Abstract
An estrogen receptor (ER) β ligand (LY3201) with a preference for ERβ over ERα was administered in s.c. pellets releasing 0.04 mg/d. The brains of these mice were examined 3 d after treatment had begun. Although estradiol-17β is known to increase spine density and glutaminergic signaling, as measured by Golgi staining, a clear reduction in spines was evident on the dendritic branches in LY3201-treated mice but no morphological alteration and no difference in the number of dendritic spines on dendritic stems were observed. In the LY3201-treatment group, there was higher expression of glutamic acid decarboxylase (GAD) in layer V of cortex and in the CA1 of hippocampus, more GAD+ terminals surrounding the pyramidal neurons and less glutamate receptor (NMDAR) on the neurons in layer V. There were no alterations in expression of Iba1 or in Olig2 or CNPase. However, GFAP+ astrocytes were increased in the LY3201-treatment group. There were also more projections characteristic of activated astrocytes and increased expression of glutamine synthetase (GS). No expression of ERβ was detectable in the nuclei of astrocytes. Clearly, LY3201 caused a shift in the balance between excitatory and inhibitory neurotransmission in favor of inhibition. This shift was due in part to increased synthesis of GABA and increased removal of glutamate from the synaptic cleft by astrocytes. The data reveal that treatment with a selective ERβ agonist results in changes opposite to those reported in estradiol-17β–treated mice and suggests that ERα and ERβ play opposing roles in the brain.
Keywords: neurotransmitter, glutamate toxicity, cerebral cortex, hippocampus
Of the two estrogen receptors (ERs), ERα appears to be the major player in regulating the reproductive system, whereas both receptors ERα and ERβ are involved in regulation of mood and affective disorders (1, 2). Several studies show that ERα activation is anxiogenic (3–5), whereas ERβ activation is anxiolytic and controls expression of fear and anxiety-like behaviors (6–9). Not surprisingly, estradiol (E2), which binds equally well to ERα and ERβ, has unpredictable effects on abnormal psychiatric behaviors, depending on dose, treatment regimens, or animal models (10–13). In hippocampal cultures both exogenous (E2) treatment and locally synthesized E2 up-regulate ERα and down-regulate ERβ expression (14). Thus, E2 has unpredictable effects on the balance between anxiogenic and anxiolytic effects, in part because E2 has opposite effects on ERα and ERβ (15). Using subtype-selective ligands, we can begin to study the outcome of selective activation or inhibition of a particular receptor subtype involved in different behaviors.
Spines and the synapses located on neurons are the sites at which neuronal communication occurs and are essential for complex behavioral phenomena and cognition. In the mature CNS, alterations in synapse structure and function continue to be a dynamic process that is of fundamental importance to learning and memory as well as other adaptive abilities of the brain. Studies in humans and animals have demonstrated that depression and stress are associated with neuronal atrophy and dendritic reorganization in the hippocampus and prefrontal cortex (16–20). In 1990, Gould et al. first reported an increase in spine density on CA1 pyramidal cells in adult ovariectomized rats receiving exogenous E2 (21). This effect of E2 on pyramidal cell spine density was later confirmed by studies from several other laboratories in both rats and monkeys (22–26). However, the effects of E2 are complex and identifying which estrogen receptor is responsible for mediating these effects is an active area of research.
Astrocytes may help to mediate the neuroprotective effect of E2 by production of growth factors, secretion of cytokines, and most importantly, by their efficient uptake and metabolism of the excitatory neurotransmitter glutamate (27). Recent studies have shown that astrocytes have important physiological roles in the synchronization of neuronal firing, ion homeostasis, neurotransmitter uptake, glucose metabolism, and regulation of the vascular tone (28). Astroglial cells clear extracellular glutamate and subsequently convert the glutamate into glutamine by the enzyme, glutamine synthetase. In addition, E2 and its receptors regulate inflammatory responses in astrocytes and microglia (29–31).
