Abstract
Gradients of the plant hormone auxin, which depend on its active intercellular transport, are crucial for the maintenance of root meristematic activity. This directional transport is largely orchestrated by a complex interaction of specific influx and efflux carriers that mediate the auxin flow into and out of cells, respectively. Besides these transport proteins, plant-specific polyphenolic compounds known as flavonols have been shown to act as endogenous regulators of auxin transport. However, only limited information is available on how flavonol synthesis is developmentally regulated. Using reduction-of-function and overexpression approaches in parallel, we demonstrate that the WRKY23 transcription factor is needed for proper root growth and development by stimulating the local biosynthesis of flavonols. The expression of WRKY23 itself is controlled by auxin through the AUXIN RESPONSE FACTOR 7 (ARF7) and ARF19 transcriptional response pathway. Our results suggest a model in which WRKY23 is part of a transcriptional feedback loop of auxin on its own transport through local regulation of flavonol biosynthesis.
Keywords: flavonoid, lateral root, WRKY
Plant growth and development are characterized by recurrent organogenesis and continuous adaptation to environmental conditions. These intriguing features rely on the ability to establish and maintain meristematic activity. Both de novo induction and maintenance of root meristematic activity are governed by gradients of the plant hormone auxin (1–3). Although several plant tissues are able to synthesize auxin (4, 5), installation and maintenance of auxin maxima are mediated mainly by polar auxin transport (6). Besides the well-known auxin import (AUXIN RESISTANT 1/LIKE AUX1) and export (PIN-FORMED [PIN] and ABCB/P-GLYCOPROTEIN/MDR) proteins (7–10), additional regulators mediate the flow of auxin throughout the plant. For example, flavonols (plant-specific polyphenolic compounds), have been proposed to act as endogenous auxin transport regulators based on their competition with the synthetic auxin transport inhibitor 1-N-naphthylphthalamic acid (11). Although the molecular targets of flavonol regulation remain unknown, genetic and pharmacologic evidence clearly demonstrate a role for these secondary metabolites as negative regulators of auxin transport (12–18). Flavonol biosynthesis was recently shown to be induced by auxin through a TRANSPORT INHIBITOR RESPONSE 1 (TIR1) auxin receptor-dependent pathway (16). However, our understanding of how flavonol biosynthesis is fine-tuned during development and in response to internal and environmental signals is still limited. Here, we report on the functional characterization of a member of the WRKY family, a large, plant-specific class of transcription factors that has been associated with responses to pathogen attack, mechanical stress, and senescence (19). Our results suggest that proper expression of WRKY23 is essential for normal root development, and that misexpression of WRKY23 causes defects in meristem organization by interfering with auxin distribution. Genetic, transcriptomic, and biochemical data suggest that WRKY23 executes its function by stimulating the biosynthesis of flavonols.
Results
WRKY23 Dosage Controls Maintenance of the Root Stem Cell Niche.
WRKY23 has been identified as an auxin-inducible gene involved in plant–parasitic nematode interactions (20). The same gene also emerged from microarray experiments as a potential key component of transcriptional networks during root meristem formation (21–23), impelling a thorough investigation of its role in a developmental context.
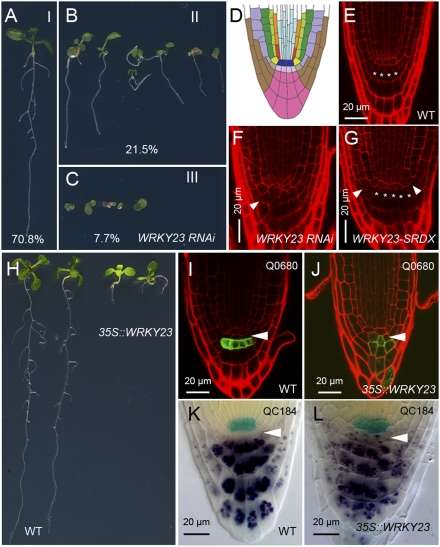
None of the publicly available T-DNA insertion lines shows obvious phenotypical differences from WT seedlings, probably because of a lack of WRKY23 transcript reductions (20). To overcome this limitation for functional analyses, we performed reduction-of-expression (RNAi) and overexpression analyses in parallel. Independent WRKY23::WRKY23RNAi lines, which displayed 80% reduction in WRKY23 transcript levels (Fig. S1A), showed variable phenotypes, which we divided into three classes: seedlings with no obvious defects (class I; 70.8% of the progeny), seedlings with a shorter and/or agravitropic root (class II; 21.5% of the progeny), and seedlings with a severely impaired development (class III; 7.7% of the progeny) (Fig. 1 A–C). Closer inspection of the class II seedlings revealed a 50% reduction in lateral root density (Fig. S1B) and defects in root tip organization (Fig. 1 D–F and Fig. S1 D–F). The root stem cell niche was less organized and contained too many cells, especially in the lateral root cap. As a result, the WRKY23::WRKY23RNAi root tips had a swollen appearance (Fig. S1E). To ensure that these developmental defects were solely due to a reduced WRKY23 transcript abundance, we first tested the expression of several close homologs of WRKY23 in WRKY23::WRKY23RNAi seedlings using quantitative (q)RT-PCR. This revealed that none of the tested WRKY23 homologs were significantly differentially expressed (Fig. S1A). Next, to confirm the reduction-of-expression phenotypes of the WRKY23::WRKY23RNAi lines, we used the SRDX repressor domain, a short 12-amino acid motif that converts transcription factors into dominant repressors (24). However, this implies that the transcription factor acts as an activator. To investigate this, we made use of a transient expression assay in tobacco cells (25). Both C-terminal and N-terminal WRKY23-GAL4 fusions were able to activate a UAS::LUCIFERASE construct (Fig. S1C), demonstrating that WRKY23 acts as a transcriptional activator. Subsequently, transgenic plants were generated in which a WRKY23-SRDX translational fusion was driven by the broadly expressed 35S promoter (26). The 35S::WRKY23-SRDX seedlings had fewer lateral roots, and their primary roots were agravitropic and exhibited a reduced growth rate (Fig. S1H). Similar to WRKY23::WRKY23RNAi, 35S::WRKY23-SRDX root tips were enlarged and showed defects in cellular patterning (Fig. 1G and Fig. S1G). Thus, two independent approaches show that WRKY23 is required for the organization of the primary root tip, lateral root development, and root gravitropic responses.
