Abstract
Cluster of differentiation 81 (CD81) is a widely expressed tetraspanin molecule that physically associates with CD4 and CD8 on the surface of human T cells. Coengagement of CD81 and CD3 results in the activation and proliferation of T cells. CD81 also costimulated mouse T cells that lack CD28, suggesting either a redundant or a different mechanism of action. Here we show that CD81 and CD28 have a preference for different subsets of T cells: Primary human naïve T cells are better costimulated by CD81, whereas the memory T-cell subsets and Tregs are better costimulated by CD28. The more efficient activation of naïve T cells by CD81 was due to prolonged signal transduction compared with that by CD28. We found that IL-6 played a role in the activation of the naïve T-cell subset by CD81. Combined costimulation through both CD28 and CD81 resulted in an additive effect on T-cell activation. Thus, these two costimulatory molecules complement each other both in the strength of signal transduction and in T-cell subset inclusions. Costimulation via CD81 might be useful for expansion of T cells for adoptive immunotherapy to allow the inclusion of naïve T cells with their broad repertoire.
Activation of naïve T cells requires two independent signals. The first is generated by interaction of the TCR with its cognate antigen presented by the major histocompatibility complex. The second, costimulatory, signal is delivered through accessory molecules on the T-cell surface and is antigen independent (1). Once activated, naïve cells proliferate and generate effector cells that can migrate into inflamed tissues (2–4). After the initial response the majority of effector cluster of differentiation 4 (CD4) T cells die via apoptosis, leaving a small population of memory cells that can confer protection and give, upon a secondary challenge, a more rapid and vigorous response (5, 6). Memory cells respond optimally to lower doses of antigen, are less dependent on accessory cell costimulation, and display effector functions with faster kinetics compared with naïve T cells (7–10).
CD28 is the major and best-studied T-cell costimulatory molecule, known to provide a powerful second signal for T-cell activation (11, 12). However, additional members of the CD28 family, such as ICOS and several members of the TNF family, such as CD27 and CD137 (4-1BB) are well known T-cell costimulatory molecules (13, 14). Less investigated is the costimulatory effect of the tetraspanin family of molecules, including CD9, CD53, CD63, CD81 and CD82 (15–24). Interestingly, costimulation either by CD9 or by CD81 occurs in CD28-deficient T cells, suggesting either a different or a redundant activation pathway (18, 19).
Tetraspanins function as lateral organizers of their associated membrane proteins. Members of this family tend to associate both with each other, and with cell-type-specific partner proteins. For example, CD81 associates in B cells with CD19 (25) whereas in T cells it associates with CD4 and CD8 (26–28). Tetraspanins and their partners assemble in the membrane in tetraspanin-enriched microdomains (TEMs), which facilitate key cellular functions. Tetraspanins play a role in membrane fusion, cell migration, and in signal transduction (29–32). The role of CD81 as a costimulatory molecule is of special interest because it has been shown to localize at the site of interaction between B cells and T cells in a central supermolecular cluster (33).
Here, we compared the costimulatory potential of CD81 to that of CD28. In doing so, we examined both the earliest steps of signal transduction, as well as the later events of T-cell activation. Also, we measured events both at the level of single cells and at the level of cell subpopulations. We found that the earliest steps of CD81-mediated signal transduction differ from those induced by CD28. Specifically, the costimulatory signal mediated through CD81 is more prolonged than that through CD28. In addition, we found that CD81 better costimulates the naïve T-cell subset than does CD28. Combined costimulation through both CD81 and CD28 results in a T-cell response that better includes the naïve T-cell subset.
Adoptive T-cell transfer, the isolation and expansion of antigen-specific cells by costimulation with CD28, followed by reinfusion is a promising approach in the induction of anti-tumor immune response (34, 35). Interestingly, the adoptive transfer of naïve T cells was shown to mediate a superior antitumor immunity (36). Our study provides insight regarding the preferential activation of naive T cells and may be useful in manipulating diseases of the immune system, such as autoimmunity and cancer.
Results
Kinetics of Early Signaling Events in T-Cell Activation Depend on the Costimulatory Molecule.
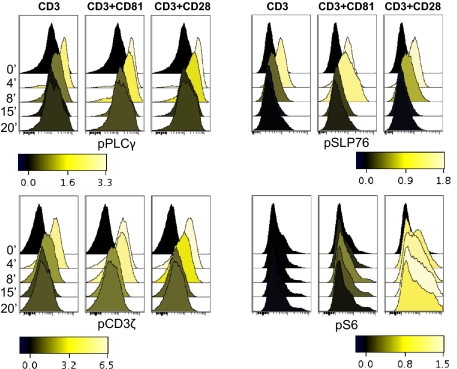
To screen for early signaling events induced by costimulation of human T cells by CD81 or by CD28 we used both a lysate array assay and Western blot analysis (Fig. S1). Costimulation by either CD28 or CD81 resulted in the expected increase in phosphorylation of signaling molecules, compared with stimulation by CD3 alone. Both activated the transcription factor NFAT as evident by its dephosphorylation and deactivated its kinase, GSKβ (Fig. S1) Interestingly, additional signaling molecules differed: Costimulation by CD81 better activated PLCγ, LAT, MEK, and ERK, whereas costimulation by CD28 better activated NFκB, IκB, and S6, a ribosomal protein phosphorylated downstream of the AKT/mTOR pathway, integrating multiple signals including the MAPK pathway (37). Phosphorylation of S6 increases translation of mRNA transcripts encoding proteins involved in cell cycle progression (38). Next, we used phosphoflow cytometry to follow the activation of signaling molecules in single cells while simultaneously determining their lineage (39). We focused on molecules with available good phosphoflow reagents. Once again, costimulation of CD4 T cells by either CD81 or by CD28 showed the expected increase in signal transduction, compared with stimulation via CD3 alone (Fig. 1). The upstream molecules CD3ζ, PLCγ, and SLP76 were better activated by CD81, as visualized by their extended phosphorylation, whereas S6 was more strongly activated upon costimulation by CD28, suggesting that the signaling pathways induced by these two costimulatory molecules indeed differ (Fig. 1 and Fig. S2). These differences in proximal signal transduction pathways might influence the outcome of later activation events and thereby affect T-cell fates.
