Abstract
Spermiogenesis is a series of poorly understood morphological, physiological and biochemical processes that occur during the transition of immotile spermatids into motile, fertilization-competent spermatozoa. Here, we identified a Serpin (serine protease inhibitor) family protein (As_SRP-1) that is secreted from spermatids during nematode Ascaris suum spermiogenesis (also called sperm activation) and we showed that As_SRP-1 has two major functions. First, As_SRP-1 functions in cis to support major sperm protein (MSP)-based cytoskeletal assembly in the spermatid that releases it, thereby facilitating sperm motility acquisition. Second, As_SRP-1 released from an activated sperm inhibits, in trans, the activation of surrounding spermatids by inhibiting vas deferens-derived As_TRY-5, a trypsin-like serine protease necessary for sperm activation. Because vesicular exocytosis is necessary to create fertilization-competent sperm in many animal species, components released during this process might be more important modulators of the physiology and behavior of surrounding sperm than was previously appreciated.
Keywords: cell motility, regulated exocytosis, sperm competition, postcopulatory sexual selection, de novo sequencing
In most, if not all, animals, males produce sperm in their gonad that are fertilization-incompetent until they leave this organ and undergo further maturation. For instance, whereas mammalian spermatozoa form in the testes, they must undergo a maturation process called capacitation in the female reproductive tract before they become fertilization-competent (1). In nematodes, spermatids do not complete maturation into spermatozoa (spermiogenesis) until after they have left the testes. In the nematode Caenorhabditis elegans, sperm are made in both males and self-fertile hermaphrodites (there are no conventional females). Hermaphrodites have a testis that proliferates sperm and then it switches into an ovary and produces oocytes. The first ovulated oocyte pushes the stored spermatids from the gonad into the spermatheca, where they are rapidly activated into spermatozoa. Upon mating with hermaphrodite, male spermatids are ejaculated and activated within the uterus by exposure to unknown factor(s) in the seminal fluid (2). Male-derived sperm are preferentially used to promote outbreeding in a typical cross (3). This sperm precedence correlates with the larger size of male-derived sperm relative to hermaphrodite-derived sperm (4) and can even occur in certain fertilization-defective mutants (5). Although in vitro and in vivo studies have suggested that protease activity is involved in C. elegans sperm activation (6, 7), neither hermaphrodite nor male sperm activators have been identified.
Unlike C. elegans, the intestinal parasitic nematode Ascaris suum (or Ascaris hereafter) has males and true females, but no hermaphrodites. However, sperm of both species do share several similarities (8). First, they are unusual in that their activated spermatozoa lack flagella. Rather, nematode spermatozoa move by using pseudopods generated during spermiogenesis, also called sperm activation (2). Second, unlike other types of amoeboid motility that are based on actin, the motility of nematode spermatozoa is based on controlled assembly/disassembly of a major sperm protein (MSP) cytoskeleton (8). Third, like male-derived sperm in C. elegans, Ascaris sperm activation occurs postinsemination. Fourth, sperm of both C. elegans and Ascaris contain structurally similar membranous organelles (MOs) (2), which is a type of intracellular vesicle with similarity to lysosomes (9). During sperm activation, fusion of MOs with the plasma membrane (PM) of spermatids is necessary for spermatozoan motility and male fertility (10, 11). However, the exact function of MOs and their components that are released into the extracellular space during fusion are not well understood.
Ascaris sperm are highly suitable for answering questions about how sperm prepare for fertilization because: sperm activation can be studied ex vivo (12), sperm motility has been reconstituted in cell-free sperm extracts (13, 14), and all relevant components can be obtained in the large quantities required for biochemical analysis (12, 15). In this study, we identified two Ascaris proteins, As_SRP-1 [a member of the Serpin (serine protease inhibitor) superfamily] and As_TRY-5 (a trypsin-like serine protease). We showed that nematode sperm maturation triggered by vas deferens-derived As_TRY-5 involves sperm-secreted As_SRP-1 and that secreted As_SRP-1 in the medium inhibits activation of surrounding spermatids. This dual function of sperm-secreted As_SRP-1 might play a significant role during postcopulatory sexual selection.
Results
As_SRP-1 (1CB4 antigen) Is Translocated During Ascaris Sperm Activation.
