Abstract
Axin is a tumor suppressor and a key negative regulator of the Wnt/β-catenin signaling pathway. Axin turnover is controlled by its poly-ADP-ribosylation catalyzed by tankyrase (TNKS), which requires the direct interaction of Axin with TNKS. This interaction is thus an attractive drug target for treating cancers, brain injuries, and other diseases where β-catenin is involved. Here we report the crystal structure of a mouse TNKS1 fragment containing ankyrin-repeat clusters 2 and 3 (ARC2-3) in a complex with the TNKS-binding domain of mouse Axin1. Surprisingly, we found that Axin contains two discrete TNKS-binding segments, both of which bind simultaneously to the two ARC2 domains in the ARC2-3 homodimer. Our crystal structure shows that in each TNKS-binding segment of Axin there is a conserved glycine residue that lies in the bottom of a narrow “gate” formed by two parallel tyrosine side chains on the TNKS surface. This glycine-selection gate is crucial for TNKS-Axin interactions, as mutation of the TNKS gate-forming residues, or mutation of either glycine residue in the two Axin segments, completely abolishes the binding of the corresponding Axin segment to TNKS. The bivalent binding of Axin to TNKS is required for Axin turnover, since mutations in either gate-binding glycine residue in Axin lead to Axin stabilization in the cell. In addition, our analyses also reveal the structural basis for TNKS substrate recruitment, and shed light on the overall structure of TNKS that should help in developing specific inhibitors of Wnt/β-catenin signaling.
Keywords: cancer, drug target, poly-ADP-ribosylation, tumor suppressor, Wnt
The Wnt/β-catenin signaling pathway plays critical roles in embryonic development, stem cell biology and tissue homeostasis in adults, and in disease. Deregulation of the canonical Wnt/β-catenin signaling pathway is tightly associated with several cancers, particularly colon cancer (1, 2). Development of Wnt/β-catenin pathway inhibitors is of great interest for the treatment of multiple diseases, including cancers and brain injuries (3–6). One potential target for small molecule inhibitors of Wnt/β-catenin signaling is Axin (a.k.a. Axin1), which is a scaffold of the β-catenin destruction complex and a known tumor suppressor (2, 7, 8). The assembly or disassembly of the destruction complex in the cytosol is a central regulatory step of the Wnt/β-catenin pathway (2, 9). Since Axin levels are much lower than most other key components of the destruction complex in the cell, Axin is believed to be the rate-limiting factor for the assembly of the β-catenin destruction complex (10). Recently, various cell-based screenings of Wnt pathway inhibitors led to the discovery of compounds that inhibit Wnt signaling by inhibiting Axin turnover, demonstrating that Axin turnover is a crucial target for developing Wnt pathway inhibitors (11, 12). In addition to the Wnt/β-catenin pathway, prevention of Axin degradation may also suppress tumor growth by promoting the turnover of the c-myc oncoprotein and stimulation of p53 function (13, 14).
Ubiquitination of Axin, which leads to its subsequent turnover, requires its poly-ADP-ribosylation (PARylation) (11). PARylation of proteins is catalyzed by a family of poly-ADP-ribose polymerases (PARPs), with 18 known members in humans, which regulate many aspects of biology including genomic stability, cell cycle, and energy metabolism (15, 16). Axin PARylation is specifically catalyzed by tankyrases 1 and 2 (a.k.a. TNKS1/PARP5a and TNKS2/PARP5b, respectively). Indeed, Wnt pathway inhibitors discovered in cell-based assays stabilize cytosolic Axin by blocking the active sites of TNKS catalytic PARP domains (17). Since TNKS1 and TNKS2 catalyze PARylation of many different protein substrates (18), TNKS active-site inhibitors are not specific for the Wnt/β-catenin pathway. It may therefore be useful to develop specific Wnt/β-catenin pathway inhibitors that prevent Axin recognition by TNKS (8).
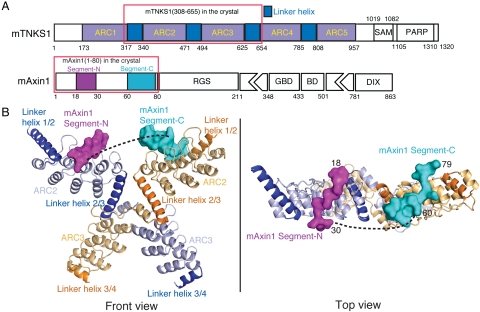
TNKS1 and TNKS2 share three conserved domains: the ankyrin-repeat domain responsible for substrate recruitment, a SAM domain that mediates homo- and hetero-oligomerization of TNKS, and a C-terminal catalytic PARP domain (Fig. 1A) (18–24). TNKS1 also contains a unique N-terminal HPS-rich domain with unknown function. The ankyrin-repeat domain is composed of five conserved subdomains, or ankyrin-repeat clusters (ARCs) (19, 20, 22–24). There are conserved linkers between neighboring ARCs. It is presently unclear what structure the linker adopts and how these five ARCs relate with one another. Several TNKS binding partners or substrate proteins contain a consensus TNKS binding motif (TBM) with a sequence of RXXPDG, which appears to be sufficient for binding to each of the five subdomains (19, 20, 22, 23). It is noteworthy that Axin uses its N-terminal domain (residues 1-80), which does not contain an apparent TBM. Previous work shows that Axin residues 19-30, the most conserved region of Axin, is required for TNKS binding (11). For understanding Wnt/β-catenin signaling mechanisms and for developing Wnt/β-catenin pathway inhibitors, it would be useful to know how Axin interacts with TNKS, which lead us to our present investigation.