Fig. 1.
Kinetics of activation of signaling molecules induced in response to costimulation by CD81 or by CD28. Isolated CD4 T cells were stimulated with 0.8 μg/mL of anti-CD3 together with 10 μg/mL of antibodies against the costimulatory molecules CD81 (5A6) or CD28 for the indicated times. The cells were permeabilized, fixed and stained for expression of the phosphoproteins pCD3ζ, pPLCγ, pSLP76 and pS6. The shading scale units represent [fold increase in MFI] − 1.
Costimulation by both CD28 and CD81 (Dual Costimulation) Increases the Number of Activated T Cells.
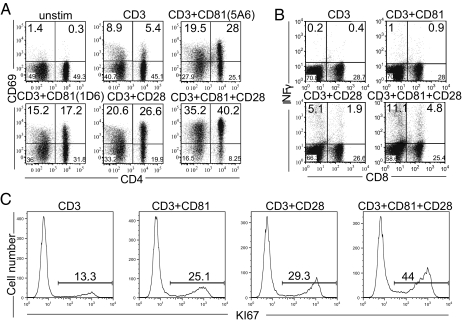
We isolated human T cells and costimulated them through CD81 and/or CD28. As measures of activation we examined CD69 expression (Fig. 2A), IFNγ production (Fig. 2B) and Ki67 expression (Fig. 2C). Individually, an anti-CD81 mAb (5A6 or 1D6) increased the number of activated cells with a magnitude similar to costimulation by the anti-CD28 mAb, as has been reported (16–18, 24). However, IFNγ was better produced following CD28 costimulation possibly due to stronger activation of NFκB (Fig. S1). Interestingly, ligation of both costimulatory molecules resulted in increased percentage of both CD4 and CD8 responding cells, compared with costimulation by either CD81 or by CD28 alone (Fig. 2A Lower Right). Similarly, analysis of IFNγ production by the dually costimulated CD4 and CD8 cells after 48 h demonstrated an increased production of IFNγ, compared with costimulation by the individual molecules (Fig. 2B). Subsequently, the number of proliferating T cells increased in response to the dual costimulation as measured by Ki67 expression after 4 d (Fig. 2C). In each case, the combined costimulation resulted in an approximately additive number of cells responding, suggesting strongly that the two forms of costimulation were addressing different cells.
Fig. 2.
Dual costimulation by CD28 and CD81 increases the number of activated T cells. Isolated T cells were cultured in the presence of anti-CD3, anti-CD81 (5A6 or 1D6) and anti-CD28 mAbs, as indicated. (A) Cells stimulated for 22 h were stained for the surface expression of CD4 and CD69. (B) Cells stimulated for 48 h were stained for surface expression of CD8 and analyzed by flow cytometry for intracellular INFγ production. (C) Cell proliferation was measured by intracellular staining for Ki67 after 4 d. Data are representative of three independent experiments.
CD28 and CD81 Costimulate Different T-Cell Subsets.