In the present study, we have analyzed the alteration of dendritic spines, glutamic acid decarboxylase (GAD)65+67, NMDA receptor, astrocytes and glutamine synthetase in vehicle- and LY3201-treated mice. The ERβ agonist LY3201, (3aS, 4R, 9bR)-2, 2-difluoro-4-(4-hydroxyphenyl)-3, 3a, 4, 9b-tetrahydro-1H-cyclopenta[c]chromen-8-ol (CAS 787621–78-7), was prepared according to methods previously described (32). After exposure to LY3201, a decrease of spines, NMDA receptors (NMDARs) and increase of GAD65+67, astrocytes, and glutamine synthesis (GS) were observed. With these data we reveal the activity of a selective ERβ ligand in the brain and propose that activation of ERβ favors inhibition over excitation.
Results
Reduction of Dendritic Spines on Cortical and Hippocampal Neurons After LY3201 Treatment.
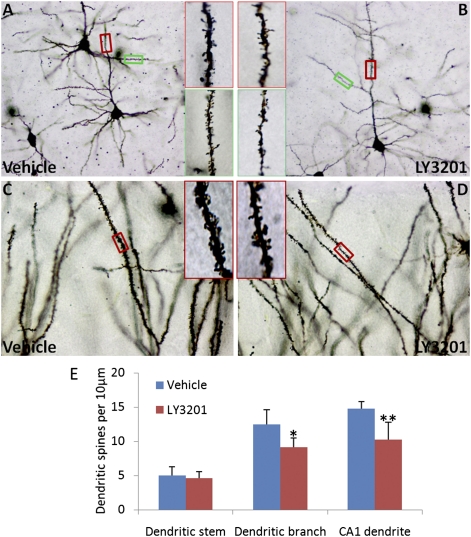
Dendritic spines were examined by Golgi staining and a comparison was made between vehicle- and LY3201-treatment groups. After 3 d of treatment with LY3201, the number and morphology of the dendritic spines on dendritic stems appear unchanged (red Inset boxes in Fig. 1 A and B). However, the number of spines on the dendritic branches in LY3201-treated mice is less than in the vehicle-treated group (green Inset boxes in Fig. 1 A and B). Moreover, the number of spines on neuronal dendrites in the hippocampus CA1 in LY3201-treated mice is less than in the vehicle-treated group (Fig. 1 C and D). There was no overall alteration of the morphology of spines and distinct types of spines (mushroom spines, stubby spines, and thin spines) could be seen in both groups (Fig. 1 A–D). When the number of dendritic spines per 10 μm was counted, we found that compared with the vehicle-treated group, there were fewer spines on dendritic branches and CA1 neuronal dendrites in the LY3201 group (Fig. 1E). There was no measurable difference between vehicle- and LY3201-treated groups in the number of spines on dendritic stems (Fig. 1E).
Fig. 1.
Alteration of dendritic spines on cortical and hippocampal neurons visualized with Golgi staining. (A and B) There is no morphological alteration and no difference in the number of the dendritic spines on dendritic stems after LY3201 treatment (red Inset boxes). However, there are fewer spines on the dendritic branches in LY3201-treated mice than in vehicle group (green Inset boxes). Magnified view of red and green boxed areas in A and B is shown on the Right side of A and the Left side of B. Number of spines on neuronal dendrites in the hippocampus CA1 in LY3201-treated mice is less than in the vehicle-treated group (C and D). Magnified view of the red boxed area in C and D is shown on the Right side of C and the Left of D. (E) Number of dendritic spines per 10 μm on dendritic stems and branches of cortical and hippocampus CA1 neurons in vehicle- and LY3201-treated mice. In the LY3201-treated group, there are fewer spines on dendritic branches and CA1 neuronal dendrites (n = 3; *P = 0.017; **P = 0.026 for LY3201) than in the vehicle-treated group (Scale bars in A and B, 50 μm; in C and D, 20 μm; Inset boxes, 10 μm.)
Increased Expression of the GAD65+67 and Reduced NMDA Receptors on the Excitatory Pyramidal Neurons in Cortex-Layer V After LY3201 Treatment.
