 were less phagocytosed by MH–S cells than WT PAK. The necessity of TLR5/MyD88 engagement for P. aeruginosa phagocytosis was also confirmed by using primary TLR5−/− and MyD88−/− AMs. Importantly, TLR4 signaling did not play any role in P. aeruginosa uptake by primary AMs. Loss of bacterial motility (18) was not a relevant factor in our model. Indeed, PAKL88 and PAKL94 are fully motile bacteria (16); however, none of the mutants was killed by AMs. Moreover, after centrifugation to force bacteria–cell contact, flagellin-mutated bacteria or unflagellated mutants were still considerably less phagocytosed than WT bacteria, demonstrating that the phagocytic phenotype was not attributable to a mobility-deficit or flagellin/TLR5 engagement but was related to diminished specific flagellum/TLR5 interaction.
Unexpectedly, the use of PAKL88 and PAKL94 revealed differences in P. aeruginosa phagocytosis and killing. Indeed, although both mutants were equally resistant to AM killing, PAKL88 stayed mostly extracellular, whereas PAKL94 was better phagocytosed by AMs, albeit not as efficiently as WT PAK. Although a short stretch of 10 aa (N-88-LQRIRDLALQ-97) in the flagellin monomer was known to be crucial for TLR5 recognition (16–18), our data demonstrate that the L88 residue is important for binding/recognition by TLR5 and intracellular entry, whereas the L94 residue may be more important for intracellular downstream events. Indeed, Franchi et al. (14) demonstrated that PAKL94 was much less efficient at inducing mature IL-1β than WT PAK in BMDMs, presumably because of a reduced interaction of bacteria with the intracellular receptor IPAF. These data demonstrated that L94 was a critical residue for the engagement of the inflammasome via IPAF, in addition to residues present at the C terminus of the D0 domain of flagellin monomer (14, 17, 19). Alternatively, other Nod-like receptors such as Naip5 (19, 20) may also be involved in the differential binding of flagellin L94.
Another important finding of our study is that TLR5 is important for production of IL-1β in AMs. The latter result contrasts with that of Franchi and coworkers (14, 21), who studied P. aeruginosa and Salmonella infection of BMDMs and showed, without studying the effect on phagocytosis and bacterial killing, that TLR5 was redundant and that IPAF and ASC were the two inflammasome constituents regulating IL-1β synthesis. Two things may explain this discrepancy: first, whereas these authors used BMDMs, we have used AMs; second, and crucially, whereas they primed BMDMs with LPS before bacterial infection to increase pro-IL-1β production, our protocol did not involve priming, and the production of mature IL-1β occurred following a single event of P. aeruginosa infection (4 h). In our study, TLR5 is essential for bacterial internalization, production, and processing of pro-IL-1β (presumably through IPAF). In the study mentioned above (14, 21), BMDM priming with LPS probably bypassed the need for TLR5 through other receptors (e.g., TLR4) for pro-IL-1β generation. The latter events involving inflammasome activation most likely occurred in a similar fashion in both studies.
The role of inflammasome has been mainly investigated using intracellular bacteria (Legionella, Shighella) (22, 23), but relatively few studies have addressed this issue in AMs infected with extracellular bacteria (e.g., P. aeruginosa). How can P. aeruginosa, then, activate the inflammasome if this bacterium fate is destruction in the phagolysosome? No system to evade the phagolysosome has been described for P. aeruginosa. However, a cytosolic leakage/transport of bacterial constituents is a possibility. The main vector for this transfer may be the T3SS, and the main ligand may be flagellin itself, although most studies used, for their demonstration, elegant but artificial transfection methods to introduce flagellin in the cytosol to activate IPAF. Indeed, we showed that extracellularly added flagellin, although able to produce pro-IL-1β, is unable to activate procaspase-1 and, consequently, to induce the mature IL-1β secretion. Moreover, PAKΔpscF, a mutant deficient in T3SS unable to engage IPAF (13, 14), was not killed by AMs and completely failed to induce the mature IL-1β production (although pro-IL-1β was produced), despite the presence of flagellum and its proper internalization. This demonstrates that IL-1β processing needs both the flagellum and T3SS to activate the inflammasome. Crucially, IL-1β production is not merely a bystander in the killing process but is causative because WT AMs treated with IL-1RA or AMs from IL-1R1−/− mice could not kill P. aeruginosa. This clearly demonstrates the existence of an autocrine loop, involving IL-1β production, IL-1β/IL-1R interaction, and subsequent P. aeruginosa clearance. It has been shown recently that the IL-1β processing (procaspase-1/pro-IL-1β) may, in fact, be operative in lysosomes (24, 25), bringing this machinery in close contact with incoming bacteria and, presumably, decreasing the time of reaction for mature IL-1β production upon bacterial contact. Moreover, this vesicular localization of procaspase-1/caspase-1 may explain why IL-1β release precedes cell lysis, because in the time frame of our experiments, no pyroptosis/cell lysis was observed. However, it is possible that AMs may be inherently more resistant to pyroptosis because this cell type performs a crucial sentinel role within tissues. Interestingly, Carvalho et al. have shown that IL-1RA is produced by AMs in response to flagellin after 4 h, suggesting that this counterregulatory molecule is only secreted at a late time point in the phagocytic process and may not impair bacterial clearance (26).
