The scientific literature is replete with studies of the influence of weak magnetic fields on biological systems. Often motivated by alleged health hazards of the stray electromagnetic fields that accompany the distribution and use of electrical power, the majority of these articles report definite effects. However, in the relatively few cases in which independent replication has been attempted, the original results have usually proved irreproducible (1, 2). The situation is not helped by the scarcity of (bio)physical mechanisms by which weak magnetic fields might interact with biology. With no hypothetical mechanism to guide experimental design, the majority of these investigations have been, to varying degrees, unsatisfactory. A striking exception is a series of articles by Anatoly Buchachenko and Dmitry Kouznetsov (BK) and their associates (3–6). In more than 10 papers dating back to 2005, including one in PNAS (3), BK have reported effects of magnetic interactions on the rate of enzymatic synthesis of ATP in vitro. These studies are conspicuous in that the reported changes are large, the interaction mechanism is physically credible, an explicit reaction scheme is proposed, and the process itself is of considerable biological importance. If genuine and applicable in vivo, these results could have significant therapeutic (if not health) implications. In PNAS, the work by Crotty et al. (7) describes attempts to replicate BK's findings.
The conversion of ADP into ATP is catalyzed by a number of magnesium-dependent enzymes. The works by BK report that the rate of ATP production by four kinases—ATP synthase, phosphoglycerate kinase, pyruvate kinase, and creatine kinase—exhibits an unusual and substantial magnesium isotope effect (3, 4). Magnesium has three stable isotopes: 24Mg (79%), 25Mg (10%), and 26Mg (11%). Rather than a monotonic dependence of the reaction rate on the isotope mass number, which would be expected for the conventional kinetic isotope effect, it was found that ATP was formed more than twice as fast in the presence of 25Mg than in the presence of either 24Mg or 26Mg. This finding was taken as the signature of the radical pair mechanism (RPM) (8) in which magnetic isotope effects (MIEs) arise not from the mass but from the magnetic moment of the atomic nucleus (9). Of the three isotopes, only 25Mg is magnetic as a result of the number and disposition of its protons and neutrons. Although much less common than the kinetic isotope effect, the MIE is a well-characterized feature of chemical transformations that have radical pairs as transient reaction intermediates. Since its discovery by Buchachenko in 1976, it has been exploited as a probe of free radical reaction pathways.
A somewhat more common manifestation of the RPM is the sensitivity of radical pair reactions to external magnetic fields (8, 10). Acknowledged for more than 30 y to be responsible for a multitude of magnetic effects on chemical reaction rates and yields, the RPM has been implicated in several experimental observations of biomolecular magnetic field effects (MFEs). Although there is little competition for the title, the RPM is arguably the most plausible mechanism by which weak magnetic interactions might affect biochemical reactions. Supporting their interpretation of the ATP data, BK found that the difference in phosphorylation rates for the magnetic and nonmagnetic isotopes of Mg increased from twofold to more than fourfold when an 80-mT magnetic field was applied (5). To put this finding in context, 80 mT is about 1,000 times stronger than the Earth's magnetic field and about 100 times weaker than the strongest fields used for clinical MRI.
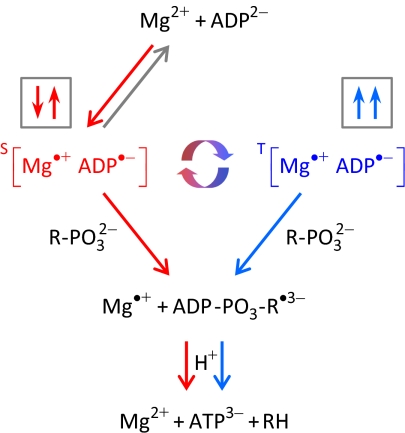
The novel reaction pathway put forward by BK (3) to account for both MIE and MFE is shown in Fig. 1. As outlined, the hyperfine interaction of the magnetic 25Mg isotope in a radical pair comprised of a Mg+ ion and an ADP radical is thought to flip the electron spin of the former, opening up a reaction pathway that would otherwise be insignificant and favoring the catalytic reaction over the unproductive back reaction. Although there seems to have been no previous suggestion that the +1 oxidation state of Mg can mediate ATP production and scant evidence that Mg has any biologically relevant redox chemistry, the general idea that magnetic nuclei and applied magnetic fields can alter the coherent spin dynamics of radical pairs and therefore affect reaction rates and product yields is beyond doubt (8).
Fig. 1.
