Abstract
An exhaustive description of the molecular recognition mechanism between a ligand and its biological target is of great value because it provides the opportunity for an exogenous control of the related process. Very often this aim can be pursued using high resolution structures of the complex in combination with inexpensive computational protocols such as docking algorithms. Unfortunately, in many other cases a number of factors, like protein flexibility or solvent effects, increase the degree of complexity of ligand/protein interaction and these standard techniques are no longer sufficient to describe the binding event. We have experienced and tested these limits in the present study in which we have developed and revealed the mechanism of binding of a new series of potent inhibitors of Adenosine Deaminase. We have first performed a large number of docking calculations, which unfortunately failed to yield reliable results due to the dynamical character of the enzyme and the complex role of the solvent. Thus, we have stepped up the computational strategy using a protocol based on metadynamics. Our approach has allowed dealing with protein motion and solvation during ligand binding and finally identifying the lowest energy binding modes of the most potent compound of the series, 4-decyl-pyrazolo[1,5-a]pyrimidin-7-one.
Keywords: ADA , well-tempered metadynamics, ligand/protein docking, path collective variables, reweighting algorithm
Adenosine Deaminase (ADA) regulates the purine metabolism by catalyzing the irreversible hydrolysis of adenosine to inosine and 2′-deoxyadenosine to 2′-deoxyinosine. Thus, this enzyme plays a crucial role in many pathologies such as inflammation, some types of cancer, and others which are strictly connected to the physiological level of these nucleosides (1–5). Despite great efforts in developing ADA inhibitors, only Pentostatin is currently in clinical use (I in Fig. S1) (3). However, recent progress has been reported by Terasaka et al. and by some of us who have developed a new generation of nonnucleoside ADA inhibitors (II, III, and IV in Fig. S1) (6–11). Unfortunately, the understanding at molecular level of the ligand/ADA interaction is hampered by the pronounced ability of the active site to accommodate different inhibitors and by the crucial role played by water molecules during ligand binding. A rational drug design is further complicated by the fact that in response to different inhibitors, ADA can assume either an open or a closed conformation by changing the position of the H3 α-helix (Thr57-Ala73). The open conformation corresponds to the apo-form (PDB ID code 3iar) and is preferred when a nonnucleoside inhibitor is bound (12, 13). In this case, the active site presents a hydrophilic subsite S0 and three hydrophobic subsites F0, F1, and F2 (Fig. 1A) (13). The S0 subsite is defined by the structural gate formed by a β-strand (Leu182-Asp185) and two leucine side chains attached to the H3 α-helix while the F0 site is formed by the hydrophobic side chains of the H3 α-helix. In the open form the H3 α-helix assumes a conformation that exposes two additional hydrophobic subsites in the upper part the helix, namely F1 and F2 (13). The binding of a ground-state inhibitor, like the hydroxylated form of adenosine (HDPR) (V in Fig. S1) (14), induces a conformational change that leads to the closed form. In this case, the gate is closed, the F1 and F2 sites are no longer accessible and the active site is composed only by S0 and F0 (Fig. 1B).
Fig. 1.
Representation of ADA in the open (A) and closed form (B).
While the structural difference between the two conformations is believed to be the result of binding to different inhibitors, the specific details of the enzyme conformational changes are still unclear. For instance, Kinoshita, et al. (15) have recently reported that the ground-state inhibitor EHNA (VI in Fig. S1), expected to bind the closed form, has been cocrystallized rather surprisingly in the open one. In fact, experimental evidences suggest that the n-hexyl group of EHNA interacts with the narrow hydrophobic entrance of the ADA active site destabilizing the closed form. Another example of the difficulty of predicting a ligand binding model comes from the inhibitor FR221647, which has also been found to bind to the open conformation in spite of having been designed as a binder of the closed form (12).
In this confusing scenario, a practical approach is to find a lead compound with whatever strategy works (high-throughput screening, ligand-based design etc.), understand at molecular level the lead/protein recognition mechanism and finally, if necessary, try to rationally improve the lead.
Here we have carried out the first two steps in this strategy by applying a combined experimental and theoretical approach. First we discovered a new class of inhibitors combining a number of functional groups that are present in many of the reported inhibitors (Table 1). Among these new inhibitors, the most potent is 4-decyl-pyrazolo[1,5-a]pyrimidin-7-one, here named 3b. In the lack of further experimental information we performed an extensive series of calculations aimed at understanding the ligand/protein molecular recognition process. We first performed standard docking calculations, which are the most economical way of obtaining molecular information on ligand/protein interaction. Unfortunately, all the docking attempts failed due to the ADA flexibility and the active role of the water molecules. Thus we had to step up the computational strategy and use metadynamics (16, 17). Metadynamics has already been applied with success to difficult docking processes (18–20). After extensive calculations, the binding mode of 3b has been understood revealing also the important role played by protein motion and solvent during the ligand binding. We are comforted in our conclusions by a number of experimental data and the similarity of our binding mode with that of other ADA inhibitors.
Table 1.