To evaluate alterations of inhibitory and excitatory neurotransmitters after treatment, we examined the expression of GAD65+67 and NMDA receptors. GAD65+67 is the enzyme responsible for the conversion of glutamic acid to gamma-aminobutyric acid (GABA), the major inhibitory transmitter in brain. After LY3201 treatment, higher expression of the GAD65+67 was observed surrounding the pyramidal neurons in layer V of cortex and CA1 pyramidal neurons in hippocampus than in the vehicle-treated group (Fig. 2 A–F). NMDARs are glutamate-gated cation channels that are highly permeable to calcium (Ca2+) and are essential for regulation of synaptogenesis, use-dependent synaptic remodeling, and long-term plastic changes in synaptic strength (33). There was less NMDAR expression on the pyramidal neurons in layer V in the LY3201-treated group than in the vehicle-treated group (Fig. 2 G and H). The above results indicate that there are more inhibitory and less excitatory signal inputs to pyramidal neurons. This difference should lead to inhibition of the excitatory pyramidal neurons.
Fig. 2.
Expression of the GAD65+67 and NMDA receptors in layer V of the cortex and hippocampus CA1 in the vehicle- and LY3201-treated groups. (A and B) In the LY3201-treated group, there is higher expression of GAD65+67 in layer V of the cortex than in the vehicle-treated group. (C and D) There is more expression of GAD65+67 in the CA1 of hippocampus in the LY3201- than in the vehicle-treated group. (E and F) Overlay of GAD65+67 on neurons in layer V. There is more GAD65+67 expression (green) surrounding the pyramidal neurons (red) in the LY3201- than in the vehicle-treated group. Nuclei were counterstained with DAPI (blue). (G and H) Overlay of NMDA receptors on neurons in layer V. The positive NMDAR area is less in the LY3201- than in the vehicle-treated group. Nuclei were counterstained with DAPI. (I) GAD+ terminal density in vehicle and LY3201 groups (n = 3; *P = 0.004; **P = 0.002 for LY3201 compared with vehicle group). (J) NMDAR+ area in vehicle and LY3201 groups (n = 3; *P = 0.002 for LY3201 compared with vehicle group). (Scale bars in A–F, 20 μm; in G and H, 50 μm.)
Increased Activated Astrocytes but No Alteration of Microglia or Oligodendrocytes.
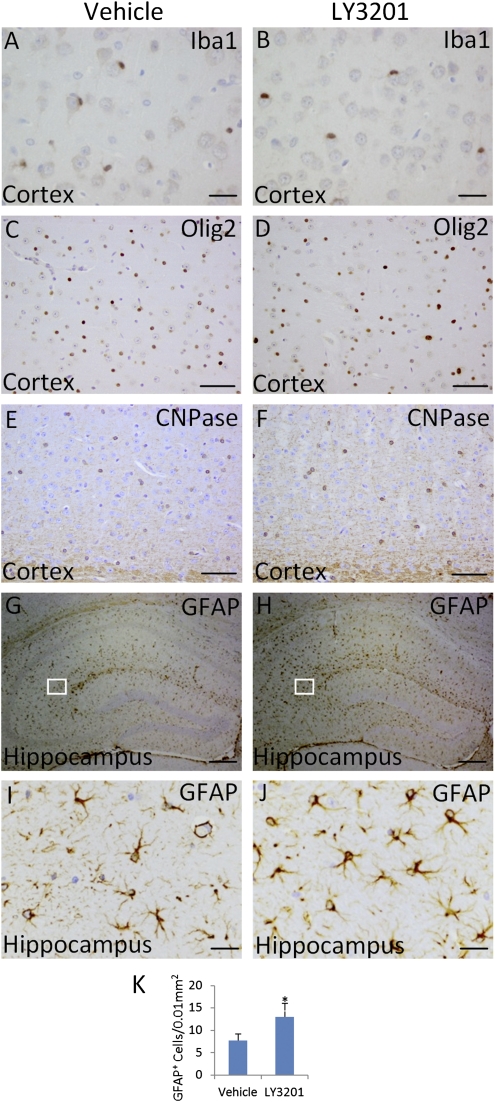
Because there are changes in neurons and neurotransmitters, we investigated whether there were changes in other types of cells in the brain after treatment with LY3201. There are three categories of neuronal-supporting cells; microglia, oligodendrocytes, and astrocytes, which can be identified with specific antibodies. After LY3201 treatment, there were no alterations of Iba1 (microglia marker), or in expression of Olig2 or CNPase (oligodendrocyte markers) (Fig. 3 A–F). However, the number of GFAP+ cells (astrocytes) was increased after LY3201 treatment (Fig. 3 G–K). In addition to an increase in astrocyte number in the LY3201 group, there were more activated astrocytes as evidenced by more astrocytic projections (Fig. 3 I and J). Astrocytes play a very important role during extracellular clearance of neurotransmitters. The cause for the increase in the number of activated astrocytes is not known but it could be due to the imbalance between excitatory and inhibitory transmitters.