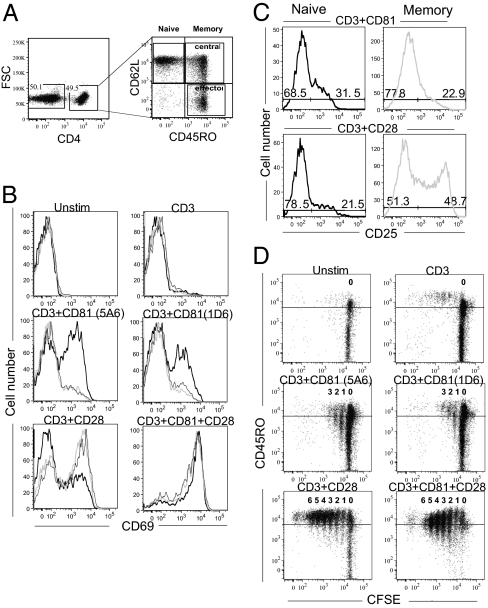
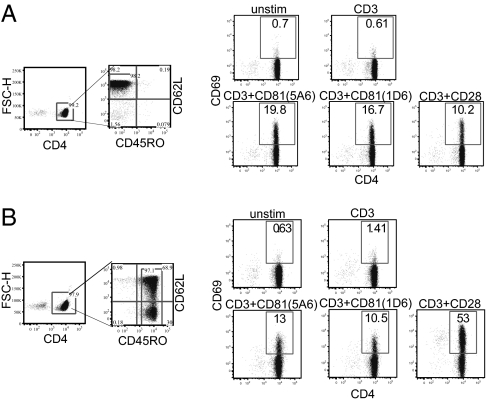
To assess whether these costimulatory molecules could affect different T-cell subsets, we used the surface markers CD45RO and CD62L to distinguish between naïve, effector memory, and central memory T-cell subsets (8) (Fig. 3A). Indeed, analysis of naïve and memory CD4 T cells revealed preferential activation by each of the costimulatory molecules. A greater percentage of naïve cells responded to CD81 costimulation, compared with the memory subsets (Fig. 3B Center); whereas both memory subsets responded more to costimulation by CD28, compared with the naïve subset (Fig. 3B Lower Left). Importantly, dual costimulation strongly activated both the naïve and memory subsets (Fig. 3B Lower Right). Analysis of up-regulation of CD25 expression on the same cells showed a similar subset preference by the individually and dually costimulated cells (Fig. 3C).
Fig. 3.
Costimulation by CD28 or CD81 targets different T-cell subsets. (A–C) Isolated T cells were cultured in the presence of anti-CD3 anti-CD81 and anti-CD28 mAbs, as indicated. The cells were stimulated for 24 h, stained for expression of the surface molecules, CD4, CD45RO, CD62L and CD69 or CD25 and analyzed by flow cytometry. (A) CD4-gated cells were further analyzed for CD45RO and CD62L expression. Shown is gating on naïve (CD62L high CD45RO low), central memory (CD62L high CD45RO high), and effector memory (CD62L low CD45RO high) T cells. (B) Increased CD69 expression on gated naïve (black solid line) CD4 cells in response to costimulation by the anti-CD81 mAbs 5A6 and 1D6 (Middle). Costimulation by CD28 affected mostly CD69 expression on gated central (light gray line) and effector (black dashed line) memory CD4 cells (Lower Left). Dual costimulation increased CD69 expression on all subsets (Lower Right). (C) CD25 expression on gated naïve and memory CD4 cells was increased on naïve cells costimulated by CD81 (Left) and on memory cells (Right) costimulated by CD28. (D) Isolated T cells were labeled with CFSE, cocultured with the indicated antibodies for 5 d and analyzed by flow cytometry. Shown is CFSE dilution in relation to CD45RO expression on CD4-gated cells. Data are representative of four independent experiments.
These findings prompted us to determine whether costimulation through CD28 and CD81 differentially affected the CD4+Foxp3+ T-cell population. We found that costimulation by CD28 increased both the number of CD4+Foxp3+ cells and their activation level. By contrast, the number of CD4+Foxp3+ cells responding to costimulation by CD81 was not increased (Fig. S3). Therefore, CD81 was less efficient in activating CD4+Foxp3+ cells compared with CD28.
The increase in expression were analyzed after 24 h, the time required to induce these activation markers (40), before shifts occurred from the original naïve phenotype and within the memory subsets. Interestingly, the preferential costimulatory effect could be observed even at longer times after initiation of the signals. CFSE-labeled T cells were costimulated and then analyzed after 5 d. Following costimulation with CD28 cells had undergone at least 7 divisions and most expressed a high level of the memory subset marker, CD45RO. After costimulation with CD81, the cells had undergone fewer divisions, but the dividing cells included those expressing low levels of CD45RO (Fig. 3D). Taken together, these studies suggest that CD81 preferentially activates naïve T cells, whereas CD28 preferentially activates the memory subsets. Dual costimulation by CD81 and CD28 resulted in activation of most naïve and memory subsets (Fig. 3D) thereby partially explaining the additive effect seen when the whole population was analyzed. The same subset preference was also seen upon analysis of CD8 cells, which tended to be generally less activated.
CD81 Is Expressed Similarly on Naïve and Memory T-Cell Subsets.
A possible explanation for a preferential activation of the naïve subset by CD81 could be a higher CD81 expression level compared with the memory subsets, as has been reported for the tetraspanin molecule CD9 (20). However, CD81 was equally expressed on both subsets, whereas CD28 expression was higher on the memory subsets (41), CD45RO expression on the isolated naïve and memory subsets confirmed their purity (Fig. S4). Thus, CD81 expression does not explain the preferential activation of the naïve subset.
Signal Transduction in Individual Naïve and Memory Cells Responding to Costimulation.
