Abstract
Following infection with Epstein–Barr virus (EBV), the virus is carried for life in the memory B-cell compartment in a silent state (latency I/0). These cells do not resemble the proliferating lymphoblastoid cells (LCLs) (latency III) that are generated after infection. It is of fundamental significance to identify how the different EBV expression patterns are established in the latently infected cell. In view of the prompt activatability of CD4+ T cells in primary EBV infection, and their role in B-cell differentiation, we studied the involvement of CD4+ T cells in the regulation of EBV latency. Lymphoblastoid cell lines (LCLs) were cocultured with autologous or allogeneic CD4+ T cells. Activated T cells influenced the expression of two key viral proteins that determine the fate of the infected B cell. EBNA2 was down-regulated, whereas LMP1 was unregulated and the cells proliferated less. This was paralleled by the down-regulation of the latency III promoter (Cp). Experiments performed in the transwell system showed that this change does not require cell contact, but it is mediated by soluble factors. Neutralizing experiments proved that the up-regulation of LMP1 is, to some extent, mediated by IL21, but this cytokine was not responsible for EBNA2 down-regulation. This effect was partly mediated by soluble CD40L. We detected similar regulatory functions of T cells in in vitro-infected lymphocyte populations. In conclusion, our results revealed an additional mechanism by which CD4+ T cells can control the EBV-induced B-cell proliferation.
Keywords: infectious mononucleosis, Hodgkin lymphoma
Following primary EBV infection, the virus is carried by its human host over a lifetime. In healthy individuals, the latent virus resides in the memory B-cell compartment (1), in cells that do not resemble the proliferating lymphoblastoid cells (LCLs) generated by in vitro infection. The carrier cells usually do not express any virally encoded proteins other than EBNA1, which is required for the maintenance of the viral episomes. They do not express the growth transformation program and have a resting phenotype and, therefore, do not represent a direct pathogenic threat.
The establishment of this type of latency by EBV is a focal point of attention, ever since the virus was discovered. According to one scenario, EBV exploits the B-cell differentiation program (2). The virus infects naïve IgD+ B cells that subsequently express 9 virally encoded proteins (EBNA1 through -6 and three LMPs) and transforms the cells into immunoblasts. This expression pattern is referred to as the “growth transformation,” or latency III program. The key proteins of this program are EBNA2 and LMP1. Because at least six of the nine proteins are immunogenic, the proliferating immunoblasts are readily recognized and eliminated by immune effector cells. A fraction of the infected cells migrate to the lymph node follicles and concurrently change their viral transcription program to express only three proteins: EBNA1, LMP1, and LMP2 (latency II). The LMPs facilitate the passage of the infected cells through the germinal center (GC). LMP1 down-regulates Bcl-6, which signals the emerging memory cell to exit from the germinal center. Because of the influence of the virus, naïve EBV-infected B cells can pass through the germinal center without encountering cognate antigen. As a consequence, EBV gains access to the memory B-cell pool, entering in a persistent, truly latent state (latency I/0) (3). By shutting down the expression of its immunogenic products, the latent virus escapes immune elimination and is carried during the entire lifetime of the individual.
This model was exclusively based on RT-PCR data, and it does not necessarily imply that the corresponding proteins are expressed during the transition of an infected B cell through the germinal center. It suggests, moreover, that EBV autoregulates its gene expression.
The regulation of the different EBV expression patterns in the latently infected cell is of fundamental significance for the understanding of the interaction of EBV with its normal and potentially malignant target cells and with the host response.
In vitro EBV infection of mononuclear cells collected from seronegative individuals (cord blood, seronegative children or adults) leads to the activation of CD4+ T cells and generates CD4+ cytotoxic T lymphocytes (CTLs), suggesting that these cells may be the earliest immune responders to primary infection. These activated CD4+ T cells may act on the virally encoded protein expression in the infected cells, representing a crucial early step in the EBV–host interaction. We have previously found that cytokines secreted by CD4+ T cells can change the EBV gene expression pattern in latently infected cells. The interleukins IL10, IL4, IL13, and IL21 could induce a shift from latency I toward latency II in Burkitt (BL) and Hodgkin (HL) lymphoma-derived cell lines (4, 5), whereas IL21 could induce a shift from latency III toward latency II in lymphoblastoid cell (LCL) lines and type III BL lines (6).
These findings prompted us to study the role of activated CD4+ T cells in the establishment of a stable interaction between the latent virus and the B cell.
Results
Activated CD4+ T Cells Down-Regulate EBNA2 Protein Expression in LCLs.