Fig. 1.
WRKY23 controls maintenance of the root stem cell niche. (A–C) WRKY23::WRKY23RNAi plants showing a phenotypic variation from WT-looking plants (class I) (A) to mildly (class II) (B) and strongly (class III) (C) affected seedlings. (D) Schematic representation of the Arabidopsis root tip. Blue, QC; light pink, columella initials; pink, columella root cap; brown, lateral root cap. (E–G) Primary root tips of WT (E) (94.1%; n = 17), class II WRKY23::WRKY23RNAi (F) (91.9%; n = 62), and 35S::WRKY23-SRDX (G) (96.7%; n = 30) seedlings. White arrowheads indicate extra divisions, and asterisks mark the rows of columella cells. (H) Ectopic expression of WRKY23 reduces root growth (Left, WT; Right, 35S::WRKY23). (I–L) Q0680 (I and J) and QC184 (K and L) marker analysis in WT (I and K) and 35S::WRKY23 (J and L) root tips. Arrowheads point toward columella initials.
To examine the effect of ectopic expression of WRKY23 on root development, we selected several lines with high WRKY23 transcript levels in which WRKY23 was driven by the global 35S promoter (35S::WRKY23) or by the RPS5A promoter, which is more restricted to sites of active cell division (27). The root length of all 35S::WRKY23 and RPS5A>>WRKY23 seedlings was reduced compared with WT (Fig. 1H and Fig. S1R). This phenotype is correlated with reduced cell division in the meristem, as shown by the B-type cyclin CycB1;1::GUS marker (Fig. S1 N and O). Moreover, in contrast to the regular and well-organized cellular pattern in WT root tips (Fig. 1 D and E), alterations in columella cell shape were observed in the 35S::WRKY23 and RPS5A>>WRKY23 root tips (Fig. 1 I and J and Fig. S1 I, J, S and T). This coincided with an altered expression domain of the Q0680 and Q1630 columella cell markers (Fig. 1 I and J and Fig. S1 P and Q), as well as with the appearance of starch granules, a characteristic of differentiated columella cells (28), in cells at the position of the columella initial cells (Fig. 1 K and L). The absence of these defects at younger stages hints at an initially correct specification of the quiescent center (QC) cells (marked by QC184) (Fig. S1 K–M), but argues for the necessity of a balanced WRKY23 expression level to maintain root stem cell and meristem activity postembryonically.
Taken together, our results demonstrate that an altered WRKY23 expression level impairs the maintenance of stem cell identity in the primary root meristem, and that WT-levels of WRKY23 expression are required for proper root growth and development.
WRKY23 Is Not a Cell-Autonomously Acting Differentiation Factor.
Root growth and meristematic activity largely depend on the correct functioning of both cell-autonomous (29–31) and non–cell-autonomous mechanisms (1, 3). Because WRKY23 is clearly involved in the maintenance of the root stem cell niche, we investigated whether the loss of stem cells in WRKY23-overexpressing roots was provoked by a local misregulation of WRKY23 expression or through a more distantly WRKY23-derived signal. Therefore WRKY23 was expressed in a cell type-specific context with the GAL4VP16-UAS transactivation system. The J2341 and Q0680 activator lines were used to drive expression in the columella initial cells and in the youngest columella layer, respectively (Fig. S2 C and D, Insets). None of these transactivation lines displayed reduced primary root growth, altered cell patterning in the root tip, or enhanced differentiation of the GAL4-expressing cells (Fig. S2 C and D) compared with the control (Fig. S2A). This is in contrast to seedlings expressing WRKY23 under the control of the 35S promoter (Fig. S1O) or the RPS5A promoter (Fig. S2B). The difference between global misregulation of WRKY23 levels and misregulation of WRKY23 levels within the columella initials suggests that WRKY23 is not a cell-autonomously acting differentiation factor.