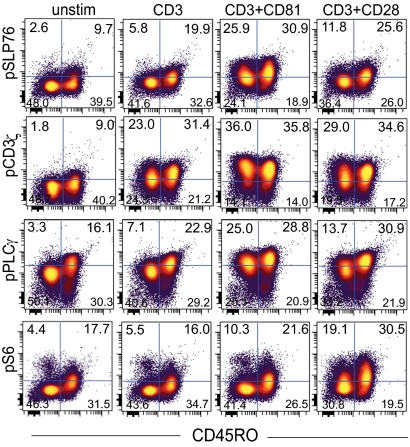
Having shown differential activation of naïve and memory cells in response to costimulation by CD81 and by CD28 (Fig. 3), we used phosphoflow cytometry to interrogate the early response of naive (CD45RO low) and memory (CD45RO high) CD4 T cells to costimulation. Naïve cells had previously been shown to require stringent activation conditions (8). Indeed, baseline phosphorylation of all four tested signaling molecules was lower in naïve (CD45RO low) cells in comparison with memory (CD45RO high) cells (Fig. 4 Left). Costimulation by either CD28 or by CD81 led to increased phosphorylation of PLCγ, CD3ζ, and SLP76 in both the naïve and memory subsets, as expected. However, the effect of CD81 on the naïve subset was relatively greater than that of CD28, as can been seen by the increase in the percentage of activated CD45RO low (naïve) compared with CD45RO high (memory) cells (Fig. 4 Upper quadrants). This might explain why costimulation by CD28 was less efficient in activating naïve cells compared with costimulation by CD81. By contrast, when phosphorylation of S6 was measured, costimulation through CD81 had less effect altogether and costimulation through CD28 had a greater effect on the memory subpopulations (Fig. 4). S6 can integrate signaling inputs from numerous upstream pathways and therefore it reflects potential alternative mechanisms of activation.
Fig. 4.
Costimulation by CD81 preferentially activates signal transduction in naïve CD4 T cells. Isolated CD4 T cells were stimulated with 0.8 μg/mL of anti-CD3 together with 10 μg/mL of antibodies against the costimulatory molecules CD81 (5A6) or CD28. After permeabilization and fixation cells were stained for expression of CD4 and CD45RO and the phosphoproteins pPLCγ, pCD3ζ, pSLP76 and pS6. Shown is phosphorylation measured in resting cells (unstim) and after 8 min stimulation of the naïve (CD45RO low) and memory (CD45RO high) subsets. Data are representative of three independent experiments.
Isolated CD4 T Subsets Are Preferentially Costimulated: Naïve Cells by CD81 and Memory Cells by CD28.
To eliminate possible effects of one subset on the other, we analyzed activation of isolated highly purified naïve and memory CD4 subsets (shown in Fig. 5 A Left and B Left). A higher percentage of isolated naïve cells responded to costimulation by CD81, compared with those induced by CD28 (Fig. 5A). During this time there was no detectable conversion of naïve to memory cells (Fig. S5). Conversely, CD28 costimulation was more effective in activating the memory subsets (Fig. 5B). Thus, naïve T cells are intrinsically more susceptible to activation through CD81, whereas memory T cells are more sensitive to CD28 costimulation.
Fig. 5.
Isolated naïve and memory T cells are preferably costimulated by CD81 and CD28 mAbs, respectively. Naïve and memory CD4 T cells were purified by negative isolation kits (A and B Left), costimulated separately with the indicated mAbs and analyzed for CD69 expression after 22 h. (A) Isolated naïve T cells. (B) Isolated memory T cells. Data are representative of three independent experiments.
Optimal Costimulation of Naïve Cells by CD81 Requires IL-6.
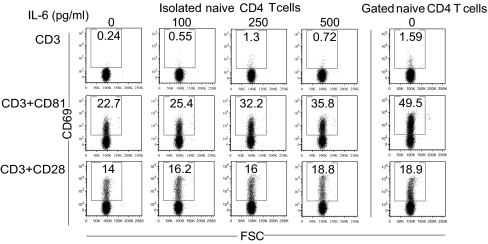
Isolated naïve CD4 T cells responded less vigorously to costimulation by CD81 (Fig. 5A), compared with naïve cells cultured in the presence of memory T cells (Fig. 3B). To determine the role of secreted factors we used the multiplex Luminex assay and identified IL-6 as a major cytokine secreted upon CD81 costimulation (Fig. S6). In addition, a previous study showed that the IL-6 receptor (IL-6r) is expressed at high levels on naïve and central memory T cells, but not on effector T cells (42). We then tested whether exogenous IL-6 can augment CD81-mediated costimulation of naïve CD4 T cells. Isolated naïve CD4 T cells were incubated with increasing concentrations of IL-6 along with the costimulatory antibodies. In the absence of IL-6, the isolated naïve CD4 T cells were less activated in response to costimulation by CD81 (Fig. 6 Left, isolated naïve T cells) compared with naïve T cells that were in contact with the memory subset during the costimulation period (Fig. 6 Right, gated naïve T cells). Importantly, addition of IL-6 to the isolated naïve CD4 T cells had a significant effect on activation in response to costimulation by CD81. The percentage of activated cells, which was 22.7% in the absence of IL-6, increased in response to increasing concentrations of the cytokine, reaching 35.8%, a level that partially restored that seen in the presence of memory T cells (49.5%) (Fig. 6 Right). By contrast, the addition of IL-6 to isolated naïve T cells that were costimulated by CD28 had a much smaller effect on their activation. Similarly, addition of IL-6 to the culture that was incubated only with anti-CD3 had no effect on activation, suggesting that IL-6 by itself was not sufficient and that its presence with the costimulatory molecules, especially CD81, augmented activation. Finally, when mixed populations of T cells were costimulated with CD81 in the presence of a neutralizing antibody to IL-6, the activation of naïve T cells was reduced (Fig. S7). Therefore, in nonseparated T cells cross talk between the subpopulations, possibly through IL-6, results in an augmented costimulatory response by the naïve T cells to CD81.