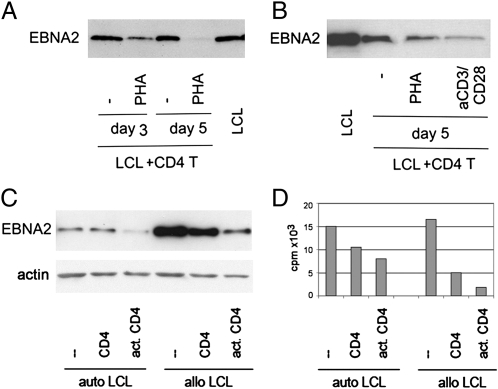
CD4+ T cells were isolated from buffy coats of healthy blood donors and cocultured with autologous or allogeneic LCLs. Unmanipulated or activated CD4+ T cells were used in the parallel cultures. Activation was induced by adding 5 μg/mL phytohemagglutinin (PHA) or anti-CD3/ anti-CD28-coated Dynabeads. After 3 and 5 d, we isolated the LCLs by CD19-coated Dynabeads and determined EBNA2 protein expression by immunoblot. LCLs recovered from the cultures intermixed with activated CD4+ T cells expressed lower levels of EBNA2 than LCLs cocultured with nonactivated CD+ T cells (Fig. 1). CD4+ T cells could induce EBNA2 down-regulation whether activated by PHA or by anti-CD3/CD28 beads (Fig. 1B). This effect occurred in both autologous and allogeneic combinations (Fig. 1C).
Fig. 1.
Activated autologous and allogeneic CD4+ cells down-regulate EBNA2 and reduce LCL proliferation. LCL07.08 was cocultured with CD4+ T cells activated with 5 μg/mL PHA or anti-CD3/CD28 beads (A and B). At the indicated time point, LCLs were isolated from the coculture by anti-CD19 beads and analyzed by immunoblot for EBNA2 protein expression. In the experiments for C and D, CD4+ T cells were activated with anti-CD3/CD28 beads. They were mixed with autologous, freshly established LCLs (10 d old) and with LCL07.08 that constituted the allogeneic LCLs. On day 5, samples were handled in the same way as described in A and B. In addition, on these separated LCLs, [3H]Thy incorporation was determined.
Down-regulation of EBNA2 indicates a shift from latency III (where EBNA2 is expressed) toward latency II or I (where EBNA2 is not expressed). In parallel with the down-regulation of EBNA2, the [3H]thymidine (Thy) incorporation of the LCLs isolated from the cocultures was also down-regulated (Fig. 1D).
Soluble Factors Released by Activated CD4+ T Cells Down-Regulate EBNA2.
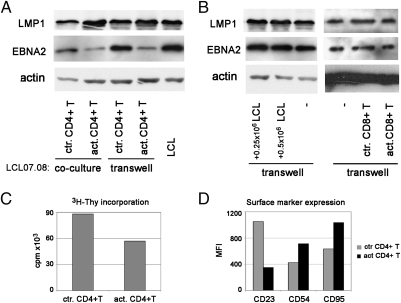
To assess whether the effect of activated CD4+ T cells on viral gene expression in the LCLs requires direct cell contact, target cells were separated from the effectors by a cell impermeable membrane [transwell (TW) system]. In this experimental setup, the EBNA2 expression of the LCLs was down-regulated by CD4+ T cells activated by anti-CD3/CD28-coated beads to a similar extent as in the cocultures (Fig. 2A). The LMP1 levels were also up-regulated. Nonactivated T cells in the TW did not change the expression of the EBV-encoded proteins. This indicates that the down-regulation of EBNA2 and up-regulation of LMP1 by activated CD4+ T cells is mediated by soluble factors (cytokines, chemokines) without requiring direct cell-to-cell contact.
Fig. 2.
CD4+ T-cell-induced down-regulation of EBNA2 and CD23 and the inhibition of proliferation do not require cell contact (TW experiments). LCL07.08 was cocultured with anti-CD3/CD28 beads activated or control CD4+ T cells for 5 d. In parallel, the same LCL was cultured in the TW system. On day 5, LCLs were isolated from the coculture with anti-CD19 beads and their EBV protein expression was determined by immunoblot along with LCLs from the TW (A). Control TW experiments were done with CD8+ T cells or with different LCLs numbers placed on the other side of the insert (B). Numbers represent the initial plating nr. of LCLs in the well surrounding the insert. [3H]Thy incorporation on day 5 of the LCLs from the insert (C). CD23, CD54, and CD95 expression was determined by FACS staining on LCLs cultured in TW (D).
The changes of LMP1 and EBNA2 protein levels of the LCLs cultured in the TW system were also shown at the single-cell level by immunofluorescent staining (Fig. S1). The results confirmed the changes recorded at population level by immunoblot. The staining also revealed that the alteration of protein expression occurred uniformly, affecting all of the cells.
In the coculture system, a decrease of the EBNA2 level occurred even in the presence of nonactivated CD4+ T cells. Most likely, this was attributable to activation of T cells by the allogeneic LCL. However, up-regulation of LMP1 did not occur in this culture, suggesting that the two proteins are regulated independently by at least two different factors (Fig. 2A).
In an identical experimental setup, LCLs cultured in the insert surrounded by activated CD8 T cells did not change the EBNA2 level (Fig. 2B). This experiment also served as a control for nonspecific down-regulation of EBNA2, e.g., by competition for nutrients. Furthermore, introduction of other actively proliferating cells outside the insert, such as LCLs, did not change the EBNA2 level either (Fig. 2B).
In line with the key role of EBNA2 for sustained LCL growth, the LCLs cultured for 5 d proliferated less, as judged by the decrease of cell numbers and [3H]Thy incorporation (Fig. 2C).