Fig. 1.
Overall structure of the TNKS(308-655)/Axin1(1-80) complex. (A) Schematic representation of the domain organization of mouse Tankyrase and Axin1. “ARC” stands for ankyrin-repeat cluster. Five ARCs are colored in gray. Linkers between them are colored dark blue. Domains SAM and PARP are also labeled. “GBD” stands for GSK3 binding domain; “BD” stands for β-catenin binding domain. RGS and DIX domains are also shown. Segment-N and –C of mAxin1(1-80) observed in the crystal structure are colored in magenta and cyan, respectively. (B) Overall structure of a complex between mTNKS ARC2-3 and mAxin1(1-80). Two copies of ARC2 and ARC3 in the dimer are shown in cartoon with light blue and light orange, respectively. Linker helices before and after ARC2 and ARC3 in two monomers are shown in blue and orange, respectively. The two Axin1 segments are shown in solid surface with magenta and cyan, respectively.
Here we report the crystal structure of an Axin-TNKS complex, which provides a unique view of structural organization of multiple ankyrin-repeat clusters in TNKS. Combined with our biochemical and functional data, the structure reveals two discrete Axin segments involved in TNKS interaction, which are also required for Axin turnover. Interestingly, our studies also reveal that the Axin-TNKS interaction involves a unique structural mechanism for Gly selectivity. Together, our work provides an enhanced foundation for understanding the molecular mechanism of Axin turnover and for TNKS substrate recruitment, and should facilitate drug discovery that targets the TNKS-Axin interface.
Results
Mapping of the TNKS-Axin Binding Domains
The N-terminal domain of mouse Axin1, which contains residues 1-80, was shown to be responsible for TNKS binding, whereas residues 19-30, which is fully conserved in all Axin1 analogues, is necessary for this interaction (11). To test if the most conserved region of Axin1 (residues 19–30) is sufficient for TNKS binding, we compared the binding of recombinant Axin1(1-80) and Axin1(1-43) with the entire TNKS ankyrin-repeat domain (ARC1-5, residues 173-961; Fig. 1A). While Axin1(1-80) forms a stable complex with ARC1-5 that can be copurified using size-exclusion chromatography (SEC) and ion-exchange chromatography (IEC), Axin1(1-43)/TNKS complexes cannot be copurified using SEC or IEC, indicating that Axin1 residues 44-80 are important for TNKS binding. Therefore, we used Axin1(1-80) for our structural analysis.
It has been proposed that the ankyrin-repeat domain of TNKS contains five ankyrin-repeat clusters (ARCs) that are separated by four conserved linkers, with each ARC containing four ankyrin repeats (23). While other models with distinct ankyrin-repeat assignments have also been proposed, this model is in general agreement with our domain structure model (Fig. 1A), derived from our crystal structure shown in Fig. 1B. We then overexpressed and purified each of the five ARCs separately and tested their Axin1 binding activities. Pulldown experiments using GST-ARCs and untagged Axin1(1-80) (Fig. S1) demonstrated that ARC2, ARC4, and ARC5, but not ARC1 and ARC3, can interact with Axin1(1-80), respectively.
Crystal Structure of an Axin-TNKS Complex
Numerous combinations of mouse TNKS-Axin complexes were tested for crystallization. High-quality crystals were obtained with the mouse TNKS1(308-655)-Axin1(1-80) complex. The crystal structure was determined by molecular replacement and refined at 2.0 Å resolution. This TNKS fragment contains ARC2 and ARC3, as well as three inter-ARC linkers: linkers 1/2, 2/3, and 3/4 (linker 1/2 represents the linker between ARC1 and ARC2, and so on; Fig. 1 A and B). The electron densities for the entire TNKS linker 1/2 and 2/3 are visible, although the density for the linker 3/4 is only visible for the N-terminal portion. There are two TNKS subunits and one Axin molecule in each asymmetric unit in the crystal lattice (see below). Each ARC consists of four ankyrin repeats, and each ankyrin repeat contains two helices and a β-hairpin linking the two. Each of the conserved inter-ARC linkers forms a single long helix and packs on the ends of neighboring ARCs (Fig. 1B). The two TNKS copies form a swapped dimer with the swap occurring behind the first helix of ARC3 (Fig. 1B and Fig. S2). In solution, TNKS(308-655) has a tendency to form a dimer, whereas the binding of Axin1(1-80) promotes homogeneous dimerization of TNKS(308-655) (Fig. S3).