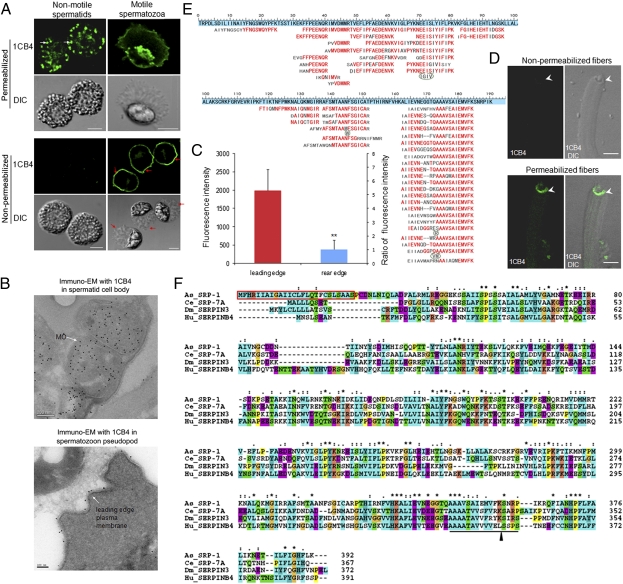
We found that the 1CB4 monoclonal antibody that recognizes C. elegans MOs (11, 16–18) also recognized Ascaris sperm MOs (Fig. 1 A and B). Immunofluorescence staining of permeabilized Ascaris spermatids or spermatozoa with 1CB4 revealed punctuate, peripherally located structures, similar to what is seen in C. elegans (11, 17). Cryo immuno-EM with 1CB4 confirmed that immuno-gold labeled tightly-packed stacks of membranes inside sperm (Fig. 1B, Upper), characteristics of MOs in C. elegans (2). Different from previous immunofluorescence studies in C. elegans, 1CB4 also stained the leading edge PM of Ascaris spermatozoon (Fig. 1A). The 1CB4 staining in the leading edge spermatozoon is further shown to be on the outer PM by three lines of our evidence. First, in nonpermeabilized spermatozoa, 1CB4 immunofluorescence was readily observed on the cell surface (Fig. 1A). Second, Cryo-immuno-EM with 1CB4 revealed the clear immunogold labeling along the outer PM of spermatozoa (Fig. 1B, Lower). Third, from an in vitro MSP motility assay (Fig. 1D), in which the leading edge PM-derived vesicles from spermatozoa extracts recruit cytosolic components to trigger MSP fiber assembly (13), we found that 1CB4 immunofluorescence could be detected only in permeabilized fiber-growing vesicles. Given that these vesicles acquire an inside-out configuration during cell lysis (13), this result is consistent with the outer PM-localization of the 1CB4 target in Ascaris spermatozoa. Moreover, immunofluorescence quantification of the 1CB4 staining in nonpermeabilized spermatozoa demonstrates that the 1CB4 on the outer PM of spermatozoa was distinctly asymmetrical, i.e., the fluorescence intensity along the leading edge PM was 5.3-fold higher than that in the rear edge PM (Fig. 1C), in agreement with previous observations by quantitative immuno-EM in C. elegans spermatozoa (11).
Fig. 1.
As_SRP-1 (protein recognized by the 1CB4 antibody) is translocated during Ascaris sperm activation. (A) The 1CB4 monoclonal antibody labeled MOs and the leading edge of spermatozoon PM in Ascaris. White arrows, MOs; red arrows, the leading edge of the pseudopod. (Scale bars, 5 μm.) (B) 1CB4 immunogold was detected in the MO of spermatid (Upper) and the outer PM of spermatozoon leading edge (Lower) by Cryo-immuno-EM. [Scale bars, 200 nm (Upper) and 100 nm (Lower).] (C) 1CB4 fluorescence intensity at the leading edge and rear edge was measured in nonpermeabilized spermatozoa (A, Lower) by MetaMorph. Results are means ± SD (n = 50 spermatozoa). **P < 0.01 (Student t test). (D) In an in vitro MSP fiber assembly assay, the 1CB4 immunostaining (green) was detected only in vesicles that were permeabilized. (Scale bars, 5 μm.) (E) The protein recognized by the 1CB4 antibody was identified as a Serpin family protein by de novo sequencing (SI Materials and Methods). Set against a blue background is the partial As_SRP-1 protein sequence translated from Ascaris expressed sequence tag (EST) sequences. Sequences deduced from mass spectra of As_SRP-1 peptides generated by trypsin. Red, matched residues; gray, unmatched residues; dash, a gap; bulge, extra residues found in the de novo peptide sequences. (F) Amino acid sequence of As_SRP-1 was aligned with three other Serpins, including Ce_SRP-7A (C. elegans, NP_001023823), Dm_SERPIN3 (D. melanogaster, NP_524956), and Hu_SERPINB4 (human, NP_002965). The reactive site loop (RSL) is underlined; arrowhead, the putative scissile bond; red box, a predicted signal peptide; asterisk, identical amino acid; colon, amino acid with high similarity; dot, amino acid with less similarity.