EBNA2 is associated with the expression of various phenotypic markers. We assayed CD23 and found that it was also down-regulated by activated CD4+ T cells (Fig. 2D). The expression of CD54 [intercellular adhesion molecule (ICAM)1] and CD95, known to be regulated by LMP1 (7, 8), was elevated in accordance with the increased LMP1 levels (Fig. 2D).
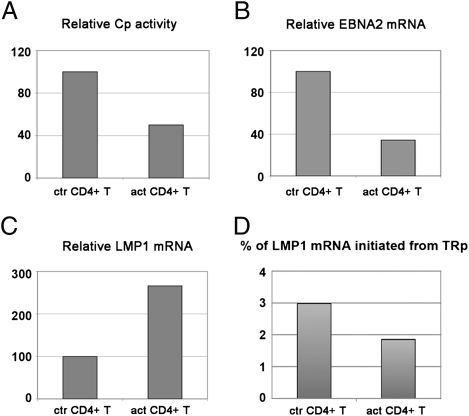
The changes of the EBV-encoded protein expression were confirmed at mRNA level by real-time quantitative PCR. (The primer pairs are listed in Table S1.) The C promoter was down-regulated, whereas LMP1 mRNA was elevated (Fig. 3 A and C). The EBNA2 mRNA levels showed a good correlation with the down-regulation seen at the level of Cp activity (Fig. 3B). LMP1 can be expressed from two alternative promoters: the EBNA2- responsive ED-L1 or the promoter located in the first terminal repeat (TR-promoter) (9). PCR with primers that amplify only the RNAs originating from the TRp showed that only a small fraction of LMP1 originated from this promoter, and this further decreased when the LCL was exposed to soluble factors produced by activated CD4+ T cells (Fig. 3D).
Fig. 3.
Changes at mRNA level after the LCL-CD4+ T-cell soluble factor encounter. LCL05.18 was subjected to activated CD4+ T-cell effects in TW system. On day 5, LCLs were collected and quantitative PCR was performed on them to determine their relative Cp activity (A), relative EBNA2 mRNA (B), and LMP1 mRNA levels (C). The relative mRNA levels transcribed from the terminal repeat promoter (TRp) of LMP1 was also determined and expressed as % of total LMP1 mRNA (D).
The changes seen in Cp activity and in LMP1 mRNA levels correspond to a change toward latency II. However, Qp activity was not elevated, suggesting that the latency type was only partially switched. To improve the conditions for a more complete switch, we reexposed the LCL to freshly activated CD4+ T cells. However, Qp activity also did not increase after these two consecutive 5-d treatments, even though the EBNA2 protein levels were further down-regulated.
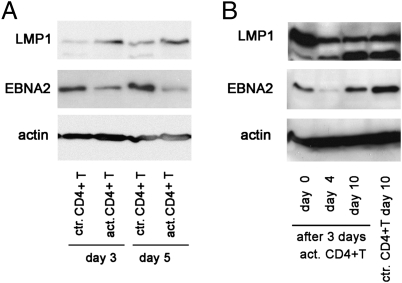
EBNA2 Down-Regulation Is Relatively Rapid and Is Maintained Even After the Removal of the Inducing Media.
We found that the EBNA2 level was already suppressed on day 3, its level decreased even more on day 5 (Fig. 4A), and it was sustained after the removal of the activated CD4+ T-cell conditioned media. Cells were collected on day 3 from the insert and plated in new media. The CD4+ T-cell effect on LCL proliferation was sustained for at least 10 d (Fig. S2). The target cells showed an additional drop in EBNA2 level on day 4 after removal, and they were still not fully recovered on day 10 (Fig. 4B).
Fig. 4.
Kinetics of EBNA2 down-regulation. LCL07.08 was cultured in the TW system with anti-CD3/CD28 bead-activated CD4+ T cells or control CD4+ T cells. LCLs were collected at different time points, and EBV protein levels were determined by immunoblot (A). Cells recovered on day 3 were replated in fresh medium (day 0 in B). On days 4 and 10, pellets were made to assess the kinetics of EBV protein recovery after the initial 3 d of activated CD4+ T-cell exposure (B).
Comparison Between CD4+ T-Cell and IL21 Effects.
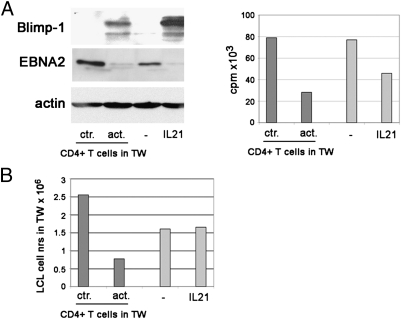
We have previously shown that IL21 can down-regulate EBNA2 and up-regulate LMP1 (6). Therefore, we compared its effects with that of the activated CD4+ T cells. Similar changes were induced with regard to EBNA2 expression, as well as [3H]Thy incorporation (Fig. 5A). However, the level of Blimp-1, a transcriptional factor induced by IL21 in LCLs, was only modestly up-regulated by the activated CD4+ T cells. Moreover, whereas activated CD4+ T cells impaired the proliferation of the LCLs markedly, IL21 had no such effect on the cell numbers (Fig. 5B), even though [3H]Thy incorporation at the end of the 5-d treatment was impaired by IL21 as well (Fig. 5A, Right). The discrepancy in the two findings is probably attributable to different kinetics. These differences suggested that the effect of activated CD4+ T cells is not, or not only, attributable to the release of IL21.