We went a step further in the dissection of the mechanism by showing that IL-1β increased phagolysosomal acidification. Because the latter is critical for the activation of cysteine proteases involved in bacterial degradation (27), we studied specifically one of them, AEP, which controls the activation of other lysosomal cysteine proteases (28). By using three independent techniques, we observed a reduction of AEP levels upon infection with WT PAK, but not with PAKL94 or PAKΔpscF or purified flagellin (Fig. S3D). AEP was shown to be a key factor participating in AM-mediated P. aeruginosa killing because AEP−/− AMs were unable to kill WT PAK.
In summary (Fig. S7), we describe a phagocytic pathway, demonstrating the role of TLR5 in AM phagocytosis after P. aeruginosa early infection. This occurs in a T3SS-dependent fashion, leading to caspase-1 and IL-1β maturation. The latter induces, after acidification of the phagolysosome, the consumption of AEP, a protease that we demonstrate to be a key factor in P. aeruginosa killing. Our results give a mechanistic insight into early events following P. aeruginosa infection of AMs in the lung and extend our understanding of the crucial role of TLR5 in the defense of the lung against this important pathogen (29, 30–32).
Materials and Methods
Mouse Primary AMs and MH–S Cells.
TLR4−/−, TLR5−/−, MyD88−/− (S. Akira, Osaka University, Osaka, Japan), and IL-1R1−/− mice (CDTA) were backcrossed 8 times with C57BL/6J. C57BL/6J mice (Janvier) were used as control mice. Care and use of the mice was in accordance with Institut Pasteur guidelines in compliance with the European Animal Welfare regulations. Mouse primary AMs were isolated after lung washing with PBS (33). AMs were plated in complete RPMI medium (supplemented with 2 mM l-glutamine, 1% antibiotic, and 5% inactivated FBS). After 2 h, medium was removed and AMs were incubated overnight with fresh medium. Mouse AM cell line MH–S (CRL-2019; ATCC) was maintained in complete RPMI medium supplemented with 1% sodium pyruvate. Primary AMs or MH–S cells were placed into serum- and antibiotic-free RPMI for 5 h before infection.
Bacterial Strains.
WT strain PAK (from S. Lory, Harvard Medical School, Boston, MA) is a commonly studied P. aeruginosa strain. All modified mutants used were derived from PAK (Table S1). Bacteria were grown overnight in LB broth at 37 °C and then transferred to fresh medium and grown by shaking at 100 rpm for 4–5 h to mid-log phase. The culture was centrifuged at 3,000 × g and the pellet was washed and resuspended in PBS. The OD600nm was adjusted to give the desired inoculum. Inoculum was verified by serial dilutions plated on LB agar to determine the number of colony-forming unit (CFU). Bacterial growth of each PAK strain used in this study was shown to be identical.
Bacterial Clearance Assay.