Consistent with the results of the mapping experiments, Axin1(1-80) interacts with TNKS ARC2 but not ARC3 in the crystal lattice. Electron densities of two Axin1 segments, Axin1(18-30) and Axin1(60-79), can be clearly visualized on the two ARC2 surfaces in the swapped TNKS dimers (Fig. S4). While the remaining parts of Axin1 cannot be seen in the electron density maps, these two Axin1 segments are likely from the same Axin1(1-80) molecule, since SEC results indicate that Axin1(1-80) stabilizes TNKS(308-655) dimerization in solution (Fig. S3), most likely by interacting with both copies of the swapped dimer. In addition, the distance between Axin1 G30 and A60 Cα atoms in our structure is 49.4 Å, which can be covered by the 29 missing residues in between. The sequences of Axin1(18-30) and Axin1(60-79) are EDAPRPPVPGEEG and TPRRSDLDLGYEPEGSASPT, respectively. It is interesting that two Axin1 segments with distinct sequences bind to identical TNKS surfaces in the TNKS dimer.
The TNKS-Axin Interfaces: Both Axin segments Bind to the Same TNKS Surface
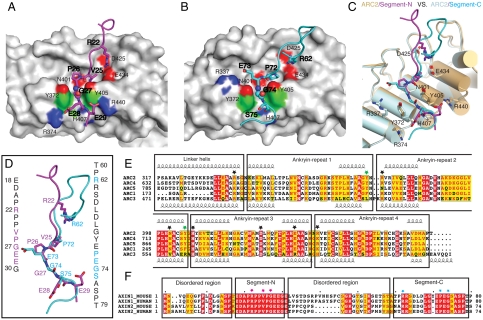
Both Axin segments interact with a groove formed by the second helices and the β-hairpins of TNKS ARC2. For the N-terminal segment, Axin1(18-30) adopts an extended conformation and runs roughly perpendicular with the groove (Fig. 2A and Fig. S5). Inside the groove, the side chains of Axin1 Arg-22 side chain forms two salt bridges with TNKS Asp-425 and Glu-434, respectively. After Pro-23 and Pro-24 that maintain the Axin1 mainchain conformation in the groove, the side chain of Axin1 Val-25 is buried in a pocket in the bottom of the groove. The main-chain carbonyl groups of Val-25 and Pro-26 interact with TNKS Tyr-405 and Asn-401 side chains, respectively. These interactions lead Gly-27 into a narrow valley formed by two parallel tyrosine residues on the surface of TNKS (Tyr-372 and Tyr-405). Past the valley, the Gly-27 mainchain carbonyl interacts with TNKS His-407, and the side chains of Glu-28 and Glu-29 interact with TNKS Arg-374 and Arg-440, respectively.
Fig. 2.
Structural details of the mouse TNKS1(308-655)/Axin1(1-80) interactions. (A) TNKS1-Axin1 segment-N interface. One copy of ARC2 of TNKS is shown in gray solid surface. Segment-N of Axin1 is shown in stick model in magenta. Residues involved in the interface are labeled. Regular font is used for labeling TNKS1 residues and bold font for Axin1 residues. The critical glycine is shown in sphere mode. (B) TNKS1-Axin1 segment-C interface. Another copy of ARC2 of TNKS is shown in gray solid surface as in A. Segment-C of Axin1 is shown in stick model in cyan. Residues are labeled in the same way as in A. (C) Superpositon of two TNKS1 ARC2-Axin1 interfaces. Two ARC2s are shown in cylindrical cartoon in light pink (interacting with Segment-N) and light gray (interacting with Segment-C). Residues of TNKS in the interface are labeled. The distance between the two parallel tyrosine side chains is approximately 7.3 Å. (D) Superposition of the two Axin1 segments. Color coding is the same as in (A) and (B). Sequences of two segments are shown in vertical text with critical residues colored in magenta and cyan, respectively. (E) Sequence alignment of five ARCs in mouse TNKS1. Individual ankyrin repeats as well as the linker helices between ARCs are boxed and labeled. Critical residues for the interaction are highlighted with black stars. The two gate-forming tyrosines (Tyr-372 and Tyr-405) are highlighted with green stars. Tyr-405 side chain also forms a hydrogen bond with the Axin mainchain. (F) Sequence alignment of the TNKS-binding domains of human and mouse Axin1/Axin2. Segment-N, Segment-C as well as the disordered region in the structure are boxed and labeled. Key residues from Segment-N and Segment-C are highlighted with red and cyan stars, respectively.