ADA Inhibition Data of Derivatives 3a–c, and 6–8
 |
 |
||
| N° |
n |
R |
Ki (nM) * |
| 3a | 8 | 27.00 ± 0.95 | |
| 3b | 9 | 1.49 ± 0.03 | |
| 3c | 10 | 3.12 ± 0.09 | |
| 6 | NH2 | n.a.† | |
| 7 | NHCOC6H4pCF3 | n.a. | |
| 8 | NHCONHC6H4pCF3 | n.a. | |
| (+)-EHNA | 1.14 ± 0.10 | ||
*The Ki values are means ± SEM (standard error of the mean).
†n.a.: non active. Inhibition occurred at a concentration higher than 10 μM.
Results
Ligand-Based Design.
Prompted by the promising results of the 4-aminopyrazolo[3,4-d]pyrimidine (APPs) series (compound III in Fig. S1) as ADA inhibitors, recently identified by some of us (9, 10), and using our synthetic expertise in the pyrazolopyrimidines field, we decided to further investigate this scaffold. Thus by modifying the heterocyclic ring, a new series of compounds bearing a pyrazolo[1,5-a]pyrimidin-7-one system has been developed. Our investigation started synthesizing compounds 3a–c, carrying a n-nonyl, n-decyl and n-undecyl chain, respectively, in the position 4 of the heterocyclic core. As we demonstrated in the APPs series (9), a long, linear, and hydrophobic alkyl chain can interact favorably with the enzyme active site. In fact, tested on the bovine spleen ADA, compounds 3a–c have been found to potently inhibit ADA, showing Ki values in the nanomolar range (Table 1). Moreover, an activity trend similar to the parent APPs was observed as the ranking of inhibitory potency being n = 8 (3a) < n = 9 (3b) > n = 10 (3c). In order to further investigate the role of the 4-alkyl chain in the interaction with the active site of the enzyme, derivatives 7 and 8, bearing bulkier and more rigid groups at position 4, were likewise synthesised and tested. In 7 the two phenyl rings are connected through an amide linker while in 8 the linker is an ureic residue. Both these functions are present in other potent ADA inhibitors (7, 12). As summarized in Table 1, neither 7 nor 8 showed any appreciable inhibitory activity, proving that in the pyrazolo[1,5-a]pyrimidin-7-one series the preferred substitution pattern is represented by the flexible alkyl chain.
In order to rationalize these experimental data and to proceed with a structure-based lead optimization, it was necessary to understand at molecular level the binding mechanism to ADA of the most potent compound of the series, 3b. This aim was achieved through an extensive computational study described in details in the following paragraphs.
Molecular Docking Studies.
We first performed a series of docking simulations of 3b in the ADA active site using the AutoDock program (vs. 4.0) (21, 22). Both the open and closed conformation were explored applying different docking settings (see SI Text). We verified that AutoDock correctly predicts the experimental pose for EHNA and HDPR in their open and closed form, respectively. The docking calculations of 3b in the ADA closed form gave among others a highly populated cluster in which 3b binds similarly to the nucleoside inhibitor HDPR. This cluster presents also the lowest energy solution. In this pose 3b coordinates the zinc ion through the oxygen of the pyrimidinone ring (see Fig. S2A). Similarly to HDPR, 3b H-bonds with Asp295 and Glu217 in the S0 site through the oxygen and nitrogen atoms. On the other hand, the n-decyl chain only partially fills the region which, in the case of HDPR, would be occupied by the ribose ring and points towards the hydrophobic region formed by the Leu62 Phe65 and Leu105 residues at the F0 site.
At first glance this is a plausible result, however in the light of recent experiments (15), which found the ground-state inhibitor EHNA to bind the open form of ADA and not the closed one as expected, and being this ligand, at variance from the other compounds of its class, not directly involved in the coordination of the metal, we decided to substantiate the proposed binding pose with further experiments. Thus, we investigated the ability of 3b to coordinate the zinc ion performing a number of UV spectroscopy measurements. The UV spectra were recorded upon addition of increasing amount of zinc(II) ion into a homogeneous solution of compound 3b (see SI Text). Surprisingly, no appreciable change in optical density of the absorption was observed, thus indicating that 3b is not able to coordinate the metal.
This experimental result lead us to carry out additional docking calculations using a water molecule as the fifth coordination group of zinc. The open form of ADA was used in all these attempts and furthermore, some water molecules known to be important for ligand binding, such as those involved in the interactions of EHNA with Gly184 and Glu217 (15), were also explicitly taken into account in some of the docking calculations. Unfortunately, in all these docking attempts, the ligand has never been found to interact with residues known to be important for ligand binding in ADA, such as Asp19, Gly184, and Glu217. Furthermore, none of the water molecules, when included in the docking calculation, has been involved in the ligand binding. These reasons and the poor convergence of the docking calculations have casted doubts on the reliability of these results (see SI Text for details).