1CB4 is a monoclonal antibody generated using homogenates of whole C. elegans (16). Although it has been extensively used for labeling MOs in C.elegans (11, 17), the molecular identity of the antigen recognized by 1CB4 has not been determined. By using Western blotting, we found that a single polypeptide (∼46 kDa) is recognized by 1CB4 in Ascaris sperm extract, and it was mostly in a soluble, cytosolic fraction (Fig. S1A). Isolation and purification of the 46 kDa protein were achieved by following the 1CB4 signal in Western blots from different cellular fractions (Fig. S1B). Initial MS analysis of the purified protein using a conventional database search strategy was ineffective because this protein was not in the database. We resorted to de novo sequencing analysis using the pNovo program (19) and extracted sequences directly from the tandem mass spectra of peptides derived from this protein (Fig. 1E and Fig. S2). We synthesized two peptides according to the pNovo result and found that the identification of these two sequences was fully supported by the parent masses and high-resolution MS/MS spectra of the synthetic peptides (Fig. S2). BLAST searches of these peptides against predicted Ascaris protein sequences in NEMBASE3 (20) revealed that the most abundant protein in the sample was a Serpin (Fig. 1E), belonging to the Serpin superfamily (we named it As_SRP-1). Using rapid amplification of cDNA ends by PCR (RACE PCR), we cloned the full-length cDNA of As_srp-1 and deduced its amino acid sequence (Fig. 1F). When the original MS data were searched against a database containing the newly cloned As_SRP-1 sequence using either Mascot or pFind, As_SRP-1 was identified as the top hit, and the overlap between the database search result and the de novo sequencing result was extensive (Figs. S3 and S4). Amino acid alignment showed that As_SRP-1 shares strong sequence homology with members of the clade B Serpin family. A highly conserved reactive site loop (RSL) containing a putative scissile bond (21) was detected in the sequence of As_SRP-1 (Fig. 1F). When expressed in E. coli, the recombinant As_SRP-1 displayed the same molecular mass as that of native As_SRP-1 and was recognized by both 1CB4 and the polyclonal antibody we raised against purified native As_SRP-1 (Fig. S1C). These data demonstrate that the target of the 1CB4 monoclonal antibody in Ascaris is As_SRP-1.
As_SRP-1 Is Essential for MSP-Based Sperm Motility in Ascaris.
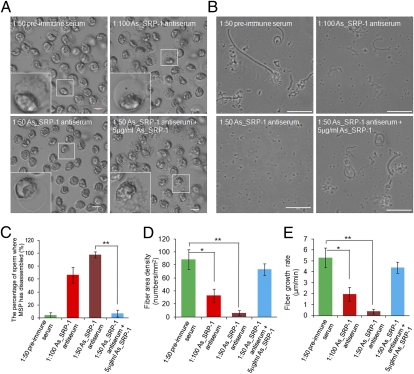
The localization of As_SRP-1 on the outer PM of spermatozoon and its asymmetrical distribution at the leading edge (Fig. 1 A–D) suggest that this protein probably plays a role in MSP cytoskeleton dynamics and sperm motility. To examine this possibility, we performed both ex vivo and in vitro experiments. When spermatozoa were perfused with the As_SRP-1 antiserum (1:100 or 1:50 dilution), spermatozoa stopped crawling, their MSP cytoskeleton disappeared (66% or 98%, respectively), and cells rounded up (Fig. 2 A and C). These defects in cytoskeleton dynamics and sperm morphology were almost completely reversed when the antiserum was first neutralized by adding purified native As_SRP-1, with < 5% of spermatozoa exhibiting defects (Fig. 2 A and C). Furthermore, As_SRP-1 localization on the inner leaflet of the vesicle membrane (equivalent to outer PM) (Fig. 1D) is important for MSP fiber assembly in vitro because extracts from As_SRP-1 antiserum-treated spermatozoa resulted in a significant decrease in both the growth rate and area density of MSP fibers. Such motility defects disappeared in the extracts from spermatozoa that were perfused with the As_SRP-1 antiserum and the neutralizing As_SRP-1 (Fig. 2 B, D, and E). Together, these data indicate that As_SRP-1 at the outer PM of sperm pseudopod leading edge plays an essential role in regulating both MSP cytoskeleton assembly and cell motility. Our immunolabeling data suggest that As_SRP-1 regulates Ascaris sperm motility probably through protein tyrosine phosphorylation (Fig. S5).
Fig. 2.
As_SRP-1 is essential for MSP-based cytoskeleton dynamics and sperm motility in Ascaris. (A) As_SRP-1 antiserum treatment of spermatozoa for 20 min caused MSP cytoskeleton disassembly and spermatozoan roundup, although adding 5 μg/mL As_SRP-1 rescued this phenotype. Upper Left, 1:50 preimmune serum treatment (control). Insets, higher magnification of outlined sperm. (Scale bars, 20 μm.) The sperm cytoskeletal disassembly was quantified (C). (B) An in vitro MSP fiber assembly assay where the extracts from spermatozoa treated with As_SRP-1 antiserum had fewer fibers and a slower fiber growth rate, whereas adding 5 μg/mL As_SRP-1 rescued the defects. Upper Left, MSP fibers assembled with the extract from spermatozoa treated with 1:50 preimmune serum (control). (Scale bars, 50 μm.) The area density and growth rates of assembled fibers were calculated by MetaMorph (D and E). The data shown in C–E are means ± SD (n = 5 experiments). *P < 0.001; **P < 0.0001 (Student t test).
Secreted As_SRP-1 Blocks Sperm Activation in Surrounding Spermatids.