For the Axin1(60-79) segment (Fig. 2B and Fig. S5), Axin1 residues 60-71 form loose interactions with the TNKS surface. Among them, Arg62 is roughly in the same spatial position as Arg-22 in the N-terminal segment, and forms two salt bridges with TNKS Asp-425 and Glu-434, respectively. Pro-72 is positioned in the bottom of the TNKS groove. Like the Axin1 N-terminal segment, main-chain carbonyl groups of Pro-72 and Glu-73 form hydrogen bonds with the side chains of TNKS Tyr-405 and Asn-401, respectively. The side chain of Glu-73 forms a salt bridge with TNKS Arg-337. These interactions place Gly-74 inside the narrow valley formed by TNKS Tyr-372 and Tyr-405. Finally, like the Axin1 N-terminal segment, the other end of Gly-74 is stabilized by a hydrogen bond between the Gly-74 main-chain carbonyl and the side chain of TNKS His-407. The interaction between Axin1 residue 75-79 and the TNKS surface are mostly weak van der Waals interactions. The folded Axin1 RGS domain starts with Pro-80 and is responsible for interacting with SAMP repeats of APC in the β-catenin destruction complex (25).
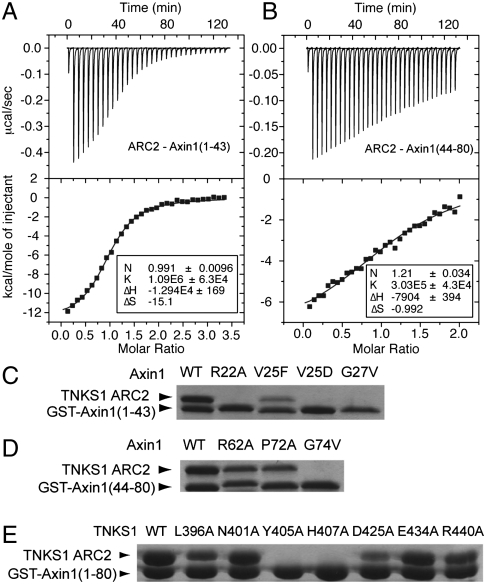
Superposition of the two Axin1 segments on ARC2 demonstrates that ARC2 structures in the dimer are mostly identical (Fig. 2C). There are variations on side chain arrangements for residues involved in Axin1 binding. The two Axin1 segments take almost identical paths on TNKS ARC2 surface; i.e., roughly perpendicular to the groove formed by the four loops and the top-ends of second helices of the four ankyrin-repeats in ARC2 (Fig. 2 A–D). To demonstrate that both Axin1 segments can bind to the same ARC surface area in solution, we performed a competition assay, in which Axin1(44-80) competes with GST-tagged Axin1(1-43) for TNKS binding in a dosage-dependent manner, thereby confirming the overlapping core binding sites (Fig. S6). The binding affinities for the ARC2-Axin1(1-43) and ARC2-Axin1(44-80) interactions are 0.92 and 3–5 μM, respectively, as measured by ITC (Fig. 3 A and B).
Fig. 3.
Biochemical characterization of TNKS-Axin interaction and the critical role of a Glycine-selection gate. Isothermal titration calorimetry (ITC) analysis of the interactions between TNKS1 ARC2 and (A) Axin1(1-43); (B) Axin1(44–80). The Kd value for Axin1(1-43) is 0.92 μM, whereas the Kd for Axin1(44-80) is in the range of 3–5 μM. (C and D) Mutagenesis of Axin1-N and Axin1-C segments and in vitro GST-pulldown assays define key interactions in each Axin1 segment. (E) Mutagenesis of Axin1-binding residues of TNKS1 and in vitro Axin1(1-80) binding assay using GST-pulldown. It is clear that the Gly-gate interaction region is most crucial for the Axin-TNKS interaction.
Mutagenesis Analysis and Structural Comparison Reveals a Crucial Glycine-Selection Gate
To examine the importance of individual residues on the TNKS-Axin interface in TNKS-Axin interaction, we analyzed the TNKS-Axin interaction using either purified TNKS1(308-485) mutant proteins or Axin1(1-80) mutant proteins in a GST pulldown assay. Consistent with the bivalent binding mode, mutations in the Axin1(1-80) fragment, R22A, V25F, G27V had greatly reduced their affinities with TNKS-ARC2, but did not completely disrupt the binding. Mutations in the Axin1 C-terminal segment, such as R62A or P72A, had even less effect (Fig. S6). In contrast, when we made corresponding mutations in Axin1(1-43) and Axin1(44-80), R22A, G27A and G74A all completely disrupted their interaction with TNKS-ARC2 (Fig. 3 C and D). These results demonstrate that the docking of glycine residues with the gates is crucial for the TNKS-Axin interaction.