We ascribe this failure to the fact that docking algorithms take into account enzyme flexibility and solvent effects in a limited way. In particular, the solvent can be taken into account either in an implicit way or by adding, as done here, an appropriate number of explicit water molecules. However, the position of these waters does not change during the calculations and if it is not known beforehand, one risks to make things worse. In the ADA case, while the position of the water coordinating the zinc ion can be defined with a good level of precision, the other water molecules, like those involved in the binding of some of the ADA inhibitors (14, 15, 23), can occupy different positions and assume a number of orientations in response to different inhibitors. Thus, the inclusion of waters through an explicit but fixed model might be not helpful and could lead to rather inaccurate results as we found to be the case here.
Well-Tempered Metadynamics.
The failure of the docking calculations suggests using atomistic simulations in explicit water in order to fully include solvent and protein flexibility. Unfortunately, standard molecular dynamics (MD) can access only a time scale of hundreds of nanoseconds, while processes like ligand/protein docking usually take from microseconds to hundreds of seconds. Thus, the use of enhanced sampling is mandatory. Among the emerging techniques, metadynamics has shown to be useful in the study of ligand docking (18–20). We used here this technique in its new variant named well-tempered metadynamics, which enhances sampling allowing the reconstruction of the free-energy profile of the process of interest by adding an adaptive bias on a selected numbers of collective variables (CVs) (17). The choice of CVs represents a crucial point because, to construct the Free-Energy Surface (FES), the CVs must describe all the slow modes relevant for the process under study (24–26).
The change from closed to open form is mostly determined by the movement of the H3 α-helix and because it represents a slow mode, use of a CV that is able to describe this movement is necessary (see Methods for details). Recently, a number of path CVs (PCV) have been developed in our research group (27, 28), which are able to reconstruct the lowest free-energy path that connects an initial and a final state. In the ADA case, assuming that the initial and the final state correspond to ADA with the H3 α-helix in the closed and open conformation, respectively, a PCV is constructed with the cartesian coordinates of the alpha carbons of the residues that determine the α-helix movement. This type of CV has been successfully applied in recent studies on complex protein motion (29, 30) and ligand/protein docking (20).
In order to specify the position of the ligand relative to the enzyme, we used a distance CV which measures the distance between the center of mass of the pyrazolo[1,5-a]pyrimidin-7-one ring and that of a group of protein atoms (see Table S1).
Under the acceleration of metadynamics, the ligand is able to explore the whole binding site moving from one free-energy minimum to the next and then going back into the previous one, thus overcoming the large free-energy barriers which are encountered during the binding process. Thanks to these recrossings between different basins, the calculated FES is accurate and quantitatively well characterized with the deepest basin corresponding to the preferred binding mode of the ligand inside the protein. We underline once more that side chain flexibility, the motion of the H3 α-helix, and solvation are fully taken into account. It is essential for the convergence of the calculations the use of the PCV which accelerates the H3 α-helix motion, allowing a continuos change from one conformation to the other.
The Energy Minima.
The full exploration of the binding site took approximately the equivalent of 0.3 μs. In Fig. 2 three different minima, A, B, and C, can be identified. Basin A is approximately 3 kcal/mol deeper than the other two minima. Here, the ligand is into the innermost binding site while in B and C it is located in more external sites. A detailed discussion on basin B and C is given in the SI Text while the movie showing the ADA binding site exploration by the ligand can be found as Movie S1.
Fig. 2.
Representation of the FES of the 3b binding process to ADA with the three main minima shown as insets, (A, B and C). The FES is represented as function of the distance and dihedral CVs using isosurfaces of 1 kcal/mol. It is important to underline that no bias has been added to the dihedral CV and the statistics collected during the metadynamics simulations on the torsion CV is used to generate the final FES using the reweighting protocol (31). In the insets the ligand and the main interacting residues are displayed as licorice while the protein is represented as cartoon with the H3 α-helix colored in yellow. The ligand/Asp19 water bridge interaction is also shown while only polar hydrogens interacting with the ligand are displayed for clarity.
Basin A.
In this basin just as in the case of the EHNA/ADA complex, the protein is in the open conformation (Fig. 3A). Here, the ligand engages a number of favorable interactions with the surrounding residues. In particular, the oxygen atom of the pyrazolo[1,5-a]pyrimidin-7-one H-bonds to the NH backbone and the hydroxyl group of Ser103, while the nitrogen atom of the pyrazole ring interacts with the Cys153 side chain (Fig. 2). Additionally, the oxygen of the pyrimidinone moiety is involved in a H-bond interaction with Asp19 via a water molecule. Besides these polar interactions, the pyrazolo[1,5-a]pyrimidin-7-one ring engages several hydrophobic contacts with the surrounding residues. In fact, the methyl group of the pyrimidinone moiety points towards the hydrophobic side chains of Phe65 and Met69, while the aromatic ring lies close to His17 and His214 where a π-π and a T-shaped interaction, respectively, are possible.
Fig. 3.
(A) Overlapping of the conformations of ADA found by metadynamics in basin A (yellow), ADA complexed with EHNA (green, PDB ID code 2z7g) (15) and ADA complexed with HDPR (orange, PDB ID code 1krm) (14). The metadynamics ADA conformation (yellow) is similar to that ligated to EHNA (open form, green) with a low rmsd value of 1.84 Å calculated for the H3 α-helix backbone atoms. (B) Overlapping in the ADA binding site of the 3b binding conformation calculated by metadynamics (cyan) and the X-ray binding conformation of FR104783 (orange) (PDB ID code 1wxy) (13).