WRKY23 Focuses Auxin Response Maxima During Organogenesis and Meristem Maintenance.
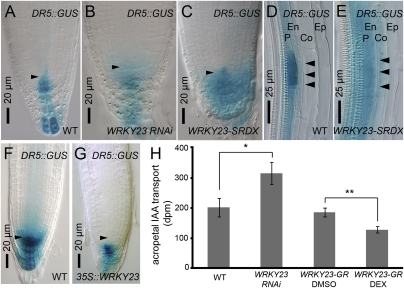
To examine the effect of WRKY23 misregulation on auxin maxima, which are known to drive meristem organization, we investigated the auxin response maximum in roots of WRKY23 reduction-of-expression and overexpression lines using the DR5::GUS reporter (32). In WT primary root tips, the auxin maximum was confined to the QC and the central columella cells (Fig. 2A). However, in WRKY23::WRKY23RNAi and WRKY23-SRDX root tips, the DR5 expression domain was expanded radially (Fig. 2 B and C). The same broadening of the auxin response maximum was observed during lateral root initiation (Fig. 2 D and E). Prior to lateral root initiation, auxin accumulates in two neighboring pericycle founder cells that subsequently divide asymmetrically (2), after which intense DR5::GUS expression can be observed in the small daughter cells (Fig. 2D) (33). However, in WRKY23-SRDX plants, DR5 expression was less confined to the founder cells and was even expanded to the cortex layer (Fig. 2E). Conversely, overexpression of WRKY23 resulted in a narrowing of the DR5-visualized auxin response domain in the primary root (Fig. 2 F and G).
Fig. 2.
WRKY23 is a negative regulator of auxin transport. (A–C) Auxin response in WT (A), WRKY23::WRKY23RNAi (B), and 35S::WRKY23-SRDX (C) root tips visualized by short staining of DR5::GUS. Arrowheads indicate QC cells. (D and E) DR5::GUS-visualized auxin response during WT (D) and 35S::WRKY23-SRDX (E) lateral root initiation. Arrowheads denote the cell walls of asymmetrically divided pericycle cells. P, pericycle; En, endodermis; Co, cortex; Ep, epidermis. (F and G) Auxin response in WT (F) and 35S::WRKY23 (G) root tips visualized by long staining of DR5::GUS. Arrowheads indicate QC cells. (H) Auxin transport measurements showing an enhanced transport in WRKY23::WRKY23RNAi and a reduced acropetal transport in 35S::WRKY23-GR roots on Dex treatment compared with the WT and DMSO control, respectively. *P < 0.05; **P < 0.01, Student t test.
To investigate whether WRKY23 might be involved in the regulation of auxin transport, we performed quantitative measurements of auxin transport. These analyses revealed that the acropetal auxin transport (toward the root tip) in WRKY23::WRKY23RNAi seedlings was higher than that in WT seedlings (Fig. 2H). Because the primary roots of the 35S and RPS5A-driven overexpression lines were too short to analyze, we constructed an inducible 35S::WRKY23-GR line that phenocopied both overexpression lines on dexamethasone (Dex) treatment (Fig. S3 A–C). Auxin transport measurements on Dex-treated 35S::WRKY23-GR seedlings revealed that ectopic WRKY23 expression negatively influenced auxin transport (Fig. 2H), whereas Dex treatment of WT or DMSO treatment of the inducible lines had no effect on transport.
Based on the aforementioned data and the phenotypic observations of WRKY23 transgenic plants, we propose that WRKY23 is part of a transcriptional network intimately associated with the local control of auxin transport required for the maintenance of auxin maxima in the root tip and during lateral root initiation.
Identification of Genes Regulated by WRKY23.
On a transcriptional level, the transport of auxin can be regulated by the expression of the PIN transport regulators (6, 18). Given the possible involvement of WRKY23 in the control of auxin transport, we evaluated the expression of the PIN efflux carriers in the WRKY23 transgenic lines, but found no explicit changes (Fig. S4). This suggests that WRKY23 might control the expression of other or upstream regulators of auxin transport. To gain insight into the WRKY23-controlled transcriptional cascade, we analyzed the transcriptome of Col-0 WT, WRKY23::WRKY23RNAi, and RPS5A>>WRKY23 roots using Affymetrix ATH1 arrays. After statistical analysis, the significantly differentially regulated genes were clustered according to their transcriptional behavior. A group of 86 genes was up-regulated in RPS5A>>WRKY23 and down-regulated in WRKY23::WRKY23RNAi, whereas 38 genes exhibited a converse transcriptional behavior. Retaining only those genes with more than 1.5-fold altered expression in RPS5A>>WRKY23, we reduced this list to a selection of 42 putative target genes (Table S1). Among these genes, only TRANSPARENT TESTA 7 (TT7) had been previously implicated in auxin transport regulation. TT7 encodes flavonoid 3′ hydroxylase, a flavonol biosynthetic enzyme that mediates the conversion of dihydrokaempferol to dihydroquercetin (Fig. S5), thereby contributing to the synthesis of quercetin, an endogenous negative regulator of auxin transport (16). Interestingly, TT7 was also found to be significantly up-regulated in a transcript profiling study of WRKY23-overexpressing poplar (Populus sp.) plants (34), suggesting that WRKY23 regulates this branch of flavonoid metabolism in divergent lineages of higher plants. Moreover, flavonols (more precisely, quercetin derivatives) accumulate at infection sites of plant-parasitic nematodes (35), where WRKY23 is highly expressed (20), justifying further investigation of TT7 as a putative target of WRKY23.