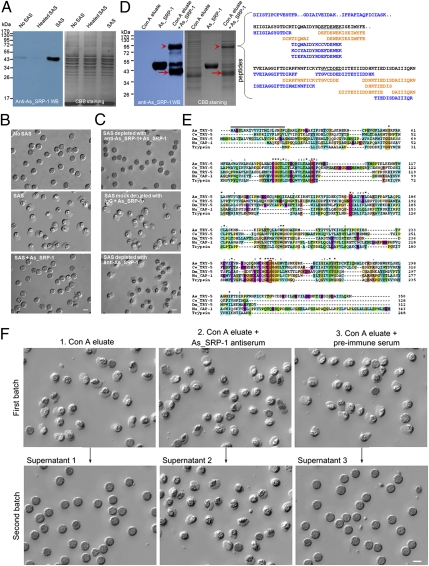
As shown in Fig. 1, the As_SRP-1 localization in Ascaris spermatids (in MOs) is different from that in spermatozoa (on the outer PM). A secretory signal peptide sequence is present at the N terminus of As_SRP-1 (Fig. 1F). Because the fusion of MOs with the PM during sperm activation is known to be a regulated exocytosis process in C. elegans (11), exocytosed As_SRP-1 could be translocated from MOs to the outer PM during Ascaris sperm activation. Indeed, when Ascaris spermatids were activated by sperm-activating substance (SAS) (the extract from vas deferens) (12), the amount of As_SRP-1 in the medium increased dramatically as shown by Western blotting analysis using anti-As_SRP-1 antibody (Fig. 3A). In contrast, only a weak signal of As_SRP-1, most likely from rare, spontaneous activation, was detected in the medium of sperm that were either not subjected to SAS or subjected to heat-inactivated SAS. The secretion of a Serpin (As_SRP-1) during sperm activation (Fig. 3A) and the ability of proteases (6, 7, 12) to activate nematode sperm led us to test whether As_SRP-1 can inhibit SAS-induced sperm activation and whether the activity of a serine protease(s) in SAS is essential for sperm activation. Not surprisingly, we found that purified As_SRP-1 was able to inhibit SAS-induced sperm activation (Fig. 3B). Further experiments showed the inhibitory target of As_SRP-1 was present in SAS but not on sperm itself because when sperm were treated with the mixture of As_SRP-1 and 0.5 or 5 μg/mL of SAS, the sperm activation rate was lower than that from sperm treated with As_SRP-1 first, then followed by SAS addition (Fig. S6). The importance of a serine protease activity in SAS-induced sperm activation is also supported by our pharmacological studies in which the effect of various specific protease inhibitors on SAS was tested. These results demonstrate that only serine protease inhibitors, and not other inhibitors, prevent SAS-induced sperm activation (Fig. S7). Furthermore, we found that As_SRP-1 could interact with the predicted serine protease(s) in SAS using immunodepletion assays (Fig. 3C). We immobilized As_SRP-1 to protein A beads through As_SRP-1 antibody, incubated the As_SRP-1 beads with SAS and separated the SAS supernatant from the beads. If physical interaction occurs, the protease should be depleted from SAS by As_SRP-1 beads, causing the supernatant to lose its activity. This was indeed what we observed (Fig. 3C, Top). Meanwhile in the control experiments, Protein A beads with mock immobilization of As_SRP-1 through control IgG (Fig. 3C, Middle) or beads preloaded with As_SRP-1 antibody alone (Fig. 3C, Bottom) failed to deplete the activity from the SAS supernatant. Therefore, As_SRP-1 can physically bind to the serine protease(s) in SAS. Collectively, these data suggest that the activity of a serine protease(s) in SAS is critical for Ascaris sperm activation and the secreted As_SRP-1 likely inhibits sperm activation through its physical interaction with this protease(s).
Fig. 3.