The atoms of the n-decyl chain were not used in defining the distance CV (see Table S1) thus different conformations of this flexible chain are to be found in the FES minima. After clustering the ligand tail conformations, two main families have been found. In one, Aa, the tail is placed in the hydrophobic F0 subsite where multiple contacts are possible with residues such as Phe61, Leu62, Phe65, Leu106, Trp117, Met155, Pro116, and His157. In the other, Ab, the n-decyl chain points towards the upper part of the H3 α-helix filling the F1 and F2 sites where different hydrophobic contacts are engaged with residues like Phe61, Leu58, and Thr269 (see Fig. S3 A and B). In both cases the pyrazolo[1,5-a]pyrimidin-7-one ring is always placed in the same position in the active site and because the protein assumes always the open conformation, the alkyl tail can occupy all these hydrophobic pockets. Due to the high flexibility of this long alkyl chain, it is natural to hypothesize that Aa and Ab are equally energetically favorable although the one with the alkyl chain placed at the bottom of the H3 α-helix, Aa (Fig. S3A), seems to be more probable as a consequence of the higher hydrophobicity of this region. The relative stability of these two different conformations has been further studied and discussed in details in the following paragraph.
The Alkyl Tail.
In order to assess the stability of the two binding conformations Aa and Ab found at basin A, one with the alkyl chain pointing towards the F0 site and the other one to the F1 and F2 sites, respectively, MD simulations over 10 ns long have been performed. The results showed a different behavior in the two cases. The binding conformation Aa was very stable during the whole simulation with a low average rmsd value for the ligand (see Fig. S3C) and with all the above described ligand/protein interactions fully conserved. In contrast, Ab first maintains its original position in the binding site but, after approximately 3.5 ns of simulation, it moves the alkyl tail down along the H3 α-helix transforming in Aa (see the lower graph in Fig. S3C).
The different behaviors of Aa and Ab observed during the MD simulations prompted us to study in more detail the stability of these two conformations. Thus, we performed a new metadynamics run using as CV a distance that is able to distinguish the two tail conformations (see Table S1). Once the calculations converged, the FES was calculated along this CV (CV2) and the distance CV used in the previous metadynamics simulations (CV1) (see Fig. S3D) using the reweighting algorithm very recently developed in our group by Bonomi, et al. (31). Thanks to this algorithm, once the free energy of the metadynamics simulation is converged, the FES can be recalculated on CVs different from those used originally in the metadynamics run. The FES shows two minima corresponding to Aa and Ab with the Aa basin wider and about 1.3 kcal/mol deeper than that of Ab. In line with the MD study, these results suggest that although both the poses are possible, Aa has a greater stability.
The Solvent Role.
In the binding mode that characterizes basin A, a water molecule plays a relevant role in the interaction between 3b and Asp19 highlighting the importance of the solvent in ligand binding. In order to assess the relevance of this water bridge interaction, the FES along the distance and the interfacial water CV (see SI Text) (32) has been calculated using the reweighting protocol (31). Looking at the FES in Fig. 4, it is worth noting that the ligand/ADA complex in basin A, corresponding approximately to the distance CV range of 7–8 Å, is always characterized by the presence of at least one interfacial water between the ligand and Asp19 and that the energetic contribution of this water bridge interaction to the ligand binding is about 6 kcal/mol (Fig. 4). This is evidence of the important role of water similarly to what has been found in other ADA inhibitors like EHNA or HDPR (14, 15). A water at similar position but not interacting directly with the ligand can be seen also in the X-ray structure of EHNA/ADA complex (15). Our results suggest that this water has a more than structural role. If in a docking calculation we now include explicitly this water and orient the Asp19 side chain so as to interact with 3b via this water, AutoDock predicts conformations very similar to that found by metadynamics among the most favorable poses (see Fig. S4A).
Fig. 4.
Representation of the FES of the 3b/ADA binding process as function of the distance and the interfacial water CVs using isosurfaces of 1 kcal/mol. Looking at the FES it is clear that when the ligand is in basin A (distance CV range of 7–8 Å) at least one water bridge interaction is present between 3b and Asp19 as shown in the inset picture. The energetic contribution to the ligand binding of this interaction is about 5.6 kcal/mol. This is computed as the free-energy difference between the states when the water bridge interaction is present (Interfacial Water CV > 1) and those when this interaction is not formed (Interfacial Water CV < 0.2), considering the ligand in basin A (distance CV range of 7–8 Å).
The Asp19 residue is involved also in the binding of other classes of inhibitors. This interaction occurs either through a direct H-bond, like in the case of HDPR (PDB ID code 1krm) (14), or via a water bridge interaction as in Coformycin (PDB ID code 3ewc) (23). Furthermore, it is worth mentioning that Kinoshita et al. have suggested that the interactions of EHNA with His17 and Asp19 are anchor points for correctly placing the inhibitor in the active site (15). As described above, both these residues play a role in the 3b binding mode. We stress that none of this information was used in setting up the simulation protocol.