A total of 105 AMs were infected with bacteria (MOI: 0.1). After 2–4 h, supernatants were collected and cells were lysed with 0.1% Triton X-100 (a concentration that did not affect PAK viability) in H2O in sequential washes to harvest total bacteria. To quantify total viable bacteria, pooled cell supernatants and lysates were diluted and plated on LB agar to determine CFU scores. Results are expressed as percentages as follows: (CFU counts recovered without AMs − CFU counts recovered after AM infection) × 100. For specific experiments, centrifugation was performed (80 × g; 4 min) to ensure synchronous contact between WT or nonmotile P. aeruginosa and AMs. To test bacterial clearance in vivo, mice were anesthetized by ketamine-xylazine intramuscular injection, and then infected intratracheally with 105 CFU (50 μL). Mice were euthanized 2 h later. BAL fluids (2 × 250 μL pooled) were performed, diluted, and plated on LB agar plates to quantify total CFU counts. The percentage of surviving bacteria in BAL was assessed as follows: (total CFU counts in BAL/CFU counts of inoculum) × 100. Total cell counts were measured with a Coulter Beckman Counter. Cell differential counts were determined after cytospin centrifugation and staining with Diff-Quik products (Medion Diagnostics).
Phagocytosis Assay.
A total of 5 × 105 AMs were infected with bacteria (MOI: 10) to 1 h. Free and adherent bacteria were removed by washing cells with PBS and were killed with tobramycin treatment (40 μg/mL; 30 min). Then, cells were washed and lysed in H2O containing 0.1% Triton X-100. The number of bacteria in lysates was determined by counting CFU on LB agar plate. The percentage of relative phagocytosis index was assessed as follows: (CFU counts in mutant PAK-treated cells/CFU counts in WT PAK-treated cells) × 100. In other experiments, bacteria were labeled with 0.1 mg/mL FITC (Sigma) in Na2CO3 buffer, pH 9.5, at 37 °C while shaking at 100 rpm for 1 h. After incubation and treatment as described above, cells were resuspended in PBS-EDTA 2 mM, and then fixed in 3% PFA. To quantify phagocytosed bacteria, fixed cells were used for FACS analysis of cell-associated fluorescence.
ELISA.
IL-1β and TNFα were assayed after stimulation using DuoSet ELISA (R&D Systems). Recombinant murine IL-1β and IL-1RA were purchased from Peprotech.
Endosomal pH Measurement.
Endosomal pH measurement assays have been described previously (28). MH–S cells were pulsed with 1 mg/mL FITC- and Alexa-647-labeled 40-kDa dextrans (Molecular Probes) for 10 min at 37 °C and washed with PBS with 1% BSA. Cells were chased and analyzed by FACS, via a FL1/FL4 gate selective for cells that have endocytosed the probes.
AEP Protease Activity.
Protease activity assays were performed on a FluoStar Optima (BMG Labtech) by measuring the release of fluorescent N-acetyl-methyl-coumarin in citrate buffer (pH 5.5) or PBS (pH 7.4) at 37 °C after incubation of AEP or total lysates with its specific substrate (Z-Ala-Ala-Asn-NHMec; Bachem).
AEP Immunofluorescence.
Primary AMs were seeded on poly-l-lysine-coated glass coverslips. Cells were infected with bacteria (MOI: 10) for 3 h, washed, fixed with 4% PFA, and quenched by adding 0.1 M glycine. Cells were permeabilized in PBS/0.05% saponin/0.2% BSA, washed, and then incubated with anti-AEP Ab. The coverslips were mounted with Fluoromount G and were analyzed by confocal microscopy (Zeiss confocal microscope LSM 700). For Z-stack acquisition, several images were acquired.
Statistics.
Data are presented as means ± SD. A one-way ANOVA with Fischer's protected least significant difference test was conducted.
Supplementary Material
Acknowledgments
We acknowledge B. Solhonne for her assistance, Prof. Z. Xing (Centre for Gene Therapeutics, McMaster University, Hamilton, Canada) for useful discussions, and Bernhard Ryffel for providing IL-1R1−/− mice (Unité Mixte de Recherche 6218, Centre National de la Recherche Scientifique, Orléans, France). We thank Vaincre la Mucoviscidose for funding this work.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108464109/-/DCSupplemental.