For TNKS mutants, mutations in the “gate” residue Y405A or in the “after-gate-anchor” residue H407A abolished any observable binding; D425A lost much of the affinity, whereas mutations L396A, N401A, E434A, and R440A had only minor effects (Fig. 3E). The gate-forming residues, Tyr-372 and Tyr-405 in ARC2, are only conserved in ARC4 and ARC5 in corresponding positions (Fig. 2E). This is in agreement with our observation that ARC2, ARC4, and ARC5, but not ARC1 and ARC3, interact with Axin (Fig. S1).
Mutation of a corresponding residue in Axin2, V26D, also known as the mouse Axin2canp mutant, leads to phenotypes characteristic for decreased Wnt/β-catenin signaling in most tissues and a loss of Axin2 turnover (26). In our in vitro binding assay, the Axin1(V25D) mutation in the Axin1(1-43) fragment completely disrupted its interaction with the TNKS ARC2 domain (Fig. 3D).
Both Axin Segments are Required for Axin Turnover in the Cell
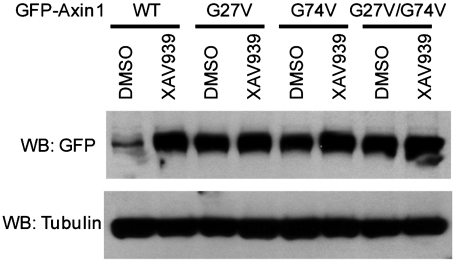
Are both Axin-TNKS-binding segments in the crystal structure required for Axin turnover in vivo? We examined this possibility by overexpressing WT Axin1 and Axin1 G27V and G74V mutants in SW480 cells. In our in vitro pulldown assay, both G27V and G74V mutants disrupted the TNKS-Axin interaction in the corresponding TNKS-binding segment (Fig. 3C). Either mutation abolished Axin1 degradation in the cell as well (Fig. 4), demonstrating that both Axin1 segments are crucial for regulating Axin1 turnover.
Fig. 4.
Both TNKS-binding segments are important for Axin turnover in vivo. Gly-27 and Gly-74 are required for XAV939-induced Axin1 protein accumulation in SW480 cells. The expression of GFP-Axin1 was under the control of the metallothionein promoter to ensure low transcription.
Axin Binding Promotes Dimerization of the TNKS Ankyrin-Repeat Domain
We observed a 2∶1 molar ratio TNKS-Axin complex in our crystal lattice, in which TNKS1 forms a swapped dimer in the first ankyrin repeat of ARC3 (Fig. S2). To examine if this dimerization also happens in solution, we analyzed the molecular weight of TNKS and TNKS-Axin complexes in solution, using a combination of SEC and dynamic light scattering. Our results indicate that the ARC4-5 fragment forms a monomer independent of Axin1(1-80) in solution (Fig. S3). In contrast, a fraction of ARC2-3 forms dimer in solution, and the presence of Axin1(1-80), or a high TNKS concentration, stabilizes TNKS in the dimeric form (Fig. S3). This supports the physiological relevance of our crystal structure and Axin-mediated TNKS dimerization.
Discussion
Axin is a crucial regulator of the Wnt/β-catenin signaling pathway, and also plays a role in myc degradation and p53 regulation. Inhibition of Axin degradation, which would enhance the degradation of β-catenin and thereby attenuate the Wnt/β-catenin pathway, is expected to be beneficial to the treatment of cancer and other clinical conditions. Structural information regarding how TNKS interacts with Axin is important not only for understanding Wnt/β-catenin signaling mechanisms and understanding TNKS substrate recognition, but also for potential drug development.
Structural Basis of TNKS-Axin Interaction and the Implication for Axin Turnover
Our work provides a first snapshot of the TNKS-Axin complex at a high resolution. Our biochemical data are fully consistent with the structure, and together they define the overall binding mode and critical residues of this interaction. Conservation of all key residues of both TNKS-binding segments in the Axin family indicates that the interactions we observed for this mouse TNKS1-Axin1 complex would be preserved for most, if not all, Axin and TNKS proteins (Fig. 2F). Our work thus provides a structural basis for interpreting the V26D mutation found in Axin2canp mouse, which is associated with Axin2(V26D) stabilization and decreased Wnt/β-catenin signaling in most tissues (26). In our structure, Axin2 V26 (V25 in Axin1) is a key residue binding to a TNKS hydrophobic pocket, and the V25D mutation completely disrupted the interaction between TNKS ARC2 and Axin1(1–43). Conversely, mouse genetic work supports the physiological relevance of our structural model.
Our crystal structure and biochemical analysis demonstrate that two discrete segments of Axin1 can individually interact with the TNKS. While the first segment, Axin1(18-30), was previously characterized (11), the discovery of the second segment, as well as the bivalent binding mode, is unexpected. Importantly, both Axin1 segments are required for TNKS-dependent Axin1 degradation, since the missense mutation of either glycine in the Axin1 segments stabilizes Axin1 (Fig. 4). The identification of two segments will be important for understanding the regulation of Wnt/β-catenin signaling and Axin1 function. For example, the second TNKS-binding segment is immediately N-terminal to the Axin1 RGS domain (residues 80-211), which is responsible for APC binding. It is interesting that APC SAMP repeats on the RGS domain surface is near Axin1 residue Pro-80 (25) and thus may be in close proximity to TNKS when both bind to Axin1.