Fig. 5.
Comparison between the effects of activated CD4+ T cells and IL21 on LCLs. LCL07.08 was exposed to the activated CD4+ T-cell medium in the TW system. Parallel LCL cultures were treated with 100 ng/mL IL21. On day 5, the LCLs were collected and analyzed for protein expression, Thy incorporation (A), and cell number (B).
We also looked for phenotypic changes induced in the LCL by the activated CD4+ T cells. As a marker for differentiation to the plasma cell direction, we tested expression of CD38. We also examined expression of germinal center markers CD10 and CD77 by flow cytometry and Bcl-6 by immunoblot. None of these molecules was induced (Fig. S3), indicating that in these experimental conditions, no significant differentiation of cells occurred.
Identification of the Factors Secreted by Activated CD4+ T Cells That Contribute to the Modulation of Viral Gene Expression.
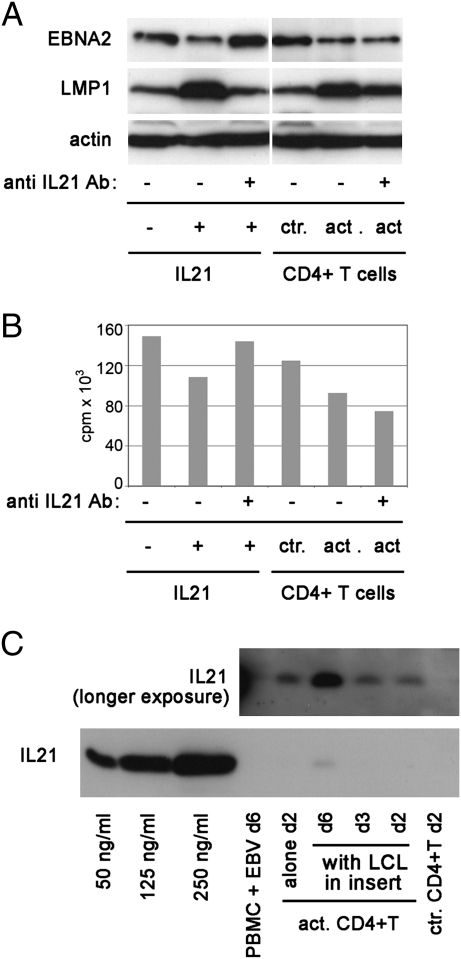
To assess the contribution of IL21 to the effects observed, we added a polyclonal rabbit anti-IL21 antibody to the culture media of activated CD4+ T cells. The functional activity of the antibody was confirmed by the inhibition of LMP1 up-regulation and of EBNA2 down-regulation by recombinant IL21 that was added to LCLs (Fig. 6A). In the TW experiment, the anti-IL21 antibody partially prevented the up-regulation of LMP1, but did not modify the down-regulation of EBNA2 (Fig. 6A). Thus, the factor that down-regulates EBNA2 in the TW system is not IL21.
Fig. 6.
Neutralization of IL21 in the activated CD4+ T-cell supernatants does not inhibit down-regulation of EBNA2 and [3H]Thy incorporation. LCL07.08 was treated with 50 ng/mL IL21 for 3 d. To parallel, IL21-treated cultures, 10 μg/mL anti-IL21 antibody was added. The same neutralizing anti-IL21 Ab was added to anti-CD3/CD28-activated CD4+ T-cell cultures that were in TW with the LCL07.08. On day 3, cells were collected and processed for EBV protein detection (A) and [3H]Thy incorporation (B). IL21 content of anti-CD3/CD28 bead-activated CD4+ T-cell supernatant (20 μL/lane) was determined by immunoblot (C). Recombinant IL21 of a known amount was also loaded on the gel. The smaller image above represents the film exposed to the blot for a longer time.
Similarly, the reduced [3H]Thy incorporation and the decrease of CD23 expression were reverted by the antibody when recombinant IL21 was used but did not modify the effect of the activated CD4+ T cells (Fig. 6B and Fig. S4A). The activation status of the T cells, judged by their proliferative capacity according to [3H]Thy incorporation, was not affected by the anti-IL21 antibody (Fig. S4B).
In this experiment, a lower dose of IL21 was used than in the earlier experiments (50 ng/mL) to ensure that the antibody can neutralize it completely (according to the recommendation of the supplier). This raised the concern that the activated CD4+ T cells may have produced higher levels of IL21 (higher that 50 ng/mL) that escaped neutralization. However, we have shown that T cells produced much lower levels of IL21 than the concentration that was successfully neutralized in the control experiment (using 50 ng/mL recombinant IL21) (Fig. 6C).