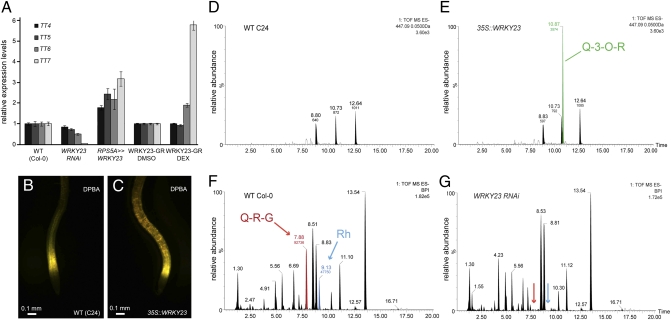
For this investigation, we first analyzed the expression of TT7 and other flavonol biosynthetic genes in WRKY23::WRKY23RNAi and WRKY23-overexpression lines. Although all four biosynthetic genes tested (TT4, TT5, TT6, and TT7) were up-regulated in WRKY23-overexpressing roots (35S::WRKY23), only a strong induction of TT7 was observed in Dex-treated 35S::WRKY23-GR seedlings (Fig. 3A). Conversely, TT7 expression was significantly down-regulated in WRKY23::WRKY23RNAi lines (Fig. 3A). Next, to investigate the spatial aspect of this regulation, we compared the distribution of flavonols, visualized by the flavonoid-specific dye diphenylboric acid 2-aminoethyl ester (DPBA), with the expression pattern of WRKY23, detected by mRNA in situ hybridization and GUS/GFP transcriptional reporter lines. Interestingly, we detected both flavonol accumulation and WRKY23 expression in root tips, in lateral root primordia, in hydathodes, and at the hypocotyl–root transition zone (Fig. S6 A–J). Moreover, in 35S::WRKY23 seedlings, ectopic flavonols accumulated throughout the entire root (Fig. 3 B and C), suggesting that WRKY23 can stimulate the biosynthesis of flavonols through transcriptional regulation of genes encoding pathway enzymes or, alternatively, of upstream factors that control pathway enzyme gene expression. To further examine the impact of WRKY23 misregulation on flavonoid production, we analyzed root extracts of WT, WRKY23::WRKY23RNAi, and 35S::WRKY23 seedlings by liquid chromatography-mass spectrometry (LC-MS). 35S::WRKY23 roots exhibited an enhanced accumulation of quercitrin (quercetin-3-O-rhamnoside) compared with WT (Fig. 3 D and E), whereas WRKY23::WRKY23RNAi roots were characterized by a highly reduced level of quercetin-rhamnoside-glucoside and rhamnetin-O-neohesperidoside (Fig. 3 F and G).
Fig. 3.
Flavonol biosynthesis is regulated by WRKY23. (A) qRT-PCR expression analysis of TT4, TT5, TT6, and TT7 in WT, WRKY23::WRKY23RNAi, RPS5A>>WRKY23, and mock-treated and Dex-treated 35S::WRKY23-GR seedlings. Error bars represent SD. (B and C) DPBA-stained WT (B) and 35S::WRKY23 (C) roots. (D and E) Extracted ion chromatograms (m/z = 447.09) showing the increased quercetin-3-O-rhamnoside (Q-3-O-R, green arrow) in 35S::WRKY23 roots (E) compared with WT C24 (D). (F and G) Base peak intensity chromatograms demonstrating the reduction in Q-R-G (red arrow) and rhamnetin-O-neohesperidoside (Rh, blue arrow) in WRKY23::WRKY23RNAi roots compared with WT Col-0.
We conclude that WRKY23 is involved in the local production of flavonol derivatives in the root tip. In particular, our results argue for a role in regulating the conversion of kaempferol to quercetin by either directly or indirectly stimulating the transcription of TT7.
Auxin Regulates TT7 Expression Through a WRKY23 Transcriptional Cascade.