Fig. 6.
IL-6 affects the extent of naïve T-cell costimulation by CD81. Total CD4 T cells (Right) and naïve CD4 T cells isolated from the same individual were cultured in the presence of anti CD3 (0.1 μg/mL), anti-CD81 (5A6) and anti-CD28 mAbs, as indicated, each at a concentration of 2.5 μg/mL and in the presence of the indicated concentrations of IL-6, the expression of CD69 was analyzed after 22 h. The cells were stained for expression of CD45RO, CD62L, CD4, and CD69 after 22 h and analyzed by flow cytometry. Shown is CD69 expression on gated naïve and memory subsets. Data are representative of three independent experiments.
Discussion
CD81 and several members of the tetraspanin family were shown to costimulate T cells (16–18, 22, 24, 43), Interestingly, costimulation delivered through CD81 differs from that delivered through CD28. Unlike CD28, triggered by interaction with CD80 and CD86 on antigen-presenting cells (APC), CD81 has no known ligand and therefore its ability to costimulate T cells differs fundamentally from CD28. In fact, the only known natural ligand for CD81 is the hepatitis C virus (HCV) envelope protein E2 (44). However, it is unknown whether the virus subverts the immune systems by nonspecifically activating naïve CD4 T cells through engagement of CD81.
There is no structural similarity between CD81 and CD28. CD28 has a single cytoplasmic domain containing the YMMM and PYAP specific motifs, which upon phosphorylation by Src family kinases bind SH2 and SH3 containing proteins thereby initiating several signal transduction cascades (reviewed in ref. 45). CD81, a four transmembrane protein with short cytoplasmic N- and C-terminal domains lacks tyrosine activation motifs (46). Nevertheless, the C-terminal domain of CD81 was shown to associate directly with ezrin-radixin-moesin (ERM) proteins (47) and to modulate their activity (48). ERM proteins enable bridging between membrane proteins and the actin cytoskeleton and in doing so facilitate membrane reorganization (reviewed in ref. 49). Indeed, ERM proteins were shown to play a role in T-cell–APC interactions (50). An additional study showed that ezrin recruited ZAP70 to the immune synapse, whereas moesin removed CD43 away from the cell-cell contact site (51). Most recently, ezrin silencing was shown to reduce cytoskeletal clustering and to modulate TCR signaling (51–53). The linkage of tetraspanins to the cytoskeleton is not limited to CD81; for example, CD82 was shown to act as a cytoskeletal-dependent costimulatory molecule, which was localized at the TCR engagement site (21).
In addition to providing a bridge to the cytoskeleton, CD81 forms lateral association with membrane proteins (reviewed in refs. 54 and 55). Tetraspanin-associated proteins have been referred to as partners and such partnerships are cell type specific (reviewed in ref. 55). The assembly of tetraspanins and their partners in TEMs has been suggested to facilitate signal transduction (reviewed in ref. 56).
The current study confirms previous findings (16, 17, 24) of the T-cell costimulatory effect mediated by CD81. We found that dual costimulation through CD81 and CD28 induced a greater number of activated cells than that induced through each of these costimulatory molecules alone. Analysis of the number of cells responding to dual costimulation revealed a marked increase in IFNγ producing cells and a substantial increase in proliferating cells, confirming an additive effect (Fig. 2). The increased number of cells responding to dual costimulation is likely due to nonoverlapping cell populations responding to CD28 or to CD81. Indeed, we showed that costimulation through CD81 better activated naïve CD4 T cells and was less efficient in activating the CD4 memory subsets, whereas CD28 activated better the memory subsets (Fig. 3). It is of note that the levels of CD81 expression do not differ between naïve and memory T cells (Fig. S4). Therefore, other mechanisms are responsible for the preferential activation of naïve cells via CD81. Interestingly, in mice, CD81 is expressed on T cells only upon activation (57), thus, naïve mouse T cells do not express CD81. This fundamental difference between human and mouse T cells suggests that CD81 plays a different role in these species, and illustrates the limitation of the Cd81−/− mouse model for understanding the role of CD81 in human T cells.