Knowing that the amount of IL21 secreted by activated CD4+ T cells was significantly lower than the concentration of recombinant IL21 added to the LCLs, now the effect of such a low IL21 concentration was tested. A dose–response experiment revealed that the EBNA2 down-regulating effect was present at 100 and 50 ng/mL but not at 25 ng/mL or a lower concentration, whereas up-regulation of LMP1 was detectable even at 5 ng/mL (Fig. S5). Therefore, the down-regulation of EBNA2 by CD4+ T cells cannot be attributable to IL21 because they do not produce it in sufficient amount.
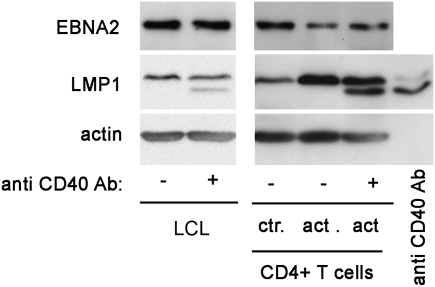
It has been shown that exposure of LCLs to CD40L down-regulates EBNA2 (10). When using an antibody against CD40 (specifically selected for its capacity to inhibit the binding, and consequently the effect of CD40L) we found that it partially inhibited the down-regulation of EBNA2, indicating that soluble CD40L is one of the contributing factors (Fig. 7). When tested on LCLs alone, this antibody by itself did not influence EBNA2 levels (Fig. 7, second lane). Because the antibody binds to CD40 on the cells, its heavy chain was detected on the immunoblot as an additional band, just below LMP1 (Fig. 7, last lane).
Fig. 7.
Soluble CD40L is partially responsible for down-regulation of EBNA2. LCL07.08 was subjected to the activated CD4+ T-cell effects in the TW system. To parallel cultures, anti-CD40 antibody was added. As a control, anti-CD40 antibody was added to LCLs cultured alone. On day 3, cells were collected and EBV proteins were detected. The lower band on the LMP1 blot represents the Ig heavy chain of the anti-CD40 antibody, as proven by the last lane on the immunoblot where pure anti-CD40 antibody was loaded.
We also tested neutralizing antibodies against IL4 and IL6, because both cytokines have been found to influence viral gene expression (5, 11). Neither of these antibodies influenced the effects of the activated CD4+ T cells on LCLs in the TW system.
Peripheral Blood Mononuclear Cells Infected with EBV Secrete Soluble Factors That Down-Regulate EBNA2 Protein Levels.
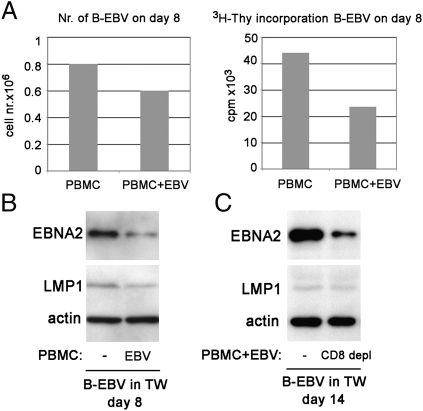
To test whether freshly infected B cells respond in a similar way to CD4+ T-cell-secreted factors as the established LCLs, EBV-infected or control peripheral blood mononuclear cells (PBMCs) were cultured in the TW system under the insert. The insert contained B-cell cultures freshly infected with EBV. It is known that EBV-infected B cells activate T cells in PBMC cultures, inducing them to produce various cytokines. These factors, if produced in sufficient amounts may act on the infected B cells in the insert. Infected B cells were collected from the inserts 8 d after infection. The number of cells that have been exposed to the supernatant of the infected PBMC was lower than those from the insert immersed into the culture of an uninfected PBMC (Fig. 8A, Left). Moreover, their proliferative capacity was reduced (Fig. 8A, Right), and they expressed a lower level of EBNA2 (Fig. 8B). However, they did not express higher levels of LMP1. We also performed an experiment where we compared the effect of EBV-infected PBMCs with a parallel CD8+ T-cell-depleted culture. This increased the absolute number of CD4+ T cells because the total cell number was the same (5 × 106). EBNA2 down-regulation was more pronounced in this experiment (day 14) (Fig. 8C).
Fig. 8.
EBV-infected PBMCs secrete soluble factors that down-regulate EBNA2 in freshly infected B cells. The experiment was performed in TW system. In the well, 5 × 106 EBV-infected PBMCs or noninfected PBMCs were cultured (A and B). In C, the well contained 5 × 106 EBV-infected PBMCs or CD8+ T-cell-depleted PBMCs infected with EBV. EBV-infected B cells (106) were placed in the insert. On day 8 (B) or 14 (C), cells from the insert were collected and tested for their EBNA2 and LMP1 protein expression. Cell number in the insert was recorded on day 8 (A, Left), and their [3H]Thy incorporation was determined during an overnight pulse.
The experiments, thus, show that soluble factors are produced during primary infection that can down-regulate the type III viral expression program. However, the level of LMP1 was not up-regulated, indicating that IL21 was not responsible for the change. Indeed, the supernatant of an EBV-infected PBMC on day 6 did not contain detectable levels of IL21 (Fig. 6C).