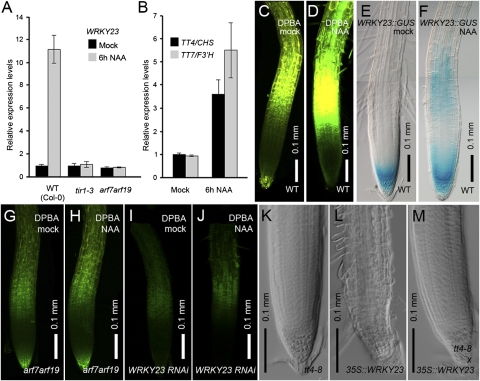
Previously, we demonstrated that WRKY23 expression can be induced by auxin in a SOLITARY ROOT/INDOLE-3-ACETIC ACID14 (SLR/IAA14)-dependent manner (20). SLR/IAA14 regulates auxin signaling by inhibiting the action of the ARF7 and ARF19 transcription factors (36). Typically, upon auxin perception by TIR1, Aux/IAA proteins, such as SLR/IAA14, are degraded (37). Accordingly, the auxin inducibility of WRKY23 expression was abolished in both arf7arf19 mutant seedlings as in the tir1 single mutant (Fig. 4A). Given that WRKY23 stimulates the biosynthesis of flavonols, we wondered whether flavonols (in particular, quercetin) could be involved in the WRKY23-dependent feedback mechanism on auxin transport. Therefore we analyzed the effect of auxin on TT4 and TT7 transcript levels as well as on DPBA fluorescence. Both TT4 and TT7 were significantly up-regulated by auxin upon a 6-h α-naphthaleneacetic acid (NAA) treatment (Fig. 4B). Moreover, compared with mock-treated seedlings, a much stronger DPBA fluorescence was observed in auxin-treated seedlings (Fig. 4 C and D). Although DPBA fluorescence was present in the elongation zone of untreated seedlings while WRKY23::GUS was not (Fig. 4 C and E), WRKY23 expression and DPBA staining perfectly coincided in this region after auxin treatment (Fig. 4 D and F). In addition, the auxin-induced increase in DPBA fluorescence observed in WT was lost in the arf7arf19 background (Fig. 4 G and H), as well as in WRKY23::WRKY23RNAi roots (Fig. 4 I and J). This indicates that the WRKY23-mediated feedback mechanism through flavonol biosynthesis is part of a SLR-ARF7-ARF19 canonical auxin-signaling pathway.
Fig. 4.
Local flavonol production is stimulated by WRKY23 in an ARF7/ARF19-dependent manner. (A) Auxin-induced WRKY23 expression is abolished in tir1-3 and arf7arf19 mutants. Black, mock-treated seedlings; gray, NAA-treated seedlings. Error bars represent SD. (B) qRT-PCR data showing auxin-induced expression of TT4 and TT7. Error bars represent SD. (C and D) DPBA-visualized flavonol accumulation induced by auxin (NAA) (D) compared with mock-treated roots (C). (E and F) Upon auxin treatment, WRKY23 expression is induced in the basal meristem, an area of strong flavonoid accumulation. Note that E and F are composite images. (G–J) Auxin-induced flavonol accumulation is not observed in the arf7arf19 background (G and H) or in WRKY23::WRKY23RNAi roots (I and J). (K–M) Early root meristem defects typical for 35S::WRKY23 seedlings are rescued by the absence of flavonols in the tt4-8 mutant. (K) tt4-8. (L) 35S::WRKY23. (M) tt4-8 × 35S::WRKY23.
As final confirmation of the regulation of flavonol biosynthesis by WRKY23, we investigated whether the change in flavonol accumulation could be causally connected to the observed WRKY23 developmental defects. We analyzed three independent tt7 insertion mutants and found a variation in root growth, similar to what was observed for the WRKY23 reduction-of-function lines. Although the majority of the seedlings exhibited a WT phenotype, ∼30% had reduced gravitropic root growth, a short root with a reduced number of lateral roots, and occasionally even stronger growth retardations (Fig. S6 K and M). To validate whether the effects of ectopic WRKY23 expression were due to an excess of flavonols, we crossed the 35S::WRKY23 line with the flavonol-deficient tt4-8 mutant. Intriguingly, the strong reduction in primary root growth and the early meristem differentiation, both characteristics of 35S::WRKY23 roots, could be rescued by introducing the tt4-8 mutation into the 35S::WRKY23 background (Fig. 4 K–M and Fig. S6 N and O) after verifying that these crosses contained the tt4 mutation and WRKY23 transgene. This finding demonstrates that overproduction of flavonol derivatives is causal to the early arrest of WRKY23-overexpressing root meristems, and thus that WRKY23 might act as a local regulator of flavonol biosynthesis.
Discussion
WRKY23 Fine-Tunes Transport-Dependent Auxin Maxima During Root Development.
Over the past decade, a large body of evidence has accumulated implicating WRKY proteins in the transcriptional reprogramming during plant defense responses (see ref. 19 for a review). Also the expression of WRKY23 is activated upon infection of plants with prokaryotic and eukaryotic pathogens (20, 38). However, WRKY23 also has appeared in several transcript profiling experiments studying plant developmental processes (21–23, 39–42). This occurrence is in agreement with the idea that WRKY transcription factors might be also involved in specific developmental programs (43–45). Using a parallel reduction-of-expression and overexpression approach, we have demonstrated that WRKY23 is of major importance for proper root growth and development.