The increased number of cells responding to dual costimulation could also be due to activation of different signal transduction pathways. Indeed, analysis of the first steps of signal transductions by phosphoflow demonstrated differences in the activation pathways of costimulation between CD81 and CD28 (Fig. 1 and supplementary Fig.1, 2), suggesting that these molecules differ in signal transduction. The prevailing notion is that prolonged costimulation is required for naïve T-cell activation (58, 59). The first step in T-cell activation is a spatial reorganization of the TCR with its immediate downstream signaling components (for a review, see ref. 60). Therefore, it is likely that CD81 activates naïve T cells more efficiently by prolonging signal transduction. Using phosphoflow cytometry to analyze signal transduction in individual naïve and memory cells we show that the phosphorylation level of resting naïve cells is lower than that of the resting memory cells (Fig. 4). This lower phosphorylation level of naïve cells may explain their stringent activation requirements. Following costimulation, the most proximal TCR signal-transducing molecules TCRζ, SLP76, as well as PLCγ, were better induced by CD81 (Fig. 4). This activation was most apparent in individual naïve T cells (Fig. 4). CD81 costimulation of naïve cells resulted in an increased and prolonged costimulation compared with that reached upon CD28 costimulation (Fig. 4). We propose that this is a possible mechanism by which CD81 more efficiently activates naïve T cells than CD28. S6 activity was induced by both CD81 and CD28 costimulation, but CD28 was more efficient (Fig. 4). Importantly, the phosphorylation of S6 by CD28 was better enhanced in the memory subsets and at later time points (Fig. 4) correlating with a more efficient activation of the memory subset by CD28. The lower activation efficiency of memory T cells by CD81 is yet to be explained. A previous study suggested that the organization of signaling molecules is different in naïve and in memory T cells (61). It is therefore possible that the distribution of TEMs in the memory subsets is less conducive to CD81 engagement. Nevertheless, colligation of both costimulatory molecules results in an enhanced activation of both naïve and the memory subsets, suggesting that CD81 ligation on memory subsets is definitely effective (Fig. 2).
Costimulation by CD81 has a differential effect on naïve vs. memory CD4 T cells. This differential effect may be explained in part by a differential sensitivity to IL-6. First, isolated naïve T cells were less activated upon CD81 costimulation (Fig. 5A) than naïve cells that were not separated from the memory subsets (Fig. 2), suggesting a cooperative effect between the cell populations. Second, IL-6 was the major cytokine secreted in response to costimulation of whole CD4 T cells by CD81 (Fig. S6) possibly due to the activation of the transcription factor NFAT (Fig. S1). Third, addition of IL-6 to isolated naïve T cells costimulated by CD81 increased significantly their activation level (Fig. 6). Fourth, a neutralizing anti-IL-6 antibody decreased the activation of naïve CD4 T cells costimulated by CD81 (Fig. S7). A previous study has shown that the IL-6 receptor (IL-6r) is expressed at high levels on naïve and central memory T cells, but not on effector T cells (42), in agreement with an effect of IL-6 on naïve T-cell activation. Nevertheless, isolated naïve T cells were also activated in the absence of memory cells and IL-6 (Fig. 6) and the anti-IL-6 antibody did not completely abolish activation by CD81 (Fig. S7). This suggests that naïve T-cell activation is affected both directly by the engagement of CD81 on the naïve cells and indirectly through IL-6 secretion by the memory cells. The addition or neutralization of IL-6 on CD28 costimulated cells was less effective compared with CD81 costimulated cells (Fig. 6 and Fig. S7), suggesting that the effect of the IL-6 cytokine plays a more important role in CD81 costimulation.
Our findings have potential application for human immunotherapy. Adoptive T-cell transfer using in vitro differentiated T cells is a powerful treatment against established cancers in humans (34, 35). It has been suggested that human effector cells derived from naïve rather than memory subsets possess superior traits for adoptive immunotherapy (36). First, naïve T cells are more efficiently transduced with genetically engineered T-cell receptors and chimeric antigen receptors (CAR) (36). Second, during persistent antigen stimulation, memory T cells undergo exhaustion, characterized by decreased proliferative ability and reduced cytotoxicity, whereas naïve derived effector cells resist terminal differentiation and posses a more robust proliferative ability and thereby retain the characteristic of effective cells for longer (25, 36). Additional studies have shown that adoptively transferred naïve CD4+ T cells differentiate into effector T cells in vivo and eradicate cancer (62) and that targeting of naïve T cells to tumor location increased the efficiency of tumor eradication (63). Thus, the therapeutic potential of naïve cell is increasingly recognized. In this sense, the preferential activation of the naïve subset by CD81, while minimizing the activation of the undesired subsets such as Tregs, may provide a way to activate and expand naïve T cells in vitro both for the transduction of the desired TCR specificity and following the transduction as a source of cells for adoptive T-cell transfer.
Materials and Methods
Detailed are provided in SI Materials and Methods.
T-Cell Purification.
T cells were purified by negative selection using isolation kits for human T cells, CD4+ T cells, and naïve and memory CD4+ T cells, according to the manufacturers’ instructions.
Phospho-Flow Analysis.
Purified T cells were rested for 1 h at 37 °C, incubated on ice for 10 min with antibodies, as indicated, followed by stimulation in a 37 °C water bath for 4–20 min. Cells were fixed, permeabilized, and stained with phospho-specific antibodies, followed by flow cytometry.
T-Cell Proliferation.
Purified T cells were stained with carboxyfluorescein succinimidyl ester (CFSE) then incubated for 5 d with the indicated antibodies, followed by staining for the surface markers CD4 and CD45RO.
Ki67 Expression.
Purified T cells were incubated with the indicated antibodies for 72 h, then harvested and stained with the anti-Ki67 mAb.
T-Cell Activation Markers.
Purified T cells were incubated with the indicated antibodies then stained and analyzed for expression of CD4, CD8, CD45RO, CD62L, CD69, and CD25.
Production of INFγ.