It is intriguing that Axin regulates its stability using two segments and a bivalent TNKS-binding mode. One simple mechanistic explanation for this is the increase of binding affinity due to the bivalent binding. This may be important for capturing Axin molecules in the cytosol that have a low concentration (10). Alternatively, but nonexclusively, Axin binding promotes the dimerization of the ankyrin-repeat domain of TNKS, which may be important for Axin turnover. TNKS contains five ARCs and Axin can interact with ARC2, ARC4, and ARC5 independently (Fig. S1). Axin promotes the dimerization of ARC2-3, but probably not for ARC4-5, although we cannot rule out the possibility that ARC4-5 may be also involved in TNKS dimerization in the context of full-length TNKS. Our modeling indicates that the two Axin1 segments in the same subunit may also bind to ARC4 and ARC5 in the same TNKS molecule simultaneously. Previous work shows that deletion of TNKS ARC1-2 does not affect Axin1 binding, likely because of ARC4-5, but leads to a loss of Wnt signaling activity (11). This may be explained by the loss of Axin-mediated TNKS dimerization that requires the binding of Axin to ARC2. TNKS is known to form large polymers in the cell (21). It has a known oligomerization SAM domain, and the additional induced dimerization in the ankyrin-repeat domain may impact on the overall networking and functioning of the TNKS polymers. In this regard, it is interesting that while TNKS catalyzes the PARylation of many substrates, Axin is the only known substrate (other than TNKS per se) that binds to TNKS bivalently and is subsequently degraded. It will be interesting to pursue the potential correlation between bivalent TNKS binding and subsequent ubiquitination and degradation.
Implications for TNKS Structure and TNKS Substrate Recruitment
The ankyrin-repeat domain of TNKS contains five ARCs, each consisting of four ankyrin repeats, which are connected by four linkers (approximately 25 residues). The structures of these linkers were previously not known. Our TNKS structure contains three linkers and two ARCs. Each of the three linkers forms a long helix and interacts with the helices in the neighboring ARCs. The C-terminal part of these linkers is conserved and has a consensus sequence of LLEAARXG (Fig. 2F). This region accounts for the C-terminal part of the long helix for both linker 1/2 and 2/3 in our structure and forms a four-helix bundle with the first three helices of the following ARC (Fig. 1B). We predict that any of the four linkers forms a long helix and uses its N-terminal and C-terminal half to interact with helices in the neighboring ARCs, respectively. The linker 3/4 is six residues longer than the other three (Fig. 2E). Since we were only able to observe the electron density before Glu-635 of this linker, it is possible that this linker contains a bended long helix or even two helices and thus may generate a pivot point for overall conformational flexibility or conformational change in TNKS.
Most known TNKS binding proteins share a conserved sequence motif (TBM). Axin is the only known TNKS binding protein that does not contain an apparent sequence homology with TBM. However, the core binding sites may be preserved amongst Axin and TBM peptides. Previous work showed that the Gly residue in RXXPDG motif is crucial for the TNKS-TBM interaction (24). The PDG tripeptide motif may correspond to a PEG tripeptide region in Axin1 segment-C, Axin1(72-74), while the missing Arg residue may be compensated by Arg-62 further away in the primary sequence (Fig. 2 B–D). Our data also show that the TBM peptide and the TNKS-binding domain containing the TBM competes with Axin for TNKS binding in a dosage-dependent manner (Fig. S7). Therefore, we predict that the PDG part of the RXXPDG motif interacts with TNKS in a way quite similar to Axin1(72-74), and the conserved arginine in this motif interacts with TNKS Asp425 and Glu434, which form salt bridges with arginine residues in both Axin binding segments (Fig. S8).
In summary, our structural and biochemical analysis allowed us to reveal the unexpected bivalent binding mode of the Axin-TNKS interaction, identify crucial interactions in the Axin-TNKS interface, and appreciate the requirement of a Gly-selection gate for Axin turnover. In addition, our structure made one solid step towards the understanding of the overall structure of TNKS, and allowed us to predict how a conserved TNKS binding motif (TBM), which is shared by many TNKS partners, may dock on the TNKS surface. All these conclusions will contribute to not only mechanistic understanding of Wnt signaling and other biological processes that Axin and TNKS are involved, but also future drug development targeting the Axin-TNKS interaction.