Secreted As_SRP-1 blocks other sperm activation by inhibiting As_TRY-5 activity in SAS. (A) Secretion of As_SRP-1 during sperm activation increased. Sperm were pelleted by centrifugation after being treated with buffer alone (No SAS), heated SAS (SAS was heat-inactivated for 10 min before use) or SAS (0.5 μg/mL SAS) for 10 min, and then the supernatants were subjected to SDS/PAGE and Western blot with anti-As_SRP-1. (B) As_SRP-1 inhibited SAS-induced sperm activation. Purified native As_SRP-1 (0.5 μg/mL) was incubated with 0.5 μg/mL SAS for 10 min and then tested for sperm activation capability. The HKB buffer (No SAS) and SAS were used as negative and positive controls, respectively. (Scale bar, 10 μm.) (C) As_SRP-1 interacts physically with a serine protease present in SAS. Before incubation with SAS, As_SRP-1 was immobilized onto protein A beads through its antibody, and the collected supernatant lost its sperm activating activity (Top). Middle and Bottom, mock depletion, either As_SRP-1 antibody was replaced with IgG or As_SRP-1 was omitted for binding with beads before incubation with SAS. (Scale bar, 10 μm.) (D) Identification of As_TRY-5. Left: purified As_SRP-1 was incubated with the Con A eluate (see Fig. S8A) at 4 °C overnight, followed by SDS/PAGE and Western blot with anti-As_SRP-1. Arrowheads, complex formed between As_SRP-1 and a protease in the Con A eluate. Arrows, cleaved As_SRP-1. Right, De novo sequencing of the 90-kDa complex revealed peptide sequences that led to the identification of As_TRY-5. The 90-kDa complex was digested with trypsin, Asp-N and Lys-N before subjected to LC-MS/MS (SI Materials and Methods). Black, consensus sequence; blue, tryptic peptides; orange, Asp-N peptides; the underlined, sequences used for designing PCR primers to clone the cDNA encoding this protein, “..” indicates where peptide sequences are disconnected. BLAST search with these extended sequences revealed that they are highly homologous to trypsin-like protease protein 5 in Brugiya malayi (XP_001894231). Sequence alignment of known nematode serine proteases helped place all except the first peptide (DIISTIPCPVESTFR) in relative order. (E) Comparison of the amino acid sequence of As_TRY-5 with other known serine proteases, including Ce_TRY-5 (C. elegans, NP_505421), Dm_TRY-5 (Drosophila melanogaster, NP_001163100), Hu_CAP-1 (human, NP_002764), and human trypsin (NP_002760). Red boxes, the catalytic triad (H, D and S); dark line, a predicted signal peptide; asterisk, identical amino acid; colon, amino acid with high similarity; dot, amino acid with less similarity. (F) Inhibitory effect of the secreted As_SRP-1 on sperm activation could be rescued by the addition of neutralizing antibody. The first batch of spermatids (upper images) was incubated with Con A eluate alone (Left), Con A eluate plus 1:50 As_SRP-1 antiserum (Center), or Con A eluate plus 1:50 preimmune serum (Right) at 37 °C for 10 min. For each of these three assay conditions, spermatids were activated (upper images) and the supernatants were collected after centrifugation to treat the second batch of spermatids (lower images). When secreted As_SRP-1 during the activation of the first batch of sperm (see A) was not neutralized by As_SRP-1 antiserum (Left and Right), the activation of the second batch of spermatids was inhibited, indicating the participation of As_SRP-1 in other sperm maturation. (Scale bar, 10 μm.)
Although our data (Fig. 3 B and C and Fig. S7) and accumulating evidence (6, 7, 12) suggest that the activation of nematode sperm involves a serine protease(s), the identity of this protease(s) has been unknown. To identify the protease, we used conventional biochemical purification strategies to enrich the target protein by following its sperm activating activity (Fig. S8A). The Con A eluate showed strong activity in inducing sperm activation, and this activity could be inhibited by the serine protease inhibitor PMSF (Fig. S8B), suggesting that the fraction from Con A contains our target serine protease(s). This proteolytic fraction was shown to interact physically with As_SRP-1 (Fig. 3C), and the interaction was predicted to produce a large covalent protein complex containing the cleaved As_SRP-1 and target protease(s), according to the well-characterized Serpin–protease interaction mechanism (21). Indeed, as shown on both SDS/PAGE and Western blot, an ∼90-kDa band (Fig. 3D, Left) appeared after As_SRP-1 (∼46 kDa) was incubated with the Con A eluate. This 90-kDa band was then subjected to MS analysis and de novo peptide sequencing to identify the protease because its sequence was not present in existing databases. Using the pNovo algorithm (19), we obtained over a dozen high-quality peptide sequences that did not belong to any previously characterized protein (Fig. 3D, Right, and Fig. S9). A synthetic peptide was obtained for one of them, and its fragmentation spectra were found to be identical to those of the endogenous peptide (Fig. S9), thus validating the de novo sequencing results. We assembled these sequences into longer segments and found by BLAST search that they share homology with a trypsin-like serine protease (Fig. 3D, Right). Based on the peptides identified from de novo sequencing, we designed degenerative primers for RACE PCR and cloned the full-length cDNA (Fig. 3E). Sequence comparisons indicate that the protein encoded by this cDNA shares a high degree of homology, including a conserved catalytic triad, with other known serine proteases (Fig. 3E). We named this protein As_TRY-5 after its closest homolog, TRY-5, in C. elegans. Again, analysis of the original MS data against full-length TRY-5 using Mascot and pFind further confirmed the accuracy of sequence identifications made by pNovo (Figs. S10 and S11).
We further tested whether the inhibitory effect of the secreted As_SRP-1 on sperm activation could be rescued by the addition of specific antiserum of As_SRP-1. We activated the first batch of sperm with the Con A eluate, then collected the supernatant. When the supernatant was added to the second batch of sperm, no sperm activation was observed (Fig. 3F, Upper and Lower Left), probably because As_TRY-5 in the Con A eluate was inhibited by As_SRP-1 secreted from the first batch of sperm. As expected, when As_SRP-1 antiserum was added to neutralize As_SRP-1 secreted by the first batch of sperm, the resulting supernatant was able to activate the second batch of sperm (Fig. 3F, Center, Upper and Lower). As a control, the preimmune serum had no such effect (Fig. 3F, Upper and Lower Right). Thus, sperm-secreted As_SRP-1 during sperm activation blocks the activation of other sperm by inhibiting the glandular vas deferens-derived serine protease As_TRY-5.