References
- 1.Mogayzel PJ, Jr, Flume PA. Update in cystic fibrosis 2009. Am J Respir Crit Care Med. 2010;181:539–544. doi: 10.1164/rccm.200912-1943UP. [DOI] [PubMed] [Google Scholar]
- 2.Gordon S. The macrophage: Past, present and future. Eur J Immunol. 2007;37(Suppl 1):S9–S17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 3.Di A, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 4.Deriy LV, et al. Disease-causing mutations in the cystic fibrosis transmembrane conductance regulator determine the functional responses of alveolar macrophages. J Biol Chem. 2009;284:35926–35938. doi: 10.1074/jbc.M109.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 7.Underhill DM, et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 8.Ozinsky A, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 10.Blander JM, Medzhitov R. On regulation of phagosome maturation and antigen presentation. Nat Immunol. 2006;7:1029–1035. doi: 10.1038/ni1006-1029. [DOI] [PubMed] [Google Scholar]
- 11.Doyle SE, et al. Toll-like receptors induce a phagocytic gene program through p38. J Exp Med. 2004;199:81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahenthiralingam E, Speert DP. Nonopsonic phagocytosis of Pseudomonas aeruginosa by macrophages and polymorphonuclear leukocytes requires the presence of the bacterial flagellum. Infect Immun. 1995;63:4519–4523. doi: 10.1128/iai.63.11.4519-4523.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao EA, Ernst RK, Dors M, Mao DP, Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc Natl Acad Sci USA. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchi L, et al. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 15.Jyot J, et al. Type II secretion system of Pseudomonas aeruginosa: In vivo evidence of a significant role in death due to lung infection. J Infect Dis. 2011;203:1369–1377. doi: 10.1093/infdis/jir045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma A, Arora SK, Kuravi SK, Ramphal R. Roles of specific amino acids in the N terminus of Pseudomonas aeruginosa flagellin and of flagellin glycosylation in the innate immune response. Infect Immun. 2005;73:8237–8246. doi: 10.1128/IAI.73.12.8237-8246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith KD, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 18.Amiel E, Lovewell RR, O'Toole GA, Hogan DA, Berwin B. Pseudomonas aeruginosa evasion of phagocytosis is mediated by loss of swimming motility and is independent of flagellum expression. Infect Immun. 2010;78:2937–2945. doi: 10.1128/IAI.00144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao EA, et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lightfield KL, et al. Differential requirements for NAIP5 in activation of the NLRC4 inflammasome. Infect Immun. 2011;79:1606–1614. doi: 10.1128/IAI.01187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 22.Amer A, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006;281:35217–35223. doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrei C, et al. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes. Proc Natl Acad Sci USA. 2004;101:9745–9750. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wewers MD. IL-1beta: An endosomal exit. Proc Natl Acad Sci USA. 2004;101:10241–10242. doi: 10.1073/pnas.0403971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho FA, Aitken JD, Gewirtz AT, Vijay-Kumar M. TLR5 activation induces secretory interleukin-1 receptor antagonist (sIL-1Ra) and reduces inflammasome-associated tissue damage. Mucosal Immunol. 2011;4:102–111. doi: 10.1038/mi.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bird PI, Trapani JA, Villadangos JA. Endolysosomal proteases and their inhibitors in immunity. Nat Rev Immunol. 2009;9:871–882. doi: 10.1038/nri2671. [DOI] [PubMed] [Google Scholar]
- 28.Sepulveda FE, et al. Critical role for asparagine endopeptidase in endocytic Toll-like receptor signaling in dendritic cells. Immunity. 2009;31:737–748. doi: 10.1016/j.immuni.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Balloy V, et al. The role of flagellin versus motility in acute lung disease caused by Pseudomonas aeruginosa. J Infect Dis. 2007;196:289–296. doi: 10.1086/518610. [DOI] [PubMed] [Google Scholar]
- 30.Feuillet V, et al. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci USA. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramphal R, Balloy V, Huerre M, Si-Tahar M, Chignard M. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J Immunol. 2005;175:3927–3934. doi: 10.4049/jimmunol.175.6.3927. [DOI] [PubMed] [Google Scholar]
- 32.Sutterwala FS, et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonçalves de Moraes VL, Singer M, Vargaftig BB, Chignard M. Effects of rolipram on cyclic AMP levels in alveolar macrophages and lipopolysaccharide-induced inflammation in mouse lung. Br J Pharmacol. 1998;123:631–636. doi: 10.1038/sj.bjp.0701649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.