Experimental Data Rationalization.
Our results rationalize the experimental data on the pyrazolo[1,5-a]pyrimidin-7-one series (Table 1), which show that the activities of 3a and 3c are lower than that of 3b. We believe that neither 3a nor 3c can fill comfortably the F0 site, the one because the alkyl tail is too short, the other because it is too long. We plan to perform further experiments to validate our hypothesis. For the time being we limit ourselves to docking calculations using the protein conformation found by metadynamics and taking explicitly into account the bridging water.
For 3b AutoDock finds that the pose predicted by metadynamics is among the best scoring ones (see Fig. S4A). Note that the docking algorithm did not find the Ab conformation suggesting once more that the preferred and most stable binding conformation is Aa.
In the case of 3a and 3c, docking calculations yielded different results. In fact, docking of 3a showed a number of different binding possibilities and only in the second most populated cluster can be found a pose comparable to that of 3b. However, in this pose the pyrazolo[1,5-a]pyrimidin-7-one ring is translated and rotated by 180 ° thus losing some favorable interactions with ADA (see Fig. S4B). In contrast, docking on 3c found a binding mode very similar to that of 3b with the pyrazolo[1,5-a]pyrimidin-7-one ring slightly displaced from its usual position but still able to form all the main interactions with the protein (see Fig. S4C). These results clearly validate our hypothesis that due to the loss or the weakening of some favorable interactions with the protein a shorter alkyl chain (3a) affects the ADA affinity more than the longer one (3c) in line with the experimental data.
Comparison with Other ADA Inhibitors.
Comparing the binding modes of different ADA inhibitors can be problematic because this enzyme is very complex and similar ligands can bind to the protein in different manners. Still we have found it useful to compare the 3b binding mode with the FR104783 (VII in Fig. S1) X-ray pose (PDB ID code 1wxy) (13). As can be seen in Fig. 3B, there is a clear similitude, in fact one of the two phenol rings of FR104783 occupies the bottom of the S0 site just like the pyrazolo[1,5-a]pyrimidin-7-one ring of 3b, and in both cases they are H-bonded to Asp19. Furthermore, in FR104783, the second phenol ring occupies the hydrophobic F0 subsite just like the alkyl tail of 3b. The alkyloxy branch of FR104783 occupies the F1 and F2 subsites in a manner similar to the Ab pose of 3b suggesting the utility of targeting this region in future lead optimization studies.
Other findings are further validated by a comparison with other crystallographic structures. For instance, the open conformation assumed by the protein when 3b is in the basin A is similar, albeit slightly more open, to what has been found in the EHNA/ADA complex (Fig. 3A) (15). Based on the metadynamics results, we can a posteriori say that the failure of all the preliminary docking trials in which we used the open form of ADA either hydrated (with three water molecules) or dry, is due to: (i) a slightly different conformation of the H3 α-helix; (ii) the flexibility of the side chains which allows different ligands to bind; and (iii) the user defined choice of water molecules to be considered in the calculation. As we have seen in our docking samplings, the latter choice is difficult and without a priori knowing the position occupied by the waters, one can easily incur into errors.
Validation of the Method.
The success of the metadynamics simulations is strongly related to the choice of the CVs and when a relevant CV is neglected the reconstruction of the FES fails (20, 33). This implies the necessity of running a number of metadynamics simulations to find out which CVs are needed for the process under study. With the aim of assessing the reproducibility of our results, we have carried out two additional metadynamics simulations using CVs different from the original one (see SI Text, Table S1, Fig. S5). The results have indicated that the H3 α-helix motion (PCV) and the ligand move (distance CV) are the most relevant degrees of freedom acting on the system and that the inclusion of other CVs, like the interfacial water CV, is not necessary to achieve the final results. Without knowing a priori which are the most important degrees of freedom in a ligand/protein docking study, it is advisable to use standard CVs, such as distance or torsion CVs, to achieve the best results in the shortest time. Eventually, as we have shown in the Solvent Role paragraph, one can construct, using the reweighting algorithm, the FES as a function of different CVs thus investigating the role of these degrees of freedom in the event under investigation. Another intriguing possibility is to use a very recently developed formalism, namely orthogonal space random walk (34). This algorithm is able to couple the CVs motion with the system dynamics through structural relaxation, thus allowing the exploration of hidden degrees of freedom. In doing so, CVs that are neglected during the docking study, such as protein motion or solvent effect, can be taken into account. This algorithm has been already used to sample small protein motion (35) and it would be interesting to apply it in more complex studies such as ligand/protein docking.
Discussion
As discussed by Leach, et al. (36), docking methodologies seem to have reached a plateau in recent years and are waiting for an important breakthrough. In this study on a series of novel ADA inhibitors we have been confronted with some of the major docking hurdles and overcome them through the use of an advanced computational technique. Particularly, a metadynamics-based protocol has been employed to unveil the binding pose of the most potent compound of the series 3b, taking into account effects such as protein flexibility and solvation whose inaccurate description renders docking not reliable. Our findings are in agreement with a number of experimental data and supported by extensive calculations. Among the encouraging experimental facts we mention the similitude of the 3b binding mode with the X-ray docking pattern of other ADA inhibitors. Furthermore, the solvent has been found to play a fundamental role in the binding of our inhibitor. In fact, a water molecule mediates the important ligand interaction with Asp19, that is known to be involved in the binding of many other potent ADA inhibitors.