Purified T cells were incubated with the indicated antibodies for 24 h, GolgiStop (BD Bioscience) was added during the last 6 h, followed by fixation and permeabilization staining with the anti- IFNγ mAb followed by analysis by flow cytometry.
Cytokine and Chemokine Secretion.
Purified CD4 T cells were incubated with the indicated antibodies for 72 h supernatants were treated with protein G Dynabeads (Invitrogen) and kept at −80 °C until analysis by Luminex Multiplex assay at the core facility at Stanford.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant CA34233, the Leukemia and Lymphoma Society (LLS 7155), and the Albert Yu and Mary Bechmann Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121307109/-/DCSupplemental.
References
- 1.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: A costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 2.Garside P, et al. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998 doi: 10.1126/science.281.5373.96. (New York) 281:96–99. [DOI] [PubMed] [Google Scholar]
- 3.Mackay CR. Homing of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993;5:423–427. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- 4.Austrup F, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 5.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed R, Gray D. Immunological memory and protective immunity: Understanding their relation. Science (New York) 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 7.Kedl RM, Mescher MF. Qualitative differences between naive and memory T cells make a major contribution to the more rapid and efficient memory CD8+ T cell response. J Immunol. 1998;161:674–683. [PubMed] [Google Scholar]
- 8.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 9.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 10.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 11.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Chen L. T lymphocyte co-signaling pathways of the B7-CD28 family. Cell Mol Immunol. 2004;1:37–42. [PubMed] [Google Scholar]
- 13.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 14.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 15.Toyo-oka K, et al. Synergy between CD28 and CD9 costimulation for naive T-cell activation. Immunol Lett. 1997;58:19–23. doi: 10.1016/s0165-2478(97)02706-5. [DOI] [PubMed] [Google Scholar]
- 16.Soldaini E, et al. T cell costimulation by the hepatitis C virus envelope protein E2 binding to CD81 is mediated by Lck. Eur J Immunol. 2003;33:455–464. doi: 10.1002/immu.200310021. [DOI] [PubMed] [Google Scholar]
- 17.Wack A, et al. Binding of the hepatitis C virus envelope protein E2 to CD81 provides a co-stimulatory signal for human T cells. Eur J Immunol. 2001;31:166–175. doi: 10.1002/1521-4141(200101)31:1<166::aid-immu166>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Witherden DA, Boismenu R, Havran WL. CD81 and CD28 costimulate T cells through distinct pathways. J Immunol. 2000;165:1902–1909. doi: 10.4049/jimmunol.165.4.1902. [DOI] [PubMed] [Google Scholar]
- 19.Tai XG, et al. A role for CD9 molecules in T cell activation. J Exp Med. 1996;184:753–758. doi: 10.1084/jem.184.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi H, et al. The tetraspanin CD9 is preferentially expressed on the human CD4(+)CD45RA+ naive T cell population and is involved in T cell activation. Clin Exp Immunol. 2004;137:101–108. doi: 10.1111/j.1365-2249.2004.02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagaudrière-Gesbert C, Lebel-Binay S, Hubeau C, Fradelizi D, Conjeaud H. Signaling through the tetraspanin CD82 triggers its association with the cytoskeleton leading to sustained morphological changes and T cell activation. Eur J Immunol. 1998;28:4332–4344. doi: 10.1002/(SICI)1521-4141(199812)28:12<4332::AID-IMMU4332>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Pfistershammer K, et al. CD63 as an activation-linked T cell costimulatory element. J Immunol. 2004;173:6000–6008. doi: 10.4049/jimmunol.173.10.6000. [DOI] [PubMed] [Google Scholar]
- 23.Shibagaki N, Hanada K, Yamashita H, Shimada S, Hamada H. Overexpression of CD82 on human T cells enhances LFA-1 / ICAM-1-mediated cell-cell adhesion: Functional association between CD82 and LFA-1 in T cell activation. Eur J Immunol. 1999;29:4081–4091. doi: 10.1002/(SICI)1521-4141(199912)29:12<4081::AID-IMMU4081>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Serra A, et al. Coligation of the hepatitis C virus receptor CD81 with CD28 primes naive T lymphocytes to acquire type 2 effector function. J Immunol. 2008;181:174–185. doi: 10.4049/jimmunol.181.1.174. [DOI] [PubMed] [Google Scholar]
- 25.Bradbury LE, Kansas GS, Levy S, Evans RL, Tedder TF. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992;149:2841–2850. [PubMed] [Google Scholar]
- 26.Imai T, Yoshie O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncytium formation by human T cell leukemia virus type 1 are both members of the transmembrane 4 superfamily and associate with each other and with CD4 or CD8 in T cells. J Immunol. 1993;151:6470–6481. [PubMed] [Google Scholar]
- 27.Imai T, Kakizaki M, Nishimura M, Yoshie O. Molecular analyses of the association of CD4 with two members of the transmembrane 4 superfamily, CD81 and CD82. J Immunol. 1995;155:1229–1239. [PubMed] [Google Scholar]
- 28.Tachibana I, Bodorova J, Berditchevski F, Zutter MM, Hemler ME. NAG-2, a novel transmembrane-4 superfamily (TM4SF) protein that complexes with integrins and other TM4SF proteins. J Biol Chem. 1997;272:29181–29189. doi: 10.1074/jbc.272.46.29181. [DOI] [PubMed] [Google Scholar]