Discussion
After the meiosis, spermatids are transcriptionally and translationally silent and, thus, sperm activation, motility acquisition, sperm competition, and fertilization are performed without new gene expression (22). Our ex vivo data provide evidence that motile spermatozoa are biochemically active in contributing a protein (As_SRP-1) to the seminal fluid and that this protein might coordinate both spermatozoon motility and sperm competition in vivo. On the one hand, for activated sperm in the uterus, As_SRP-1 is necessary for MSP cytoskeleton assembly and sperm motility acquisition (Fig. 2), thus improving the competitiveness of spermatozoa. Although the mechanism by which As_SRP-1 modulates cytoskeleton dynamics remains unclear, our data suggest that As_SRP-1 might act through protein tyrosine phosphorylation (Fig. S5), which has been known as a molecular switch in the regulation of MSP-based cell motility (14). On the other hand, for nonactivated sperm in the uterus from other males, As_SRP-1 irreversibly terminates the activity of a vas deferens-derived serine protease, As_TRY-5 (Fig. 3), thus inhibiting the activation of other spermatids. The spatially and temporally controlled encounter of nonmotile spermatids with the activating protease As_TRY-5 and the regulated release of the dual-function serine protease inhibitor As_SRP-1 during sperm activation constitute an elaborate opposing but complementary mechanism to coordinate sperm maturation and likely sperm competition in vivo.
Sperm competition in polyandrous species has been widely recognized as one of most potent driving forces in the evolution (23). Studies on sperm-competition mechanisms have focused on the physical traits of sperm [reviewed in (23, 24)], such as number of sperm inseminated, cell size, swimming velocity, and on seminal fluid produced by several accessory glands in the male body [reviewed in (25, 26)]. Several seminal fluid proteins in insects were involved in sperm competition by sperm displacement, sperm incapacitation or sperm ejection by females (27–30). Real-time live cell-imaging studies of sperm competition in transgenic flies with different fluorescent protein-labeled sperm support the sperm displacement mechanism, but not sperm incapacitation mechanism (31). Interestingly, the presence of sperm in addition to seminal fluid from second males would significantly enhance the magnitude of sperm displacement compared with that caused only by seminal fluid from spermless males (32). Seminal fluid is produced principally by accessory glands in the male body (25, 26), but our data show that sperm can secrete a component of this fluid. An interesting avenue for future investigation may be to determine whether other animal species besides nematodes also use sperm-secreted components mechanism to modulate sperm competition.
Some of our data from Ascaris not only agree with those from C.elegans but also further the understanding of C. elegans sperm activation. For example, it has been known for over three decades that nematode sperm can be activated in vitro by proteases, but the physiological relevance of this in vitro phenomenon is uncertain. A recent genetic study suggests that C. elegans sperm activation involves protease activities regulated by SWM-1 (7), which contains two trypsin inhibitor-like domains. The purification and identification of As_TRY-5 and As_SRP-1 as important regulators of sperm activation in Ascaris provide unequivocal evidence that proteases and protease inhibitors indeed regulate sexual reproduction. Interestingly, proteolytic activity in seminal fluid is required for the activation of insect sperm in the female reproductive tract (33, 34). Nine and 8 out of 83 predicted seminal fluid proteins in Drosophila are proteases and inhibitors, respectively, implying their important roles in male fertility (35). Functional processing of fertilin, a metalloprotease associated with mammalian sperm, by convertase during sperm transit in the epididymis of mice is essential for sperm activation and male fertility (36). Lack of the serine protease inhibitor nexin-1 in the seminal fluid of mutant mice impaired male fertility (37). Therefore, proteolysis-mediated sperm activation might have broad phylogenetic conservation and the proteolytic activity outside of sperm is essential for male reproductive success. As_SRP-1 has dual functions in the modulation of nematode sperm maturation, providing insights for the fine-tuning of sperm function and male fertility before and postinsemination. In taxa outside Nematoda that produce flagellated sperm, regulated exocytosis is also required to create fertilization-competent sperm and to achieve reproductive success (38). Thus, sperm from different taxa might use this active secretion mechanism to alter their immediate environment to enhance their own competitiveness.
Materials and Methods
Ascaris sperm were obtained by dissecting males to recover seminal vesicles, which were processed to release seminal fluid into HKB buffer [50 mM Hepes, 70 mM KCl, 10 mM NaHCO3 (pH 7.1)]. Spermatozoa were obtained by activating spermatids with the addition of SAS (vas deferens extract) (12). We observed sperm after various treatments as described in SI Materials and Methods using a DIC microscope (Axio Imager M2, Carl Zeiss) and MSP fibers assembled in vitro (13) using a phase-contrast microscope (Axio Observer, Carl Zeiss). All images were processed using MetaMorph (Universal Imaging). For additional details on fiber assembly in vitro, native protein purification, MS analysis, recombination protein expression, gene cloning, antibody preparation, immunodepletion, immunofluorescence, and cryo-immuno-EM assays, see SI Materials and Methods.