Reaction scheme proposed by BK for the enzymatic conversion of ADP to ATP shown here for creatine kinase. When performed with one of the nonmagnetic magnesium isotopes, 24Mg or 26Mg, the reaction proceeds mainly by the steps indicated by red arrows. Electron transfer from ADP to a Mg2+ ion in the active site of the enzyme leads to the (red) [Mg•+ ADP•−] radical pair. Subsequent reaction of the ADP radical with phosphocreatine forms ATP and creatine and regenerates Mg2+. Because radical pair reactions must conserve electron spin, the radical pair is produced in a singlet (S) state with antiparallel electron spins (↓↑), one in each radical. The forward catalytic process (red arrows) competes with back electron transfer (gray arrow). When 25Mg is used, its nuclear magnetic moment couples to the magnetic moment of the valence electron of Mg•+, converting the singlet (red) radical pair into its triplet form (T; blue) in which the electron spins are parallel (↑↑). As implied by the circular red-blue arrows, this process is coherent and quantum mechanical. The requirement to conserve spin angular momentum means that the triplet radical pair cannot revert directly to the reactants. Its formation in the presence of 25Mg, therefore, opens up an additional forward reaction pathway (blue arrows) and do increases the rate of ATP production.
The replication study by Crotty et al. (7) focuses on creatine kinase (CK), which converts phosphocreatine and ADP to creatine and ATP (7). Measurements of reaction yields and rates were performed independently in Dublin and Colchester using different techniques to study the same enzyme (MM1-type CK) from either rabbit muscle (Dublin) or human heart (Colchester). Only after their experiments were largely complete did the two groups become aware of each other's work and agree to publish jointly. Neither group is able to observe the twofold MIE in ambient magnetic fields or any MFE, even using fields as strong as 1,000 mT. The only detectable MIE is a small (15%) reduction in ATP production when 25Mg is used instead of natural abundance Mg. Crotty et al. (7) argue that their failure to find large effects is unlikely to be due to the different sources of protein [BK studied a monomeric CK isozyme from a snake venom, whereas MM1 variants are dimeric in the work by Crotty et al. (7)]. The replication study (7) duplicated as faithfully as possible the conditions of the original experiments. There seems to be no obvious explanation for the irreproducibility of BK's results.
There have been at least three other negative replication studies of seemingly robust biological radical pair MFEs. The works by Jones et al. (11, 12) failed to reproduce sizeable MFEs for two enzyme reactions in vitro: the conversion of ethanolamine to acetaldehyde by the bacterial enzyme ethanolamine ammonia lyase (13) and the reduction of hydrogen peroxide by HRP (14). In both cases, the changes in catalytic rates reported in the original articles were large, and the radical pair chemistry was apparently plausible. The third case was an unsuccessful attempt by Harris et al. (15) to replicate the observation that the growth of Arabidopsis thaliana seedlings was significantly affected by magnetic fields as weak as 500 μT (16). The authors of the three original studies (13, 14, 16) have yet to respond in print; in no case is it clear why independent replication proved impossible.
There have been a few more cases in which radical pairs have been invoked in a biological context, but one in particular
The replication study by Crotty et al. focuses on creatine kinase (CK), which converts phosphocreatine and ADP to creatine and ATP.
stands out. For many years, a variety of radical pair effects have been exploited to shed considerable light on the energetics of charge separation and energy stabilization in photosynthetic reaction center proteins (17). Radical pairs arise naturally as a result of photo-induced electron transfer reactions, but in all cases, forward electron transport must be artificially blocked before RPM effects can be observed. Although there is no doubt at all that these effects are real, no one seriously believes that photosynthesis in vivo might be magnetically sensitive.
So, are there no reliable instances of radical pair effects in biology? Over the last 10 y, evidence has been accumulating to support the proposal that the ability of birds to sense the direction of the Earth's magnetic field (∼50 μT) is based on radical pair photochemistry (18). The most likely candidate magnetoreceptor for this compass is the photoactive protein, cryptochrome, in which apparently suitable flavin-tryptophan radical pairs are formed in vitro after excitation by blue light (19). In diagnostic tests for the involvement of the RPM, the ability of robins to orient in the Earth's field was found to be disrupted by the presence of a 1.3-MHz radiofrequency magnetic field with a strength of only 15 nT (i.e., 3,000 times weaker than the Earth's field) (20). This result strongly suggests that radical pairs play a role in avian magnetoreception, although not necessarily as the primary sensor, and is so remarkable that it, too, must be subjected to the gold standard of independent replication.
If laboratory investigations of radical pairs in biomolecular systems do reveal reproducible MFEs, this finding should not be taken to imply a similar effect at the cellular or whole-organism level. In the enzyme reactions mentioned above, including ATP synthesis (6, 7), magnetic responses are only observed under nonphysiological conditions. With the possible exception of a radical pair magnetoreceptor, which presumably would have evolved to be exquisitely sensitive to weak magnetic fields, it seems at present unlikely, given the efficiency of homeostatic buffering, that effects observed in vitro, even under physiological conditions, would persist in vivo.