Fig. 3.
Expression of antibodies for microglia, oligodendrocytes, and astrocytes. (A–F) There are no alterations of Iba1 (microglia marker), Olig2, or CNPase (oligodendrocyte maker). (G and H) However, the expression of GFAP (astrocyte marker) is increased in the LY3201-treatment group compared with the vehicle group. (I and J) Amplified views of boxed areas in G and H. In addition to more astrocytes in the LY3201 group, there are more projections on the astrocytes than in the vehicle group. (K) GFAP+ density in vehicle and LY3201 groups (n = 3; *P = 0.016 for LY3201 compared with vehicle group) (Scale bars in A, B, I, and J, 20 μm; in C–F, I, and J, 50 μm; in G and H, 200 μm.)
Astrocytes Are ERβ− and Glutamine Synthetase+.
Previous in vitro studies have reported the expression of ERβ in fetal and neonatal astrocytes (34). In the present study ERβ could not be detected in astrocytes in the adult mouse brain, but some other nerve cells do express ERβ (Fig. 4 A–D). Therefore, it appears that activation of astrocytes seen after treatment with the ERβ agonist LY3201, represents an indirect action on astrocytes. Glutamine synthetase, the enzyme that converts glutamate to glutamine, is expressed predominantly in astrocytes. The glutamine synthetase in the present study was colocalized with GFAP and its level was increased with the increase in astrocyte number and activation after LY3201 treatment (Fig. 4 E–L).
Fig. 4.
No expression of ERβ but strong expression of glutamine synthetase in astrocytes. (A–D) There is no expression of ERβ (green) on astrocytes (red) but the nuclei of some other nerve cells are ERβ+. The arrowheads show ERβ− astrocytes. Nuclei are counterstained with DAPI (blue). (E–L) The glutamine synthetase (red) is colocalized with GFAP (green). The colocalized color is orange and nuclei are counterstained with DAPI (Scale bars in A–D, 20 μm; in E–L, 50 μm.)
Discussion
The present study demonstrates that a selective ERβ ligand, LY3201, can induce the plasticity of dendritic spines and increase axosomatic inhibitory synapses in the pyramidal neurons of cortex and the CA1 area of hippocampus. In addition, the receptors for the excitatory transmitter, glutamate, are much reduced after LY3201 treatment. Concomitant with the change in the balance between excitatory and inhibitory synaptic transmission, more astrocytes were activated and expression of glutamine synthetase was elevated in these astrocytes. No abnormalities were found in the number of immature or mature oligodendrocytes or in the shape or number of microglia after LY3201 treatment.
Structural plasticity of dendritic spines and synapses is a fundamental mechanism governing neuronal circuits and is thought to be involved in several neurologic diseases such as seizures (35), schizophrenia (36), and anxiety (37). We found a decrease in the number of dendritic spines of pyramidal neurons in layer V of cortex and in the CA1 of hippocampus in male mice treated with LY3201. The role of estrogen and its receptors in changes of dendritic spines is not clear. Srivastava et al. have shown that WAY-200070, an ERβ agonist, increased spine density (38), whereas Szymczak et al. (39) found that estrogen induces spine density but that overexpression of ERβ in hippocampal neurons from embryonic rat brains by means of gene transfection, opposed the induction by estrogen. The discrepancies between the different results from these studies may be due to different treatment methods, models, and duration of treatment.