Note Added in Proof
While this paper was under review at PNAS, Smith and Stanfield (39) reported that C. elegans TRY-5, found in the male seminal fluid, is required for male-mediated sperm activation, consistent with our data described here.
Supplementary Material
Acknowledgments
We thank Dr. Li-Lin Du for valuable ideas regarding the BLAST search; Dr. Jian Ren for advice with Fig. 3F; Ning Yang for help with Fig. 1E; Gail Ekman for advice in degenerate PCR; Dr. Taotao Wei for providing various protease inhibitors; Drs. Hengbin Wang and Yixian Zheng for critically reading the manuscript; and Wei Zhuang for excellent technical assistance. This research was supported by Grants 2012CB94502, 2010CB912303, and 30971648 (to L.M.), 2007AA02Z1A7 and 2010CB835203 (to M.-Q.D.), 2010CB912701 (to S.-M.H.), 30871226 and 31071180 (to Y.Z.) from the government of the People's Republic of China. L.M. is supported by the Chinese Academy of Sciences 100-Talents Program. S.W.L. was supported by National Institutes of Health Grant GM082932.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.L.K. is a guest editor invited by the Editorial Board.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. JF894302 (As_srp-1) and JF894303 (As_srp-1)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109912109/-/DCSupplemental.
References
- 1.Fraser LR. The “switching on” of mammalian spermatozoa: molecular events involved in promotion and regulation of capacitation. Mol Reprod Dev. 2010;77:197–208. doi: 10.1002/mrd.21124. [DOI] [PubMed] [Google Scholar]
- 2.L'Hernault SW. Spermatogenesis. The C. elegans Research Community, WormBook. 2006 doi: 10.1895/wormbook.1.85.1. Available at http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward S, Carrel JS. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev Biol. 1979;73:304–321. doi: 10.1016/0012-1606(79)90069-1. [DOI] [PubMed] [Google Scholar]
- 4.LaMunyon CW, Ward S. Larger sperm outcompete smaller sperm in the nematode Caenorhabditis elegans. Proc Biol Sci. 1998;265:1997–2002. doi: 10.1098/rspb.1998.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singson A, Hill KL, L'Hernault SW. Sperm competition in the absence of fertilization in Caenorhabditis elegans. Genetics. 1999;152:201–208. doi: 10.1093/genetics/152.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward S, Hogan E, Nelson GA. The initiation of spermiogenesis in the nematode Caenorhabditis elegans. Dev Biol. 1983;98:70–79. doi: 10.1016/0012-1606(83)90336-6. [DOI] [PubMed] [Google Scholar]
- 7.Stanfield GM, Villeneuve AM. Regulation of sperm activation by SWM-1 is required for reproductive success of C. elegans males. Curr Biol. 2006;16:252–263. doi: 10.1016/j.cub.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 8.L'Hernault SW, Roberts TM. Cell biology of nematode sperm. Methods Cell Biol. 1995;48:273–301. doi: 10.1016/s0091-679x(08)61392-8. [DOI] [PubMed] [Google Scholar]
- 9.Zhu GD, et al. SPE-39 family proteins interact with the HOPS complex and function in lysosomal delivery. Mol Biol Cell. 2009;20:1223–1240. doi: 10.1091/mbc.E08-07-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward S, Argon Y, Nelson GA. Sperm morphogenesis in wild-type and fertilization-defective mutants of Caenorhabditis elegans. J Cell Biol. 1981;91:26–44. doi: 10.1083/jcb.91.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Washington NL, Ward S. FER-1 regulates Ca2+ -mediated membrane fusion during C. elegans spermatogenesis. J Cell Sci. 2006;119:2552–2562. doi: 10.1242/jcs.02980. [DOI] [PubMed] [Google Scholar]
- 12.Abbas M, Cain GD. In vitro activation and behavior of the ameboid sperm of Ascaris suum (Nematoda) Cell Tissue Res. 1979;200:273–284. doi: 10.1007/BF00236419. [DOI] [PubMed] [Google Scholar]
- 13.Italiano JE, Jr, Roberts TM, Stewart M, Fontana CA. Reconstitution in vitro of the motile apparatus from the amoeboid sperm of Ascaris shows that filament assembly and bundling move membranes. Cell. 1996;84:105–114. doi: 10.1016/s0092-8674(00)80997-6. [DOI] [PubMed] [Google Scholar]
- 14.Miao L, Vanderlinde O, Stewart M, Roberts TM. Retraction in amoeboid cell motility powered by cytoskeletal dynamics. Science. 2003;302:1405–1407. doi: 10.1126/science.1089129. [DOI] [PubMed] [Google Scholar]