Certainly in terms of speed and computational cost, docking protocols remain the first choice technique in lead discovery and optimization, nevertheless in some cases, like ADA, the use of more advanced techniques is necessary. Unfortunately, metadynamics-based protocols are computationally expensive and are not applicable for high-throughput screening purposes. For instance, in the present case, considering only the final 3b/protein docking metadynamics, the calculation required about 105 CPU hours on High Performance Computing cluster, thus being feasible if performed on just a few ligands. However, the high scalability of molecular dynamics simulation codes and the increasing computational power can help to achieve results in more reasonable time.
The information derived from this study will be very useful for the following lead optimization strategies. In fact, a low inhibitory activity, nanomolar or picomolar, is a prerequisite to achieve potent enzymatic inhibition and to develop drug candidates. However, as very recently discussed by Gleeson, et al. (37), the level of activity of molecules is not the only determining factor to achieve drug efficacy because even other properties of molecules (e.g., log P, pharmacokinetics profile) play crucial roles. Our results provide the possibility to rationally optimize the potent lead compound 3b, representing an important opportunity to improve the protein/ligand interaction and the pharmacokinetics properties of the new designed compounds.
Methods
Metadynamics Simulations.
Before doing metadynamics simulations the 3b/ADA complex was equilibrated through 5 ns MD under NPT conditions at 1 atm and 300 K using the parmff99SB Amber force field (38, 39), as implemented in version 2.7 of the NAMD code (40). For the zinc subsite the bound model was used adding to the force field the bond and angle parameters reported by Coi, et al. (41). The Amber charges were applied to all the protein and waters atoms except for the ligand and the zinc subsite. For these the restrained electrostatic potential (RESP) charges were used (see SI Text). All the simulations were carried out in explicit solvent using the TIP3P water model (42) using periodic boundary conditions. The PLUMED plugin was used to carry out metadynamics calculations with the NAMD code (43).
It is important to underline that because the ligand starting conformation does not affect the final results of a well converged metadynamics simulation, we can arbitrarily decide to use one of the poses predicted by the docking program. In our case, a pose from the most populated cluster in an open form docking run was taken as the starting conformation.
The estimation F(s,t) at time t of the free-energy surfaces F(s) as a function of the CV s was determined by metadynamics (15) in its new well-tempered variant (16), using the following formula:
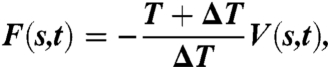 |
where V(s,t) is the bias potential added to the system and T is the temperature of the simulation. ΔT is the difference between the fictitious temperature of the CV and the temperature of the simulation. The bias potential is made up by the sum of the Gaussians deposited along the trajectories of the CVs. Thanks to this new formalism, one can increase barrier crossing and facilitate the exploration in the CVs space by tuning ΔT. A Gaussians deposition rate of 0.5 kcal/mol per picosecond was initially used and gradually decreased on the basis of the adaptive bias with a ΔT of 3,300 K.
The Path Collective Variables.
In order to simulate the movement of the H3 α-helix, we used the path CV (PCV) (27) using as metrics the rmsd of the Cα atoms of residues from Ile50 to Pro70. The PCVs are extremely powerful whenever one wants to study a transition between two states A and B. As state A we chose the X-ray conformation of ADA in the closed state (PDB ID code 1krm) (14), while state B represents the open form (PDB ID code 2z7g) (15). Now, let S(R) be a reduced representation of a generic configuration R. If the choice of S is appropriate, we would expect the reactive trajectories to be bundled in a narrow tube around the path. To trace this path, we follow the procedure of Branduardi et al. introducing the two variables s(R) and z(R):
 |
which measure the intercept and the distance of a microscopic configuration R from the reference path S(l), respectively. P is the number of frames that define S(l), ||S(R)—S(l)|| is calculated as the rmsd and λ is proportional to the inverse of the mean square displacement between successive frames. As the structure of the H3 α-helix has only slight differences between the open and closed conformation, we described the transition between the closed and open conformation of the enzyme only using two frames S(1) and S(2), being the closed (state A) and the open conformation (state B), respectively. The λ value was set to 0.75 Å-2. We performed metadynamics only in the space of s(R) while z(R) was constrained to z(R) < 2Å2. This choice gives the possibility for the system to explore conformations different from the reference states, avoiding at the same time the loss of the alpha helical secondary structure.
In addition to s(R), we have also used a distance and, in some metadynamics runs, a torsion CV to describe the different conformations of the ligand during the simulations (see Table S1).
Additional Materials and Methods information is given in the SI Text and in Table S2, Table S3, and Table S4.
Supplementary Material
Acknowledgments.
The authors thank Massimiliano Bonomi, Anna Berteotti, and Antonio Randazzo for useful discussions and acknowledge funding from the European Union (Grant ERC-2009-AdG-247075) and Swiss National Supercomputing Center—(CSCS) under project ID s233.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112181108/-/DCSupplemental.