Discussion
EBV is regarded as the most efficient transforming virus. Despite its dangerous, proliferation-inducing property, the virus has achieved a largely nonpathogenetic interaction with its human host. This is attributable to a complex lifecycle that consists of several distinct phases: (i) initial “expansion” of virally activated B cells that may reach high level as in mononucleosis. Nine growth transformation-associated virally encoded proteins are expressed, geared to stimulate proliferation. At least six of the nine are immunogenic. (ii) This is followed by a phase of “rejection” that curbs expansion, protecting the host and thereby also the virus. (iii) The third phase, “persistent viral latency,” sustains the viral genome, mainly in nonproliferating memory B cells in a hidden state, unrecognizable by the host response. This finely poised adaptation of what may have been originally a highly pathogenic virus, to an equilibrated existence that favors the survival of both virus and its host, is the result of a long evolutionary process.
The interaction of the virally infected B cell with the host is dynamic but strictly regulated. Throughout the history of EBV research, cellular immunity, particularly cell-mediated killing, was considered the main mechanism that eliminates the dangerous, proliferating EBV-infected B cells. However, our results suggest that additional mechanisms, other than elimination of the infected B cell, can be executed by the immune system to render the cells relatively harmless.
We have shown that soluble products of activated CD4+ T cells (IL21, soluble CD40L, and possibly others) can down-regulate expression of EBNA2, an important player in the virally induced immunoblast proliferation program. These cytokines are produced in the germinal centers. They can support the transition from the full set of growth transformation-associated proteins (latency III) to the more restricted program with low EBNA2 and high LMP1 expression, similar to latency II. By this, the cytokines produced by activated CD4+ T cells counteract the viral growth program. Interestingly, the switch to latency II was not complete because Qp activity was not induced, indicating that additional factors, not provided by our experimental setup, are needed for the complete switch.
Much work has been devoted to the study of EBV-infected B-immunoblast proliferation in infectious mononucleosis (IM) patients and the host mechanisms that curb this proliferation, making IM a self-limiting disease (12). In addition to antibodies, the proliferating EBV-infected cells are confronted with powerful cellular immune response, executed by natural killer (NK) cells (13) and MHC class I -restricted EBV-specific CD8+ cytotoxic T cells. The latter are believed to play a central role in bringing immunoblast proliferation to a halt and making this chronic infection asymptomatic in healthy adults.
Relatively little is known about the role of CD4+ T cells in controlling EBV infected B-cell proliferation. It is not clear whether they have a direct effector function or merely serve as a source of immunological help for the CD8+ CTLs.
A number of publications have shown that CD4+ T cells can recognize and kill EBV transformed B cells. T cells derived from cord blood and from EBV-seronegative adults can develop EBV specific cytotoxicity in vitro (14, 15). In contrast to the EBV specific, MHC class I restricted cytotoxicity that can be induced in autologous mixed lymphocyte cultures of EBV seropositive adults, mediated by CD8+ T cells, the cytotoxicity induced in the seronegatives by the same method is mediated by CD4+ CTLs (14, 16, 17). This killing has been attributed to the interaction of CD95 and CD95 ligand (18).
Our results suggest that, in addition to their role in killing the EBV-infected cells, CD4+ T cells control EBV-induced B cell growth by modifying the expression of EBV-encoded proteins. According to the derived scenario, CD4+ T cells are activated during primary EBV infection and release soluble factors that modulate EBV gene expression. This leads to reduced EBNA2, increased LMP1 expression, and reduced proliferative capacity. Our findings, thus, point to an additional mechanism whereby CD4+ T cells can participate in the control of EBV infection.
Different viral latency types in the progeny of a single infected B cell was found by Kurth et al. (19) in tonsils. They detected EBV-infected B cells with different latency types (I, II, and III) within the same clone, as identified by V gene rearrangement. This suggests that the viral gene expression of an infected B cell can be influenced by the interaction with environment, which may change after infection. However, in vitro models that would allow the study of these latency changes have not been successfully established, indicating that exogenous factors may be required for this process.
During infectious mononucleosis (IM), the number of CD4+ T cells is only marginally elevated (20). It is possible that asymptomatic primary EBV infection is accompanied by a pronounced increase in the CD4+ T-cell numbers. This could lead to a more efficient switch of latency III to latency II and eventually latency 0, attributable to higher levels of the key cytokines. The remaining lower numbers of proliferating transformed B cells would lead to lower number of activated CD8+ T cells, avoiding the pathological manifestations of mononucleosis that is caused by an exacerbated immune response.
On the other hand, cytokines produced by the CD4+ T cells (such as IL4, IL10, IL13, IL21) may up-regulate LMP1 and, thus, contribute to the survival of faultily differentiated B cells that would normally die (21). Signals delivered by LMP1 can support cell survival, leading to tumor formation. It has been shown that after IM, the risk for developing EBV-positive Hodgkin lymphoma (HL) increases (22), which is characterized by particularly high levels of LMP1 in the malignant cells (latency II). It is possible that this is mediated by LMP1-inducing, thus survival-promoting, cytokines produced during IM. A finely tuned level and timing of cytokine release may, thus, influence the outcome of primary EBV infection and its consequences.