- 29.Yáñez-Mó M, Barreiro O, Gordon-Alonso M, Sala-Valdés M, Sánchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19:434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Le Naour F, et al. Tetraspanins connect several types of Ig proteins: IgM is a novel component of the tetraspanin web on B-lymphoid cells. Cancer Immunol Immunother. 2004;53:148–152. doi: 10.1007/s00262-003-0477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarrant JM, Robb L, van Spriel AB, Wright MD. Tetraspanins: Molecular organisers of the leukocyte surface. Trends Immunol. 2003;24:610–617. doi: 10.1016/j.it.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Hemler ME. Targeting of tetraspanin proteins—potential benefits and strategies. Nat Rev Drug Discov. 2008;7:747–758. doi: 10.1038/nrd2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittelbrunn M, Yáñez-Mó M, Sancho D, Ursa A, Sánchez-Madrid F. Cutting edge: dynamic redistribution of tetraspanin CD81 at the central zone of the immune synapse in both T lymphocytes and APC. J Immunol. 2002;169:6691–6695. doi: 10.4049/jimmunol.169.12.6691. [DOI] [PubMed] [Google Scholar]
- 34.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aqui NA, June CH. Post-transplant adoptive T-cell immunotherapy. Best Practice Res. 2008;21:503–519. doi: 10.1016/j.beha.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinrichs CS, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Peterson RT, Schreiber SL. Translation control: Connecting mitogens and the ribosome. Curr Biol. 1998;8:R248–R250. doi: 10.1016/s0960-9822(98)70152-6. [DOI] [PubMed] [Google Scholar]
- 39.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: Monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 40.Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: An in vitro model to monitor cellular immune function. J Immunol Methods. 2004;293:127–142. doi: 10.1016/j.jim.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 41.De Rosa SC, Herzenberg LA, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: Identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7:245–248. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- 42.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebel-Binay S, Lagaudrière C, Fradelizi D, Conjeaud H. CD82, member of the tetra-span-transmembrane protein family, is a costimulatory protein for T cell activation. J Immunol. 1995;155:101–110. [PubMed] [Google Scholar]
- 44.Pileri P, et al. Binding of hepatitis C virus to CD81. Science (New York) 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 45.Boomer JS, Green JM. An enigmatic tail of CD28 signaling. Cold Spring Harb Perspect Biol. 2010;2:a002436. doi: 10.1101/cshperspect.a002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seigneuret M. Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: Conserved and variable structural domains in the tetraspanin superfamily. Biophys J. 2006;90:212–227. doi: 10.1529/biophysj.105.069666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sala-Valdés M, et al. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J Biol Chem. 2006;281:19665–19675. doi: 10.1074/jbc.M602116200. [DOI] [PubMed] [Google Scholar]
- 48.Coffey GP, et al. Engagement of CD81 induces ezrin tyrosine phosphorylation and its cellular redistribution with filamentous actin. J Cell Sci. 2009;122:3137–3144. doi: 10.1242/jcs.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charrin S, Alcover A. Role of ERM (ezrin-radixin-moesin) proteins in T lymphocyte polarization, immune synapse formation and in T cell receptor-mediated signaling. Front Biosci. 2006;11:1987–1997. doi: 10.2741/1940. [DOI] [PubMed] [Google Scholar]
- 50.Faure S, et al. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol. 2004;5:272–279. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 51.Ilani T, Khanna C, Zhou M, Veenstra TD, Bretscher A. Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin. J Cell Biol. 2007;179:733–746. doi: 10.1083/jcb.200707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lasserre R, et al. Ezrin tunes T-cell activation by controlling Dlg1 and microtubule positioning at the immunological synapse. EMBO J. 2010;29:2301–2314. doi: 10.1038/emboj.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta N, et al. Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nat Immunol. 2006;7:625–633. doi: 10.1038/ni1337. [DOI] [PubMed] [Google Scholar]
- 54.Hemler ME. Specific tetraspanin functions. J Cell Biol. 2001;155:1103–1107. doi: 10.1083/jcb.200108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 56.Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: Molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- 57.Maecker HT, Todd SC, Kim EC, Levy S. Differential expression of murine CD81 highlighted by new anti-mouse CD81 monoclonal antibodies. Hybridoma. 2000;19:15–22. doi: 10.1089/027245700315752. [DOI] [PubMed] [Google Scholar]
- 58.Liwski RS, et al. Prolonged costimulation is required for naive T cell activation. Immunol Lett. 2006;106:135–143. doi: 10.1016/j.imlet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 60.Dehmelt L, Bastiaens PI. Spatial organization of intracellular communication: Insights from imaging. Nat Rev Mol Cell Biol. 2010;11:440–452. doi: 10.1038/nrm2903. [DOI] [PubMed] [Google Scholar]
- 61.Watson AR, Lee WT. Differences in signaling molecule organization between naive and memory CD4+ T lymphocytes. J Immunol. 2004;173:33–41. doi: 10.4049/jimmunol.173.1.33. [DOI] [PubMed] [Google Scholar]
- 62.Xie Y, et al. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu P, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5:141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.