The effect of misregulation of WRKY23 expression on root development, auxin response marker localization and intensity, and auxin transport indicates that WRKY23 negatively influences auxin transport and its dependent physiological processes. WRKY23 is expressed in root tips, and although its expression is strongly responsive to changes in auxin levels, its expression domain does not coincide exactly with the DR5 expression region, but also includes cells surrounding the auxin response maximum, such as the outer columella and lateral root cap cells. By regulating directional auxin transport in these cells, WRKY23 could contribute to the maintenance of the established auxin gradient and hence root meristematic activity. Accordingly, a reduced WRKY23 expression level was found to result in less-focused auxin responses as visualized by DR5, compromising the maintenance of the root stem cell niche organization upon germination. WRKY23 might thus be part of a feedback mechanism through which auxin can induce the focusing of its own distribution in the stem cells of the root apex. The hypothesis that WRKY23 acts in an extra fine-tuning system independent of the auxin gradient-organizing programs (46) is in line with the observations that WRKY23 does not affect expression of the PIN genes.
Transcriptional Cascade from Auxin to Flavonol Accumulation.
The expression of WRKY23 is regulated by auxin in a SLR-ARF7/ARF19-dependent manner (20). Genetic, transcriptomic, and biochemical data suggest that WRKY23 affects auxin distribution by local control of the biosynthesis of flavonols. Previously, it was shown that the accumulation of flavonols is also elevated in response to auxin (16, 46). We could show that auxin-induced flavonol production is ARF7/ARF19-dependent and put forward a transcriptional cascade in which flavonol production is controlled by auxin through the action of WRKY23. Consistent with this model, the auxin induction of flavonol synthesis is substantially reduced in the WRKY23::WRKY23RNAi line. However, given that TT7 expression could be induced only after a 12-h Dex treatment of 35S::WRKY23-GR seedlings, WRKY23 might affect TT7 rather indirectly.
Although we observed induced expression of four flavonol biosynthetic genes in 35S::WRKY23, when considering the results for all of the WRKY23 misexpression lines, WRKY23 seems to most strongly regulate the synthesis of quercetin and its derivatives through changes in TT7 transcription. Quercetin-rhamnoside accumulates in WRKY23 overexpression roots, whereas WRKY23 reduction-of-expression roots lack quercetin-rhamnoside-glucoside (Q-R-G). Interestingly, these compounds are among the most abundant flavonol derivatives in WT roots (47), and in line with our results, Q-R-G is also strongly reduced in tt4 and tt7 mutant seedlings (47). Moreover, it has been shown that the ratio of quercetin to kaempferol in the Arabidopsis thaliana root tip increases after auxin treatment (16), and that quercetin is the most effective flavonol in competing with NPA for auxin transporter-binding sites (11). A recent report comparing the phenotypes of tt4 (which produces no flavonols) and the tt7 mutant (which accumulates kaempferol but not quercetin) posited that quercetin is the active flavonol that regulates basipetal auxin transport (toward the shoot–root transition zone) and the auxin-dependent physiological processes of root elongation and gravitropism (16). This suggests that the WRKY23-regulated production of quercetin derivatives through modulation of TT7 levels might be important for the control of auxin-mediated root growth and development. However, it remains to be investigated whether glycosylated flavonols can act as auxin transport inhibitors directly or whether the changes in flavonol derivatives reflect the available aglycone pool. In addition, because plants with a reduced WRKY23 function, generated either by RNAi or SRDX approaches, showed more severe phenotypes than the single flavonoid biosynthesis mutants, we anticipate that WRKY23 may affect other pathways as well.
As mentioned earlier, WRKY23 was originally identified in a promoter-trap experiment as a plant-parasitic nematode–inducible gene (20). By means of gland secretions, plant-parasitic nematodes are able to modify gene expression in selected root cells of their host plant, ultimately establishing a nematode feeding site (for a review, see ref. 48). During this process, several developmental programs of the host are hijacked, which has led to the hypothesis that WRKY23 might be part of a captured developmental program rather than a pathogen-induced response (20). Interestingly, plant-parasitic nematodes are known to actively manipulate polar auxin transport (49) and flavonoids (more precisely, quercetin derivatives) accumulate at nematode infection sites (35). Therefore, the WRKY23-quercetin regulation of auxin transport could be the original developmental mechanism that has been hijacked by plant pathogens during evolution.
Materials and Methods
Plant Material and Growth Conditions.
A. thaliana (L.) Heynh. seeds were sterilized, germinated, and grown as described previously (20). The mutants and transgenic lines used in this study are described in SI Materials and Methods.
Histological Analyses and Microscopy.
GUS staining was done as described previously (20). Whole-mount in situ hybridization was performed as described previously (50) using a full-length WRKY23 RNA probe. For visualization of starch granules, roots were incubated for 5–10 min in Lugol solution [4 g of potassium iodide and 2 g of iodine crystals in 200 mL of MilliQ H2O (Millipore)], washed with MilliQ H2O, cleared with chloral hydrate, and analyzed immediately under differential interference contrast microscopy. Confocal microscopy was performed using a Zeiss LSM 510 confocal microscope; roots were briefly incubated in propidium iodide (3 mg/L), and then washed with and subsequently mounted in MilliQ H2O.