Methods
Expression, Purification, and Crystallization of TNKS/Axin Complex
GST-tagged mouse tankyrase-1 (TNKS) with a TEV protease-cleavage site following the GST tag or His-tagged mouse Axin1 with a TEV protease-cleavage site following the His-tag were purified separately by affinity column, cleaved overnight with TEV, and further purified by ion-exchange and gel-filtration. The two proteins were then incubated together on ice for 1 h with excess Axin1 and the complex separated from uncomplexed Axin1 by a final gel-filtration step. The fractions containing the complex were concentrated to 15 mg/mL. Optimized growth conditions produced single crystals at 22 °C in 4–5 d by mixing 1 μL of protein solution to 1 μL of reservoir solution consisting of 0.06 M sodium citrate tribasic dihydrate pH 5.6, 0.12 M ammonium acetate, 18% MPD, and 20 mM DTT.
X-ray Data Collection and Structure Determination
For data collection, crystals of the mouse TNKS1(308-655)/Axin1(1-80) complex were dehydrated by soaking overnight in the crystallization buffer with 25% MPD, and flash frozen in liquid nitrogen. Crystals belong to the space group C2 with cell parameters a = 131.78 Å, b = 106.59 Å, c = 73.48Å, β = 105.76°. X-ray data were collected at ALS, BL8.2.2, and processed with Mosflm in the CCP4 package (27). The structure of the complex was determined by molecular replacement with program PHASER (28) using a partial mouse Notch-1 ankyrin domain (accession code 1YMP) (29) as a search model. After several cycles of refinement by REFMAC5 (30) and building by Coot (31), the electron density maps (2fo-fc and fo-fc) at the 2.0 Å resolution were clear enough to build two Axin1 regions from residues 18-30 and residues 60-76. Final crystallographic refinement was done with REFMAC5 with TLS parameters (27). The refinement statistics are summarized in Table S1.
In Vitro Binding Assays
GST-tagged Axin1 or GST-tagged TNKS1 was first incubated for 20 min on ice with 40 μL glutathione beads; washed twice in 200 μL PBS, 0.1% Triton X-100, 1 mM DTT (pulldown buffer); and incubated for 20 min with 40 μg of either untagged TNKS1 or Axin1. The mixture was then washed four times in 200 μL pulldown buffer, before boiled for SDS-PAGE. For competition assays, 5–10 μM GST-tagged Axin1, excessive ARC2 and variable amounts of the competitor protein were mixed, before glutathione beads were added.
Mutagenesis and Mutant Protein Purification
Point mutations were introduced into TNKS or Axin by the QuikChange procedure (Stratagene). N-terminal GST-tagged Axin1 with a TEV-cleavage site following the tag were expressed in a modified pGEX-4T-1 vector. All TNKS constructs contained an N-terminal GST-tag with a TEV-cleavage site following the tag and were expressed in pGEX-4T-1. Growth, induction, and harvesting of cells was identical to wild-type.
Isothermal Titration Calorimetric Studies
All titrations were performed at 30 °C using a VP-isothermal calorimeter (MicroCal). Protein samples were dialyzed for 18 h against 10mM HEPES pH 7.5, 150 mM NaCl, 1 mM DTT and degassed. TNKS was placed into the 1.42 mL cell of the calorimeter at 15 μM and titrated with 300 μM of Axin1. Each titration started with a 2 μL injection followed by 32 subsequent injections (5 μL) at 4 min intervals. The data were analyzed and thermodynamic parameters were determined using the Origin software package (MicroCal) with one-site mode.
Cellular Axin1 Stabilization Assay
GFP-Axin1 was cloned into a lentiviral construct under control of the metallotheonein promoter. SW480 cells were infected with GFP-Axin1 lentivirus, and treated with DMSO or 5 μM XAV939 overnight. Cells were lysed using RIPA buffer supplemented with protease inhibitors and phosphatase inhibitors. Cell lysates were resolved by SDS-PAGE, and blotted with anti-GFP antibody (Clontech) or anti-Tubulin antibody (Sigma).
Supplementary Material
Acknowledgments.
We are grateful to the staff at ALS beamlines BL 8.2.1 and 8.2.2 for assistance with synchrotron data collection. This work was supported by the National Institutes of Health Grant CA90351 to W.X. R.T.M. is an investigator of the HHMI.
Footnotes
The authors declare a conflict of interest. R.T.M. is a cofounder of, and consultant with, FATE Therapeutics. The other authors declare no competing financial interests.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116618109/-/DCSupplemental.
Data deposition: Diffraction data and coordinates of the mouse TNKS1(306-655)-Axin1(1-80) complex structure have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3UTM).