One question raised by our observation of a reduction in dendritic spines by LY3201 is whether these spines are excitatory or inhibitory. We addressed this question with an investigation of the expression of glutamic acid decarboxylase (GAD65+67). This is the enzyme responsible for the conversion of glutamic acid to GABA, the major inhibitory transmitter in brain. Our data show that GAD65+67 is expressed predominantly around the axosomatic pyramidal neurons and that there is increased expression of GAD65+67 upon LY3201 treatment. Because GAD is expressed on presynapses that surround the pyramidal neurons, there should be more GABA and thus more inhibition with more inhibitory spines on the pyramidal neurons. Our data further show that glutamate receptors, NMDARs, are much reduced on neurons in layer V of the cortex after ERβ agonist treatment. If there are more inhibitory and fewer excitatory signals received by the pyramidal neurons (which are excitatory neurons) there will be less excitatory signal output of these excitatory pyramidal neurons. Thus, upon treatment with LY3201, there is a shift in the balance between excitatory and inhibitory signaling in favor of inhibition. Because there is an overall reduction in the number of spines and yet an increase in the number of inhibitory spines, we can assume that excitatory spines were lost after LY3201 treatment.
Glutamate is the primary excitatory neurotransmitter in the CNS and is synthesized in all CNS cells as a key metabolite in the citric acid cycle. Glutamate is produced in high concentrations in neurons, packed into vesicles, and stored in synapses, from where it can be released into excitatory synapses. This synaptic release is the major extracellular source of glutamate in the normal CNS. In gray matter, astrocytes are major players in glutamate uptake. Upon LY3201 treatment there was a marked increase in the number of GFAP+ astrocytes. In addition, from the increase in the number of projections, these astrocytes appeared to be activated. The increase in the number of activated astrocytes would be expected to increase the uptake of glutamate. In astrocytes, glutamine synthetase catalyzes conversion of glutamate to glutamine (40). Because there is no detectable ERβ in astrocytes, the change in the number of reactive astrocytes seen upon LY3201 treatment is likely not caused by direct action of the ligand on astrocytes. The action appears to be indirect. One possible indirect mechanism may be that astrocyte activation is secondary to the increased extracellular level of glutamate. Lehmann et al. (41) have shown that higher extracellular glutamate can induce an increase in glutamine synthetase expression. In our experiment, because glutamine synthetase is expressed in all astrocytes and there are more astrocytes and more astrocytic projections after treatment with LY3201, glutamine synthetase expression is increased correspondingly and the result is that more glutamate is taken up and converted into glutamine.
In ERβ−/− mice there are morphological abnormalities in the brain, associated with defects in neuronal migration, which indicates that ERβ plays a very important role in the central nervous system and brain diseases (42). The present results indicate that ERβ also has important functions in the brain through regulation of the plasticity of dendritic spines and synapses. The results raise the possibility that specific ERβ agonists may be a useful approach in the treatment of neurological diseases caused by an imbalance between excitatory and inhibitory transmission.
Materials and Methods
Materials, Animals, and Tissue Preparation.
The ERβ agonist LY3201, (3aS,4R,9bR)-2,2-difluoro-4-(4-hydroxyphenyl)-3,3a,4,9b-tetrahydro-1H-cyclopenta[c]chromen-8-ol (CAS 787621–78-7), was prepared according to methods previously described (32). This study was conducted in accordance with the University of Houston guidelines on the use of laboratory animals. All animals were 6 mo old, male, and wild-type (WT) mice (B6), which were purchased from Taconic. The mice were divided randomly into two groups, a vehicle group and a LY3201 group. LY3201 was used as pellets (0.04 mg/d), which were made by Innovative Research of America and implanted on the lateral side of the neck between the ear and the shoulder. The pellet is made of a matrix fused with an active product. The ingredients are: cholesterol, cellulose, lactose, phosphates, and stearates. After continuous release of the pellet for 3 d, all mice were anesthetized deeply with CO2 and perfused with PBS followed by 4% paraformaldehyde (in 0.1 M PBS, pH 7.4). All brains were dissected and postfixed in the same fixative overnight at 4 °C. After fixation, brains were processed for paraffin (5 μm) sections.
Golgi Staining and Immunohistochemistry.