Materials and Methods
CD4+ T-Cell Separation.
PBMCs were separated from buffy coats of healthy blood donors by Ficoll-Paque (Pharmacia) separation. CD4+ T cells were purified from PBMCs by negative selection with a Dynabeads MyPure CD4 kit 2 (Invitrogen), according to the instructions of the manufacturer.
Cell Lines and EBV Infection of Primary B Cells.
LCL05.08 and LCL07.08 are established EBV-transformed LCLs. For fresh infection, B cells were positively separated with CD19 Dynabeads PanB (Invitrogen) from buffy coats and incubated with B95-8 cell supernatant for 1.5 h at 37 °C. Cells were washed and resuspended in complete RPMI medium.
[3H]Thy Incorporation.
At the indicated time points, 0.05 × 106 cells were plated in 200 μL of medium in a 96-well plate. [3H]Thy (1 μCi) was added to each well, and the plates were incubated at 37 °C in 5% CO2 for 16 h. Cells were harvested on a glass fiber filter, and radioactivity was measured in a liquid scintillation counter. All data represent the mean value given by three to five parallel wells.
Coculture of LCLs with CD4+ T Cells.
For the coculture experiments, 106 CD4+ T cells were incubated with 0.3 × 106 LCLs in a total volume of 2.5 mL of complete RPMI medium. Parallel cultures were treated with 5 μg/mL PHA (Sigma-Aldrich) or 106 Dynabeads T-cell expander (Invitrogen) (1 bead/1 T cell). At the end of the coculture period, LCLs were recovered from the mixture of CD19+ Dynabeads.
In specific experiments, cell–cell contact was prevented by using a TW insert with a 0.4-μm pore-size polycarbonate membrane (Costar) in a 12-well plate. In these experiments, 106 CD4+ T cells were cultured below the insert, whereas the 0.3 × 106 LCLs were placed in the insert.
Immunoblotting.
The cells were lysed in loading buffer, and aliquots corresponding to 1.5 × 105 cells were loaded on each lane. Immunoblotting has been carried out as described previously (23). For detection of different proteins, the following antibodies were used: PE-2 (anti-EBNA2; DAKO), NCL-EBV-CS1-4 (mouse anti-LMP1; Novocastra Laboratories), 3H2-E8 (mouse anti-human Blimp-1; Novus Biologicals), and d-8 (mouse anti-BCL-6; Santa Cruz Biotechnology). As a control for equal amounts of protein loaded, we detected β-actin by mAb Clone AC-15 (Sigma-Aldrich).
Treatment of Cells and Use of Neutralizing Antibodies.
The IL21 treatment of LCLs was done at 100 ng/mL IL-21 (Peprotech) at 0.3 × 106 cells/3 mL complete RPMI medium density in a 12 well plate. In the IL21 neutralizing experiment, 50 ng/mL IL21 was used. The concentration of anti-IL21 (polyclonal rabbit antibody; 500-P191; Peprotech) was 10 μg/mL. The neutralizing anti-CD40 (mouse mAb; MAB6322; R&D Systems) was added to the medium at a concentration of 10 μg/mL. The anti-IL6 neutralizing antibody (polyclonal goat IgG; AB-206-NA; R&D Systems) was used at 5 μg/mL, whereas the concentration for anti-IL4 neutralizing antibody (monoclonal mouse IgG1; MAB304; R&D Systems) was 1 μg/mL.
Flow Cytometry.
Staining for FACS analysis was performed with anti-CD23-PE or anti-CD23 FITC (Dako), anti-CD38-FITC (Dako), anti-CD54-PE (BD), anti-CD95-APC (BD), and anti-CD10-PE (BD) according to standard procedures.
Supplementary Material
Acknowledgments
We thank M. Rowe (University of Birmingham) and R. Küppers (University of Duisburg-Essen) for valuable discussion and suggestions. This work was supported by the Swedish Cancer Society and by the Cancer Research Institute (New York)/Concern Foundation (Los Angeles, CA).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120587109/-/DCSupplemental.