References
- 1.Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M. 20 years of gene therapy for SCID. Nat Immunol. 2010;6:457–460. doi: 10.1038/ni0610-457. [DOI] [PubMed] [Google Scholar]
- 2.LePage G-A, Worth I-S, Kimball A-P. Enhancement of the antitumor activity of arabinofuranosyladenine of 2′-Deoxy-coformicin. Cancer Res. 1976;36:1481–1485. [PubMed] [Google Scholar]
- 3.Flinn I-W, et al. Long-term follow-up of remission duration, mortality, and second malignancies in hairy cell leukemia patients treated with pentostatin. Blood. 2000;96:2981–2986. [PubMed] [Google Scholar]
- 4.Law W-R, Valli V-E, Conlon B-A. Therapeutic potential for transient inhibition of Adenosine Deaminase in systemic inflammatory response syndrome. Crit Care Med. 2003;31:1475–1481. doi: 10.1097/01.CCM.0000063259.09580.D8. [DOI] [PubMed] [Google Scholar]
- 5.Oskouie F-G, et al. High levels of Adenosine Deaminase on dendritic cells promote autoreactive T cell activation and diabetes in nonobese diabetic mice. J Immunol. 2011;186:6798–6806. doi: 10.4049/jimmunol.1004222. [DOI] [PubMed] [Google Scholar]
- 6.Terasaka T, et al. Structure-based design and synthesis of non-nucleoside, potent, and orally bioavailable Adenosine Deaminase inhibitors. J Med Chem. 2004;47:2728–2731. doi: 10.1021/jm0499559. [DOI] [PubMed] [Google Scholar]
- 7.Terasaka T, et al. Structure-based design, synthesis, and structure-activity relationship studies of novel non-nucleoside Adenosine Deaminase inhibitors. J Med Chem. 2004;47:3730–3743. doi: 10.1021/jm0306374. [DOI] [PubMed] [Google Scholar]
- 8.Terasaka T, et al. Rational design of non-nucleoside, potent, and orally bioavailable Adenosine Deaminase inhibitors: predicting enzyme conformational change and metabolism. J Med Chem. 2005;48:4750–4753. doi: 10.1021/jm050413g. [DOI] [PubMed] [Google Scholar]
- 9.Da Settimo F, et al. Novel, highly potent Adenosine Deaminase inhibitors containing the pirazolo[3,4-d]pyrimidine ring system, synthesis, structure-activity relationships, and molecular modeling studies. J Med Chem. 2005;48:5162–5174. doi: 10.1021/jm050136d. [DOI] [PubMed] [Google Scholar]
- 10.Antonioli L, et al. Inhibition of Adenosine Deaminase attenuates inflammation in experimental colitis. J Pharmacol Exper Ther. 2007;322:435–442. doi: 10.1124/jpet.107.122762. [DOI] [PubMed] [Google Scholar]
- 11.La Motta C, et al. Exploiting the pyrazolo[3,4-d]pyrimidin-4-one ring system as a useful template to obtain potent Adenosine Deaminase inhibitors. J Med Chem. 2009;52:1681–1692. doi: 10.1021/jm801427r. [DOI] [PubMed] [Google Scholar]
- 12.Terasaka T, Kinoshita T, Kuno M, Nakanishi I. A highly potent non-nucleoside Adenosine Deaminase inhibitor: efficient drug discovery by intentional lead hybridization. J Am Chem Soc. 2004;126:34–35. doi: 10.1021/ja038606l. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita T, et al. Structural basis of compound recognition by Adenosine Deaminase. Biochemistry. 2005;44:10562–10569. doi: 10.1021/bi050529e. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita T, Nishio N, Nakanishi I, Sato A, Fujii T. Structure of bovine Adenosine Deaminase complexed with 6-hydroxy-1,6-dihydropurine riboside. Acta Crystallogr D. 2003;59:299–303. doi: 10.1107/s090744490202190x. [DOI] [PubMed] [Google Scholar]
- 15.Kinoshita T, Tada T, Nakanishi I. Conformational change of Adenosine Deaminase during ligand-exchange in a crystal. Biochem Biophys Res Commun. 2008;373:53–57. doi: 10.1016/j.bbrc.2008.05.180. [DOI] [PubMed] [Google Scholar]
- 16.Laio A, Parrinello M. Escaping free-energy minima. Proc Natl Acad Sci USA. 2002;99:12562–12566. doi: 10.1073/pnas.202427399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barducci A, Bussi G, Parrinello M. Well-tempered metadynamics: a smoothly converging and tunable free-energy method. Phys Rev Lett. 2008;100:020603. doi: 10.1103/PhysRevLett.100.020603. [DOI] [PubMed] [Google Scholar]
- 18.Gervasio F-L, Laio A, Parrinello M. Flexible docking in solution using metadynamics. J Am Chem Soc. 2005;127:2600–2607. doi: 10.1021/ja0445950. [DOI] [PubMed] [Google Scholar]
- 19.Masetti M, Cavalli A, Recanatini M, Gervasio F-L. Exploring complex protein-ligand recognition mechanisms with coarse metadynamics. J Phys Chem B. 2009;113:4807–4816. doi: 10.1021/jp803936q. [DOI] [PubMed] [Google Scholar]