In this study we used Rapid Golgi staining to examine the histology of dendritic spines with light microscopy. The procedure followed the instructions of FD Rapid GolgiStain kit (FD NeuroTechnologies; PK401). Sections were cut on a cryostat (Leica) with a thickness of 100 μm. Paraffin sections were deparaffinized in xylene, rehydrated through graded alcohol, and processed for antigen retrieval by boiling in 10 mM citrate buffer (pH 6.0) for 2–3 min. The sections were incubated in 1% H2O2 in PBS for 15 min at room temperature to quench endogenous peroxidase. To block nonspecific binding, sections were incubated in 3% BSA for 10 min and then a biotin blocking system (Dako) was used to block endogenous biotin. Sections were then incubated with anti-Iba1 (1:100; Abcam), anti-Olig2 (1:500; Millipore), anti-CNPase (1:500; Abcam), anti-GFAP (1:400; Santa Cruz Biotechnology), and anti-GAD65+67(1:500; Abcam) in 1% BSA overnight at room temperature. BSA replaced primary antibodies in negative controls. After washing, sections were incubated with the corresponding secondary antibodies: rabbit on rodent HRP polymer kit (Biocare Medical; RMR622), MM HRP polymer kit (Biocare Medical; MM510), and goat HRP polymer kit (Biocare Medical; GHP516) for 15–20 min at room temperature, followed by the 3, 3′-diaminobenzidine tetrahydrochloride as the chromogen. For immunofluorescence, steps for quenching of endogenous peroxidase and blocking of endogenous biotin were omitted. Sections were incubated overnight with primary antibody anti-GFAP (1:400; Santa Cruz Biotechnology), anti-GAD65+67 (1:500; Abcam), anti-NeuN (1:500; Millipore), and anti-ERβ (1:200; made by our laboratory) at 4 °C after blocking nonspecific binding in 3% BSA. Primary antibodies were detected with donkey antigoat Cy3 (1:400; Jackson ImmunoResearch), donkey antirabbit FITC (1:400; Santa Cruz Biotechnology), donkey antichicken FITC (1:400; Jackson ImmunoResearch), and donkey antimouse Cy3 (1:400; Jackson ImmunoResearch). Sections were later counterstained with Vectashield mounting medium containing 4', 6'-diamidino-2-phenylindole (DAPI) (Vector) to label nuclei (43, 44).
Data Analysis.
For dendritic spines, density of cell and GAD+ terminals, the software of MicroSuite Basic Edition and Image Pro Plus 6.0 were used, respectively. The number of mice in each experiment was at least three per group. Data are presented as mean ± SD. The statistical significance of differences between LY3201 and vehicle samples was assessed by using Student's t test.
Acknowledgments
This work was supported by grants from the Robert A. Welch Foundation, Eli Lilly, the Swedish Cancer Fund, and the Texas Emerging Technology Fund.
Footnotes
This study was partially funded by a grant from Eli Lilly. Some of the authors are employed by Eli Lilly.
References
- 1.Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Harris HA, et al. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144:4241–4249. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 3.Toufexis DJ, Myers KM, Bowser ME, Davis M. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. J Neurosci. 2007;27:9729–9735. doi: 10.1523/JNEUROSCI.2529-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- 5.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 6.Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta-deficient mice. Proc Natl Acad Sci USA. 2001;98:12278–12282. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- 8.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. 2008;122:974–981. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frye CA, Koonce CJ, Edinger KL, Osborne DM, Walf AA. Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice. Horm Behav. 2008;54:726–734. doi: 10.1016/j.yhbeh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mora S, Dussaubat N, Díaz-Véliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 11.Morgan MA, Pfaff DW. Estrogen's effects on activity, anxiety, and fear in two mouse strains. Behav Brain Res. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 12.Nomikos GG, Spyraki C. Influence of oestrogen on spontaneous and diazepam-induced exploration of rats in an elevated plus maze. Neuropharmacology. 1988;27:691–696. doi: 10.1016/0028-3908(88)90077-9. [DOI] [PubMed] [Google Scholar]