Transient Expression Assays.
Transient expression assays were performed as described previously (25). Details are presented in SI Materials and Methods.
Auxin Transport Measurements.
Eight-d-old seedlings were used for the transport assays. Acropetal auxin transport in the roots was measured as described previously (51). For the Dex-inducible lines, 35S::WRKY23-GR seedlings were transferred on half-strength Murashige and Skoog medium supplemented with 10 μM Dex (Sigma-Aldrich) or an equal amount of solvent (DMSO) at 26 h before the start of the assay.
qRT-PCR and Microarray Analyses.
RNA extraction and qRT-PCR were performed as described previously (52). All qRT-PCR values represent three biological replicates, each containing three technical replicates. A detailed description of how the qRT-PCR data shown in Fig. S6 were generated is provided in SI Materials and Methods along with the sequence of the primers used to quantify gene expression levels (Table S2). Details on the microarray setup and analysis are provided in SI Materials and Methods.
Flavonol Accumulation Analyses.
Flavonol accumulation in seedlings was visualized as described previously (16) using DPBA. Either combined quercetin and kaempferol fluorescence (Fig. 3) or individual channels for quercetin and kaempferol (Fig. 4) were captured. Details of the LC-MS analyses are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ben Scheres and Renze Heidstra for providing RPS5A::GAL4 seeds and a UAS- and a GR-containing plasmid. I.D.S. is supported by the Research Foundation Flanders and a Biotechnology and Biological Sciences Research Council David Phillips Fellowship. B.D.R. was financed by the Special Research Fund of Ghent University (predoctoral scholarship). W.B. thanks the Hercules Foundation for the Synapt Q-Tof for metabolomics (Grant AUGE/014) and C.L. thanks the Laboratory for Radioisotopes (LARI) Goettingen. D.R.L. and G.K.M. are supported by National Science Foundation Arabidopsis 2010 Program Grant IOB-0820717, and G. Gheysen is supported by Research Foundation Flanders Grant G.0031.08. W.G. is a postdoctoral fellow of the Research Foundation Flanders.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121134109/-/DCSupplemental.
References
- 1.Sabatini S, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 2.Dubrovsky JG, et al. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friml J, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- 4.Ljung K, et al. Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell. 2005;17:1090–1104. doi: 10.1105/tpc.104.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersson SV, et al. An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell. 2009;21:1659–1668. doi: 10.1105/tpc.109.066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport–mediated development. Trends Plant Sci. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Bennett MJ, et al. Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science. 1996;273:948–950. doi: 10.1126/science.273.5277.948. [DOI] [PubMed] [Google Scholar]
- 8.Geisler M, Murphy AS. The ABC of auxin transport: The role of p-glycoproteins in plant development. FEBS Lett. 2006;580:1094–1102. doi: 10.1016/j.febslet.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 9.Grunewald W, Friml J. The march of the PINs: Developmental plasticity by dynamic polar targeting in plant cells. EMBO J. 2010;29:2700–2714. doi: 10.1038/emboj.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swarup R, et al. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 2001;15:2648–2653. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs M, Rubery PH. Naturally occurring auxin transport regulators. Science. 1988;241:346–349. doi: 10.1126/science.241.4863.346. [DOI] [PubMed] [Google Scholar]
- 12.Bailly A, et al. Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. J Biol Chem. 2008;283:21817–21826. doi: 10.1074/jbc.M709655200. [DOI] [PubMed] [Google Scholar]
- 13.Brown DE, et al. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 2001;126:524–535. doi: 10.1104/pp.126.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buer CS, Djordjevic MA. Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. J Exp Bot. 2009;60:751–763. doi: 10.1093/jxb/ern323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buer CS, Muday GK. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell. 2004;16:1191–1205. doi: 10.1105/tpc.020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis DR, et al. Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. Plant Physiol. 2011;156:144–164. doi: 10.1104/pp.111.172502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy A, Peer WA, Taiz L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta. 2000;211:315–324. doi: 10.1007/s004250000300. [DOI] [PubMed] [Google Scholar]
- 18.Peer WA, et al. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell. 2004;16:1898–1911. doi: 10.1105/tpc.021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci. 2010;15:247–258. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Grunewald W, et al. A role for AtWRKY23 in feeding site establishment of plant-parasitic nematodes. Plant Physiol. 2008;148:358–368. doi: 10.1104/pp.108.119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brady SM, et al. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science. 2007;318:801–806. doi: 10.1126/science.1146265. [DOI] [PubMed] [Google Scholar]