- 20.Limongelli V, et al. Molecular basis of cyclooxygenase enzymes (COXs) selective inhibition. Proc Natl Acad Sci USA. 2010;107:5411–5416. doi: 10.1073/pnas.0913377107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris G-M, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
- 22.Huey R, Morris G-M, Olson A-J, Goodsell D-S. A semiempirical free energy force field with charge-based desolvation. J Comput Chem. 2007;28:1145–1152. doi: 10.1002/jcc.20634. [DOI] [PubMed] [Google Scholar]
- 23.Ho M-C, et al. Structural and metabolic specificity of methylthiocoformycin for malarial Adenosine Deaminases. Biochemistry. 2009;48:9618–9626. doi: 10.1021/bi9012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gear C-W, Kevrekidis I-G, Theodoropoulos C. ‘Coarse’ integration/bifurcation analysis via microscopic simulators: micro-Galerkin methods. Comput Chem Eng. 2002;26:941–963. [Google Scholar]
- 25.Hummer G, Kevrekidis I-G. Coarse molecular dynamics of a peptide fragment: free energy, kinetics, and long-time dynamics computations. J Chem Phys. 2003;118:10762–10773. [Google Scholar]
- 26.Parrinello M. In: Physical Biology From Atoms to Medicine. Zewail AH, editor. London: Imperial College Press; 2008. pp. 247–265. [Google Scholar]
- 27.Branduardi D, Gervasio F-L, Parrinello M. From A to B in free energy space. J Chem Phys. 2007;126:054103. doi: 10.1063/1.2432340. [DOI] [PubMed] [Google Scholar]
- 28.Bonomi M, Branduardi D, Gervasio F-L, Parrinello M. The unfolded ensemble and folding mechanism of the C-terminal GB1 beta-hairpin. J Am Chem Soc. 2008;130:13938–13944. doi: 10.1021/ja803652f. [DOI] [PubMed] [Google Scholar]
- 29.Berteotti A, et al. Protein conformational transitions: the closure mechanism of a kinase explored by atomistic simulations. J Am Chem Soc. 2009;131:244–250. doi: 10.1021/ja806846q. [DOI] [PubMed] [Google Scholar]
- 30.Pfaendtner J, Branduardi D, Parrinello M, Pollard T-D, Voth G-A. Nucleotide-dependent conformational states of actin. Proc Natl Acad Sci USA. 2009;106:12723–12728. doi: 10.1073/pnas.0902092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonomi M, Barducci A, Parrinello M. Reconstructing the equilibrium Boltzmann distribution from well-tempered metadynamics. J Comput Chem. 2009;30:1615–1621. doi: 10.1002/jcc.21305. [DOI] [PubMed] [Google Scholar]
- 32.Pietrucci F, Marinelli F, Carloni P, Laio A. Substrate binding mechanism of HIV-1 protease from explicit-solvent atomistic simulations. J Am Chem Soc. 2009;131:11811–11818. doi: 10.1021/ja903045y. [DOI] [PubMed] [Google Scholar]
- 33.Laio A, Gervasio F-L. Metadynamics: a method to simulate rare events and reconstruct the free energy in biophysics, chemistry and material science. Rep Prog Phys. 2008;71:126601–126622. [Google Scholar]
- 34.Zheng L, Chen M, Yang W. Random walk in orthogonal space to achieve efficient free-energy simulation of complex systems. Proc Natl Acad Sci USA. 2008;105:20227–20232. doi: 10.1073/pnas.0810631106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S, Chen M, Yang W, Richards NGJ. Sampling long time scale protein motions: OSRW simulation of active site loop conformational free energies in formyl-CoA:oxalate CoA transferase. J Am Chem Soc. 2010;132:7252–7253. doi: 10.1021/ja101446u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leach AR, Shoichet BK, Peishoff CE. Prediction of protein-ligand interactions. Docking and scoring: successes and gaps. J Med Chem. 2006;49:5851–5855. doi: 10.1021/jm060999m. [DOI] [PubMed] [Google Scholar]
- 37.Gleeson MP, Hersey A, Montanari D, Overington J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat Rev Drug Discov. 2011;10:197–208. doi: 10.1038/nrd3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cornell W-D, et al. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc. 1995;117:5179–5197. [Google Scholar]
- 39.Hornak V, et al. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips J-C, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coi A, Tonelli M, Ganadu M-L, Bianucci A-M. Binding free energy calculations of Adenosine Deaminase inhibitors. Bioorg Med Chem. 2006;14:2636–2641. doi: 10.1016/j.bmc.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 42.Jorgensen W-L, Madura J-D. Solvation and conformation of methanol in water. J Am Chem Soc. 1983;105:1407–1413. [Google Scholar]
- 43.Bonomi M, et al. PLUMED: a portable plugin for free-energy calculations with molecular dynamics. Computer Physics Communications. 2009;180:1961–1972. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