- 13.Tomihara K, et al. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav. 2009;96:300–306. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuiper GGM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugiyama N, Barros RP, Warner M, Gustafsson JA. ERbeta: Recent understanding of estrogen signaling. Trends Endocrinol Metab. 2010;21:545–552. doi: 10.1016/j.tem.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 17.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 18.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 19.Czéh B, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- 20.Banasr M, et al. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci USA. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao J, et al. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- 24.Leranth C, Shanabrough M, Horvath TL. Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience. 2000;101:349–356. doi: 10.1016/s0306-4522(00)00369-9. [DOI] [PubMed] [Google Scholar]
- 25.Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447:34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- 26.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azcoitia I, Garcia-Ovejero D, Chowen JA, Garcia-Segura LM. Astroglia play a key role in the neuroprotective actions of estrogen. Prog Brain Res. 2001;132:469–478. doi: 10.1016/S0079-6123(01)32096-4. [DOI] [PubMed] [Google Scholar]
- 28.Seifert G, Steinhäuser C. Neuron-astrocyte signaling and epilepsy. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.08.024. 10.1016/j.expneurol.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Lewis DK, Johnson AB, Stohlgren S, Harms A, Sohrabji F. Effects of estrogen receptor agonists on regulation of the inflammatory response in astrocytes from young adult and middle-aged female rats. J Neuroimmunol. 2008;195:47–59. doi: 10.1016/j.jneuroim.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145:584–595. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawlak J, Brito V, Küppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain Res Mol Brain Res. 2005;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 32.Richardson TI, et al. Benzopyrans as selective estrogen receptor beta agonists (SERBAs). Part 3: Synthesis of cyclopentanone and cyclohexanone intermediates for C-ring modification. Bioorg Med Chem Lett. 2007;17:4824–4828. doi: 10.1016/j.bmcl.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 33.Benarroch EE. NMDA receptors: Recent insights and clinical correlations. Neurology. 2011;76:1750–1757. doi: 10.1212/WNL.0b013e31821b7cc9. [DOI] [PubMed] [Google Scholar]
- 34.Giraud SN, Caron CM, Pham-Dinh D, Kitabgi P, Nicot AB. Estradiol inhibits ongoing autoimmune neuroinflammation and NFkappaB-dependent CCL2 expression in reactive astrocytes. Proc Natl Acad Sci USA. 2010;107:8416–8421. doi: 10.1073/pnas.0910627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González-Burgos I, López-Vázquez MA, Beas-Zárate C. Density, but not shape, of hippocampal dendritic spines varies after a seizure-inducing acute dose of monosodium glutamate in rats. Neurosci Lett. 2004;363:22–24. doi: 10.1016/j.neulet.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 36.Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: Molecular evidence for a pathology of dendritic spines. Am J Psychiatry. 2004;161:1848–1855. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- 37.Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava DP, Woolfrey KM, Liu F, Brandon NJ, Penzes P. Estrogen receptor ß activity modulates synaptic signaling and structure. J Neurosci. 2010;30:13454–13460. doi: 10.1523/JNEUROSCI.3264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szymczak S, et al. Increased estrogen receptor beta expression correlates with decreased spine formation in the rat hippocampus. Hippocampus. 2006;16:453–463. doi: 10.1002/hipo.20172. [DOI] [PubMed] [Google Scholar]
- 40.Liaw SH, Kuo I, Eisenberg D. Discovery of the ammonium substrate site on glutamine synthetase, a third cation binding site. Protein Sci. 1995;4:2358–2365. doi: 10.1002/pro.5560041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehmann C, Bette S, Engele J. High extracellular glutamate modulates expression of glutamate transporters and glutamine synthetase in cultured astrocytes. Brain Res. 2009;1297:1–8. doi: 10.1016/j.brainres.2009.08.070. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci USA. 2003;100:703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HJ, et al. Liver X receptor beta (LXRbeta): A link between beta-sitosterol and amyotrophic lateral sclerosis-Parkinson's dementia. Proc Natl Acad Sci USA. 2008;105:2094–2099. doi: 10.1073/pnas.0711599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan XJ, et al. Liver X receptor β and thyroid hormone receptor α in brain cortical layering. Proc Natl Acad Sci USA. 2010;107:12305–12310. doi: 10.1073/pnas.1006162107. [DOI] [PMC free article] [PubMed] [Google Scholar]