References
- 1.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verkaar F, Zaman GJ. New avenues to target Wnt/beta-catenin signaling. Drug Discov Today. 2011;16:35–41. doi: 10.1016/j.drudis.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 4.de Sousa EM, Vermeulen L, Richel D, Medema JP. Targeting Wnt signaling in colon cancer stem cells. Clin Cancer Res. 2010;17:647–653. doi: 10.1158/1078-0432.CCR-10-1204. [DOI] [PubMed] [Google Scholar]
- 5.Dihlmann S, von Knebel Doeberitz M. Wnt/beta-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int J Cancer. 2005;113:515–524. doi: 10.1002/ijc.20609. [DOI] [PubMed] [Google Scholar]
- 6.Fancy SP, et al. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;14:1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo W, Lin SC. Axin: a master scaffold for multiple signaling pathways. Neurosignals. 2004;13:99–113. doi: 10.1159/000076563. [DOI] [PubMed] [Google Scholar]
- 8.Fearon ER. PARsing the phrase “all in for Axin”—Wnt pathway targets in cancer. Cancer Cell. 2009;16:366–368. doi: 10.1016/j.ccr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Kimelman D, Xu W. beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 10.Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1(1) doi: 10.1371/journal.pbio.0000010. E10. Epub 2003 Oct 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 12.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold HK, et al. The Axin1 scaffold protein promotes formation of a degradation complex for c-Myc. EMBO J. 2009;28:500–512. doi: 10.1038/emboj.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rui Y, et al. Axin stimulates p53 functions by activation of HIPK2 kinase through multimeric complex formation. EMBO J. 2004;23:4583–4594. doi: 10.1038/sj.emboj.7600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): Novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 16.Heeres JT, Hergenrother PJ. Poly(ADP-ribose) makes a date with death. Curr Opin Chem Biol. 2007;11:644–653. doi: 10.1016/j.cbpa.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 17.Karlberg T, et al. Structural basis for the interaction between tankyrase-2 and a potent Wnt-signaling inhibitor. J Med Chem. 2010;53:5352–5355. doi: 10.1021/jm100249w. [DOI] [PubMed] [Google Scholar]
- 18.Hsiao SJ, Smith S. Tankyrase function at telomeres, spindle poles, and beyond. Biochimie. 2008;90:83–92. doi: 10.1016/j.biochi.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 19.De Rycker M, Venkatesan RN, Wei C, Price CM. Vertebrate tankyrase domain structure and sterile alpha motif (SAM)-mediated multimerization. Biochem J. 2003;372:87–96. doi: 10.1042/BJ20021450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sbodio JI, Lodish HF, Chi NW. Tankyrase-2 oligomerizes with tankyrase-1 and binds to both TRF1 (telomere-repeat-binding factor 1) and IRAP (insulin-responsive aminopeptidase) Biochem J. 2002;361:451–459. doi: 10.1042/0264-6021:3610451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Rycker M, Price CM. Tankyrase polymerization is controlled by its sterile alpha motif and poly(ADP-ribose) polymerase domains. Mol Cell Biol. 2004;24:9802–9812. doi: 10.1128/MCB.24.22.9802-9812.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seimiya H, Muramatsu Y, Smith S, Tsuruo T. Functional subdomain in the ankyrin domain of tankyrase 1 required for poly(ADP-ribosyl)ation of TRF1 and telomere elongation. Mol Cell Biol. 2004;24:1944–1955. doi: 10.1128/MCB.24.5.1944-1955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seimiya H, Smith S. The telomeric poly(ADP-ribose) polymerase, tankyrase 1, contains multiple binding sites for telomeric repeat binding factor 1 (TRF1) and a novel acceptor, 182-kDa tankyrase-binding protein (TAB182) J Biol Chem. 2002;277:14116–14126. doi: 10.1074/jbc.M112266200. [DOI] [PubMed] [Google Scholar]
- 24.Sbodio JI, Chi NW. Identification of a tankyrase-binding motif shared by IRAP, TAB182, and human TRF1 but not mouse TRF1. NuMA contains this RXXPDG motif and is a novel tankyrase partner. J Biol Chem. 2002;277:31887–31892. doi: 10.1074/jbc.M203916200. [DOI] [PubMed] [Google Scholar]
- 25.Spink KE, Polakis P, Weis WI. Structural basis of the Axin-adenomatous polyposis coli interaction. EMBO J. 2000;19:2270–2279. doi: 10.1093/emboj/19.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian L, Mahaffey JP, Alcorn HL, Anderson KV. Tissue-specific roles of Axin2 in the inhibition and activation of Wnt signaling in the mouse embryo. Proc Natl Acad Sci USA. 2011;108:8692–8697. doi: 10.1073/pnas.1100328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CCP4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 28.McCoy AJ, et al. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubman OY, Kopan R, Waksman G, Korolev S. The crystal structure of a partial mouse Notch-1 ankyrin domain: repeats 4 through 7 preserve an ankyrin fold. Protein Sci. 2005;14:1274–1281. doi: 10.1110/ps.041184105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murshudov GN, Vagin AA, Lebedev A, Wilson KS, Dodson EJ. Efficient anisotropic refinement of macromolecular structures using FFT. Acta Crystallogr D Biol Crystallogr. 1999;55:247–255. doi: 10.1107/S090744499801405X. [DOI] [PubMed] [Google Scholar]
- 31.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.