References
- 1.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 2.Thorley-Lawson DA. Epstein-Barr virus: Exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 3.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 4.Kis LL, Takahara M, Nagy N, Klein G, Klein E. IL-10 can induce the expression of EBV-encoded latent membrane protein-1 (LMP-1) in the absence of EBNA-2 in B lymphocytes and in Burkitt lymphoma- and NK lymphoma-derived cell lines. Blood. 2006;107:2928–2935. doi: 10.1182/blood-2005-06-2569. [DOI] [PubMed] [Google Scholar]
- 5.Kis LL, et al. STAT6 signaling pathway activated by the cytokines IL-4 and IL-13 induces expression of the Epstein-Barr virus-encoded protein LMP-1 in absence of EBNA-2: Implications for the type II EBV latent gene expression in Hodgkin lymphoma. Blood. 2011;117:165–174. doi: 10.1182/blood-2010-01-265272. [DOI] [PubMed] [Google Scholar]
- 6.Kis LL, et al. IL-21 imposes a type II EBV gene expression on type III and type I B cells by the repression of C- and activation of LMP-1-promoter. Proc Natl Acad Sci USA. 2010;107:872–877. doi: 10.1073/pnas.0912920107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henriquez NV, Floettmann E, Salmon M, Rowe M, Rickinson AB. Differential responses to CD40 ligation among Burkitt lymphoma lines that are uniformly responsive to Epstein-Barr virus latent membrane protein 1. J Immunol. 1999;162:3298–3307. [PubMed] [Google Scholar]
- 8.Le Clorennec C, et al. Molecular basis of cytotoxicity of Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) in EBV latency III B cells: LMP1 induces type II ligand-independent autoactivation of CD95/Fas with caspase 8-mediated apoptosis. J Virol. 2008;82:6721–6733. doi: 10.1128/JVI.02250-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadler RH, Raab-Traub N. The Epstein-Barr virus 3.5-kilobase latent membrane protein 1 mRNA initiates from a TATA-Less promoter within the first terminal repeat. J Virol. 1995;69:4577–4581. doi: 10.1128/jvi.69.7.4577-4581.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pokrovskaja K, et al. CD40 ligation downregulates EBNA-2 and LMP-1 expression in EBV-transformed lymphoblastoid cell lines. Int J Cancer. 2002;99:705–712. doi: 10.1002/ijc.10417. [DOI] [PubMed] [Google Scholar]
- 11.Altmeyer A, et al. Reversal of EBV immortalization precedes apoptosis in IL-6-induced human B cell terminal differentiation. Immunity. 1997;7:667–677. doi: 10.1016/s1074-7613(00)80387-8. [DOI] [PubMed] [Google Scholar]
- 12.Hislop AD, Annels NE, Gudgeon NH, Leese AM, Rickinson AB. Epitope-specific evolution of human CD8(+) T cell responses from primary to persistent phases of Epstein-Barr virus infection. J Exp Med. 2002;195:893–905. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams H, et al. Epitope-specific evolution of human CD8(+) T cell responses from primary to persistent phases of Epstein-Barr virus infection. Br J Haematol. 2005;129:266–274. doi: 10.1084/jem.20011692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savoldo B, et al. Generation of EBV-specific CD4+ cytotoxic T cells from virus naive individuals. J Immunol. 2002;168:909–918. doi: 10.4049/jimmunol.168.2.909. [DOI] [PubMed] [Google Scholar]
- 15.Liu A, Arbiser JL, Holmgren A, Klein G, Klein E. PSK and Trx80 inhibit B-cell growth in EBV-infected cord blood mononuclear cells through T cells activated by the monocyte products IL-15 and IL-12. Blood. 2005;105:1606–1613. doi: 10.1182/blood-2004-06-2406. [DOI] [PubMed] [Google Scholar]
- 16.Sun Q, Burton RL, Pollok KE, Emanuel DJ, Lucas KG. CD4(+) Epstein-Barr virus-specific cytotoxic T-lymphocytes from human umbilical cord blood. Cell Immunol. 1999;195:81–88. doi: 10.1006/cimm.1999.1514. [DOI] [PubMed] [Google Scholar]
- 17.Misko IS, et al. Composite response of naive T cells to stimulation with the autologous lymphoblastoid cell line is mediated by CD4 cytotoxic T cell clones and includes an Epstein-Barr virus-specific component. Cell Immunol. 1991;132:295–307. doi: 10.1016/0008-8749(91)90029-b. [DOI] [PubMed] [Google Scholar]
- 18.Wilson AD, Redchenko I, Williams NA, Morgan AJ. CD4+ T cells inhibit growth of Epstein-Barr virus-transformed B cells through CD95-CD95 ligand-mediated apoptosis. Int Immunol. 1998;10:1149–1157. doi: 10.1093/intimm/10.8.1149. [DOI] [PubMed] [Google Scholar]
- 19.Kurth J, et al. EBV-infected B cells in infectious mononucleosis: Viral strategies for spreading in the B cell compartment and establishing latency. Immunity. 2000;13:485–495. doi: 10.1016/s1074-7613(00)00048-0. [DOI] [PubMed] [Google Scholar]
- 20.Williams H, et al. Analysis of immune activation and clinical events in acute infectious mononucleosis. J Infect Dis. 2004;190:63–71. doi: 10.1086/421276. [DOI] [PubMed] [Google Scholar]
- 21.Bräuninger A, et al. Molecular biology of Hodgkin's and Reed/Sternberg cells in Hodgkin's lymphoma. Int J Cancer. 2006;118:1853–1861. doi: 10.1002/ijc.21716. [DOI] [PubMed] [Google Scholar]
- 22.Hjalgrim H, et al. Infectious mononucleosis, childhood social environment, and risk of Hodgkin lymphoma. Cancer Res. 2007;67:2382–2388. doi: 10.1158/0008-5472.CAN-06-3566. [DOI] [PubMed] [Google Scholar]
- 23.Nagy N, et al. SH2D1A and SLAM protein expression in human lymphocytes and derived cell lines. Int J Cancer. 2000;88:439–447. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.