- 15.Yi K, Buttery SM, Stewart M, Roberts TM. A Ser/Thr kinase required for membrane-associated assembly of the major sperm protein motility apparatus in the amoeboid sperm of Ascaris. Mol Biol Cell. 2007;18:1816–1825. doi: 10.1091/mbc.E06-08-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto H, Thomson JN. Monoclonal antibodies which distinguish certain classes of neuronal and supporting cells in the nervous tissue of the nematode Caenorhabditis elegans. J Neurosci. 1985;5:643–653. doi: 10.1523/JNEUROSCI.05-03-00643.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arduengo PM, Appleberry OK, Chuang P, L'Hernault SW. The presenilin protein family member SPE-4 localizes to an ER/Golgi derived organelle and is required for proper cytoplasmic partitioning during Caenorhabditis elegans spermatogenesis. J Cell Sci. 1998;111:3645–3654. doi: 10.1242/jcs.111.24.3645. [DOI] [PubMed] [Google Scholar]
- 18.Al Rawi S, et al. Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science. 2011;334:1144–1147. doi: 10.1126/science.1211878. [DOI] [PubMed] [Google Scholar]
- 19.Chi H, et al. pNovo: De novo peptide sequencing and identification using HCD spectra. J Proteome Res. 2010;9:2713–2724. doi: 10.1021/pr100182k. [DOI] [PubMed] [Google Scholar]
- 20.Parkinson J, Whitton C, Schmid R, Thomson M, Blaxter M. NEMBASE: A resource for parasitic nematode ESTs. Nucleic Acids Res. 2004;32(Database issue):D427–D430. doi: 10.1093/nar/gkh018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye S, Goldsmith EJ. Serpins and other covalent protease inhibitors. Curr Opin Struct Biol. 2001;11:740–745. doi: 10.1016/s0959-440x(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 22.Grunewald S, Paasch U, Glander HJ, Anderegg U. Mature human spermatozoa do not transcribe novel RNA. Andrologia. 2005;37:69–71. doi: 10.1111/j.1439-0272.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 23.Birkhead TR, Pizzari T. Postcopulatory sexual selection. Nat Rev Genet. 2002;3:262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- 24.Wigby S, Chapman T. Sperm competition. Curr Biol. 2004;14:R100–R102. [PubMed] [Google Scholar]
- 25.Chapman T, Davies SJ. Functions and analysis of the seminal fluid proteins of male Drosophila melanogaster fruit flies. Peptides. 2004;25:1477–1490. doi: 10.1016/j.peptides.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: Identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price CS, Dyer KA, Coyne JA. Sperm competition between Drosophila males involves both displacement and incapacitation. Nature. 1999;400:449–452. doi: 10.1038/22755. [DOI] [PubMed] [Google Scholar]
- 28.Chapman T, Neubaum DM, Wolfner MF, Partridge L. The role of male accessory gland protein Acp36DE in sperm competition in Drosophila melanogaster. Proc Biol Sci. 2000;267:1097–1105. doi: 10.1098/rspb.2000.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snook RR, Hosken DJ. Sperm death and dumping in Drosophila. Nature. 2004;428:939–941. doi: 10.1038/nature02455. [DOI] [PubMed] [Google Scholar]
- 30.den Boer SP, Baer B, Boomsma JJ. Seminal fluid mediates ejaculate competition in social insects. Science. 2010;327:1506–1509. doi: 10.1126/science.1184709. [DOI] [PubMed] [Google Scholar]
- 31.Manier MK, et al. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328:354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- 32.Gilchrist AS, Partridge L. Why it is difficult to model sperm displacement in Drosophila melanogaster: The relation between sperm transfer and copulation duration. Evolution. 2000;54:534–542. doi: 10.1111/j.0014-3820.2000.tb00056.x. [DOI] [PubMed] [Google Scholar]
- 33.Osanai M, Kasuga H. Role of endopeptidase in motility induction in Apyrene silkworm spermatozoa - micropore formation in the flagellar membrane. Experientia. 1990;46:261–264. [Google Scholar]
- 34.Friedländer M, Jeshtadi A, Reynolds SE. The structural mechanism of trypsin-induced intrinsic motility in Manduca sexta spermatozoa in vitro. J Insect Physiol. 2001;47:245–255. doi: 10.1016/s0022-1910(00)00109-8. [DOI] [PubMed] [Google Scholar]
- 35.Swanson WJ, Clark AG, Waldrip-Dail HM, Wolfner MF, Aquadro CF. Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc Natl Acad Sci USA. 2001;98:7375–7379. doi: 10.1073/pnas.131568198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho C, et al. Fertilization defects in sperm from mice lacking fertilin beta. Science. 1998;281:1857–1859. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- 37.Murer V, et al. Male fertility defects in mice lacking the serine protease inhibitor protease nexin-1. Proc Natl Acad Sci USA. 2001;98:3029–3033. doi: 10.1073/pnas.051630698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vacquier VD. Evolution of gamete recognition proteins. Science. 1998;281:1995–1998. doi: 10.1126/science.281.5385.1995. [DOI] [PubMed] [Google Scholar]
- 39.Smith JR, Stanfield GM. TRY-5 is a sperm-activating protease in Caenorhabditis elegans seminal fluid. PLoS Genet. 2011;7(11):e1002375. doi: 10.1371/journal.pgen.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.