- 22.Okushima Y, et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: Unique and overlapping functions of ARF7 and ARF19. Plant Cell. 2005;17:444–463. doi: 10.1105/tpc.104.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanneste S, et al. Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell. 2005;17:3035–3050. doi: 10.1105/tpc.105.035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiratsu K, Matsui K, Koyama T, Ohme-Takagi M. Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. Plant J. 2003;34:733–739. doi: 10.1046/j.1365-313x.2003.01759.x. [DOI] [PubMed] [Google Scholar]
- 25.De Sutter V, et al. Exploration of jasmonate signalling via automated and standardized transient expression assays in tobacco cells. Plant J. 2005;44:1065–1076. doi: 10.1111/j.1365-313X.2005.02586.x. [DOI] [PubMed] [Google Scholar]
- 26.Benfey PN, Chua N-H. The cauliflower mosaic virus 35S promoter: Combinatorial regulation of transcription in plants. Science. 1990;250:959–966. doi: 10.1126/science.250.4983.959. [DOI] [PubMed] [Google Scholar]
- 27.Weijers D, et al. An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development. 2001;128:4289–4299. doi: 10.1242/dev.128.21.4289. [DOI] [PubMed] [Google Scholar]
- 28.van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature. 1997;390:287–289. doi: 10.1038/36856. [DOI] [PubMed] [Google Scholar]
- 29.De Smet I, et al. Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science. 2008;322:594–597. doi: 10.1126/science.1160158. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar AK, et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446:811–814. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- 31.Stahl Y, Wink RH, Ingram GC, Simon R. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr Biol. 2009;19:909–914. doi: 10.1016/j.cub.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 32.Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benková E, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 34.Levée V, et al. Expression profiling and functional analysis of Populus WRKY23 reveals a regulatory role in defense. New Phytol. 2009;184:48–70. doi: 10.1111/j.1469-8137.2009.02955.x. [DOI] [PubMed] [Google Scholar]
- 35.Jones JT, Furlanetto C, Phillips MS. The role of flavonoids produced in response to cyst nematode infection of Arabidopsis thaliana. Nematology. 2007;9:671–677. [Google Scholar]
- 36.Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell. 2007;19:118–130. doi: 10.1105/tpc.106.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau S, Jürgens G, De Smet I. The evolving complexity of the auxin pathway. Plant Cell. 2008;20:1738–1746. doi: 10.1105/tpc.108.060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong JX, Chen CH, Chen ZX. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 2003;51:21–37. doi: 10.1023/a:1020780022549. [DOI] [PubMed] [Google Scholar]
- 39.Goda H, et al. Comprehensive comparison of auxin-regulated and brassinosteroid-regulated genes in Arabidopsis. Plant Physiol. 2004;134:1555–1573. doi: 10.1104/pp.103.034736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee DJ, et al. Genome-wide expression profiling of ARABIDOPSIS RESPONSE REGULATOR 7(ARR7) overexpression in cytokinin response. Mol Genet Genomics. 2007;277:115–137. doi: 10.1007/s00438-006-0177-x. [DOI] [PubMed] [Google Scholar]
- 41.Morant M, et al. Metabolomic, transcriptional, hormonal, and signaling cross-talk in superroot2. Mol Plant. 2010;3:192–211. doi: 10.1093/mp/ssp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hachez C, Ohashi-Ito K, Dong J, Bergmann DC. Differentiation of Arabidopsis guard cells: Analysis of the networks incorporating the basic helix-loop-helix transcription factor, FAMA. Plant Physiol. 2011;155:1458–1472. doi: 10.1104/pp.110.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson CS, Kolevski B, Smyth DR. TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell. 2002;14:1359–1375. doi: 10.1105/tpc.001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo M, Dennis ES, Berger F, Peacock WJ, Chaudhury A. MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc Natl Acad Sci USA. 2005;102:17531–17536. doi: 10.1073/pnas.0508418102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueda M, Zhang Z, Laux T. Transcriptional activation of Arabidopsis axis patterning genes WOX8/9 links zygote polarity to embryo development. Dev Cell. 2011;20:264–270. doi: 10.1016/j.devcel.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Blilou I, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 47.Stracke R, et al. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007;50:660–677. doi: 10.1111/j.1365-313X.2007.03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grunewald W, et al. Manipulation of auxin transport in plant roots during Rhizobium symbiosis and nematode parasitism. Plant Cell. 2009;21:2553–2562. doi: 10.1105/tpc.109.069617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grunewald W, Cannoot B, Friml J, Gheysen G. Parasitic nematodes modulate PIN-mediated auxin transport to facilitate infection. PLoS Pathog. 2009;5:e1000266. doi: 10.1371/journal.ppat.1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hejátko J, et al. In situ hybridization technique for mRNA detection in whole mount Arabidopsis samples. Nat Protoc. 2006;1:1939–1946. doi: 10.1038/nprot.2006.333. [DOI] [PubMed] [Google Scholar]
- 51.Kitakura S, et al. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell. 2011;23:1920–1931. doi: 10.1105/tpc.111.083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grunewald W, et al. Expression of the Arabidopsis jasmonate signalling repressor JAZ1/TIFY10A is stimulated by auxin. EMBO Rep. 2009;10:923–928. doi: 10.1038/embor.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.