Acknowledgments
My work and the work of my colleagues in Oxford is supported by the Electromagnetic Fields Biological Research Trust and the Defense Advanced Research Projects Agency.
Footnotes
The author declares no conflict of interest.
See companion article on page 1437.
References
- 1.Lacy-Hulbert A, Metcalfe JC, Hesketh R. Biological responses to electromagnetic fields. FASEB J. 1998;12:395–420. doi: 10.1096/fasebj.12.6.395. [DOI] [PubMed] [Google Scholar]
- 2.Crumpton MJ. The Bernal Lecture 2004. Are low-frequency electromagnetic fields a health hazard? Philos Trans R Soc Lond B Biol Sci. 2005;360:1223–1230. doi: 10.1098/rstb.2005.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchachenko AL, Kouznetsov DA, Orlova MA, Markarian AA. Magnetic isotope effect of magnesium in phosphoglycerate kinase phosphorylation. Proc Natl Acad Sci USA. 2005;102:10793–10796. doi: 10.1073/pnas.0504876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchachenko AL, Kouznetsov DA, Breslavskaya NN, Orlova MA. Magnesium isotope effects in enzymatic phosphorylation. J Phys Chem B. 2008;112:2548–2556. doi: 10.1021/jp710989d. [DOI] [PubMed] [Google Scholar]
- 5.Buchachenko AL, Kuznetsov DA. Magnetic field affects enzymatic ATP synthesis. J Am Chem Soc. 2008;130:12868–12869. doi: 10.1021/ja804819k. [DOI] [PubMed] [Google Scholar]
- 6.Buchachenko AL, Kuznetsov DA, Breslavskaya NN. Ion-radical mechanism of enzymatic ATP synthesis: DFT calculations and experimental control. J Phys Chem B. 2010;114:2287–2292. doi: 10.1021/jp909992z. [DOI] [PubMed] [Google Scholar]
- 7.Crotty D, et al. Reexamination of magnetic isotope and field effects on adenosine triphosphate production by creatine kinase. Proc Natl Acad Sci USA. 2012;109:1437–1442. doi: 10.1073/pnas.1117840108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steiner UE, Ulrich T. Magnetic field effects in chemical kinetics and related phenomena. Chem Rev. 1989;89:51–147. [Google Scholar]
- 9.Salikhov KM. Magnetic Isotope Effect in Radical Reactions. Vienna: Springer-Verlag; 1996. [Google Scholar]
- 10.Rodgers CT. Magnetic field effects in chemical systems. Pure Appl Chem. 2009;81:19–43. [Google Scholar]
- 11.Jones AR, Scrutton NS, Woodward JR. Magnetic field effects and radical pair mechanisms in enzymes: A reappraisal of the horseradish peroxidase system. J Am Chem Soc. 2006;128:8408–8409. doi: 10.1021/ja060463q. [DOI] [PubMed] [Google Scholar]
- 12.Jones AR, Hay S, Woodward JR, Scrutton NS. Magnetic field effect studies indicate reduced geminate recombination of the radical pair in substrate-bound adenosylcobalamin-dependent ethanolamine ammonia lyase. J Am Chem Soc. 2007;129:15718–15727. doi: 10.1021/ja077124x. [DOI] [PubMed] [Google Scholar]
- 13.Harkins TT, Grissom CB. Magnetic field effects on B12 ethanolamine ammonia lyase: Evidence for a radical mechanism. Science. 1994;263:958–960. doi: 10.1126/science.8310292. [DOI] [PubMed] [Google Scholar]
- 14.Taraban MB, Leshina TV, Anderson MA, Grissom CB. Magnetic field dependence of electron transfer and the role of electron spin in heme enzymes: Horseradish peroxidase. J Am Chem Soc. 1997;119:5768–5769. [Google Scholar]
- 15.Harris S-R, et al. Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J R Soc Interface. 2009;6:1193–1205. doi: 10.1098/rsif.2008.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad M, Galland P, Ritz T, Wiltschko R, Wiltschko W. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta. 2007;225:615–624. doi: 10.1007/s00425-006-0383-0. [DOI] [PubMed] [Google Scholar]
- 17.Volk M, Ogrodnik A, Michel-Beyerle ME. The recombination dynamics of the radical pair P+H− in external magnetic and electric fields. In: Blankenship RE, Madigan MT, Bauer CE, editors. Anoxygenic Photosynthetic Bacteria. Boston: Kluwer; 1995. pp. 595–626. [Google Scholar]
- 18.Rodgers CT, Hore PJ. Chemical magnetoreception in birds: The radical pair mechanism. Proc Natl Acad Sci USA. 2009;106:353–360. doi: 10.1073/pnas.0711968106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritz T, et al. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys J. 2009;96:3451–3457. doi: 10.1016/j.bpj.2008.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]