Abstract
Processes that promote cancer progression such as angiogenesis require a functional interplay between malignant and nonmalignant cells in the tumor microenvironment. The metalloprotease aminopeptidase N (APN; CD13) is often overexpressed in tumor cells and has been implicated in angiogenesis and cancer progression. Our previous studies of APN-null mice revealed impaired neoangiogenesis in model systems without cancer cells and suggested the hypothesis that APN expressed by nonmalignant cells might promote tumor growth. We tested this hypothesis by comparing the effects of APN deficiency in allografted malignant (tumor) and nonmalignant (host) cells on tumor growth and metastasis in APN-null mice. In two independent tumor graft models, APN activity in both the tumors and the host cells cooperate to promote tumor vascularization and growth. Loss of APN expression by the host and/or the malignant cells also impaired lung metastasis in experimental mouse models. Thus, cooperation in APN expression by both cancer cells and nonmalignant stromal cells within the tumor microenvironment promotes angiogenesis, tumor growth, and metastasis.
Keywords: lung cancer, melanoma, proteolytic activity, shRNA, tumorigenesis
Aminopeptidase N (APN, CD13; EC 3.4.11.2) is a widely expressed type II membrane-bound metalloprotease (1, 2). It functions in the enzymatic cleavage of peptides, in endocytosis, and as a signaling molecule and has been implicated in the regulation of complex and diverse processes, including cell migration, cell survival, viral uptake, and angiogenesis (3). APN has also been linked specifically to cancer, having been identified as a cell-surface marker for malignant myeloid cells (4–7) and reaching high levels of expression in association with the progression of tumors, including breast, ovarian, and prostate cancer (8–14). Indeed, vascular endothelial growth factor (VEGF), a key angiogenesis regulator, induces the expression of APN at an early stage of tumor growth (15), again highlighting the role of this enzyme in angiogenesis, a process crucial for sustained growth of most solid tumors (16). Studies of bestatin, a CD13 inhibitor and antiangiogenic agent, also suggest that APN enzymatic activity is relevant for tumorigenesis (17, 18). Nevertheless, the substrates of APN in the context of angiogenesis are still unknown. The only well-defined substrate is angiotensin III in the renin–angiotensin pathway, in which APN cleaves the NH2-terminal arginine residue of angiotensin III to form angiotensin IV. Consistent with several lines of evidence, we have previously identified APN as a target for inhibition of tumor vascularization and growth (18–20).
Tumor growth relies on a complex microenvironment in which malignant cells cooperate with various other cell types: endothelial cells of the blood and lymphatic circulation, mesenchymal stromal cells/cancer-associated fibroblasts, and a variety of bone marrow-derived cells such as myeloid-derived suppressor cells and lymphocytes (21, 22). Some of these cell populations cooperate in inducing desmoplasia and angiogenesis. A body of indirect evidence suggests a role for APN in regulating these aspects of tumor progression. Indeed, APN is expressed by both cancer cells and nonmalignant cells such as environmental vascular endothelial cells, neutrophils, and stromal cells (8, 14), but it remains unclear where and how APN acts to regulate tumor angiogenesis.
We recently generated APN-null mice and found them to have impaired neovascularization in response to hypoxia or growth factor stimulation in the absence of tumor cells (23). We reasoned that the APN-null mouse combined with syngeneic tumor allografts would provide an ideal model system to sort out the roles of tumor and alternative sources of APN in tumor growth and metastasis. Here, we assessed the tumorigenic properties of B16F10 melanoma and Lewis lung carcinoma (LLC) in which APN expression was specifically silenced (knocked down) with small hairpin (sh)RNA. Analysis of APN-expressing and APN-shRNA grafts in wild type (WT) and APN-null mice indicated that the contribution of both tumor and host APN sources is essential for tumor vascularization. Notably, both primary tumor growth and experimental lung metastases were impaired when APN was absent from either host or tumor cells, and its absence from both cell sources had a cooperative effect. These allograft data indicate that APN is an important angiogenic factor supplied by both nonmalignant host and malignant tumor cells and that both sources contribute to cancer progression.
Results
Generation of APN Knockdown Tumor Cell Lines.
Given that APN is widely expressed, we first analyzed its expression levels in different mouse cancer cell lines of C57BL/6 background by Western blotting to identify suitable candidates for analyzing APN function. Various levels of APN expression were detected in B16F1 and B16F10 melanoma cells, LLC cells, and transgenic adenocarcinoma of the mouse prostate (TRAMP) clones C2 and C3 (SI Appendix, Fig. S1A). We chose B16F10 and LLC lines for further studies because both exhibited intermediate expression levels of APN and have long been established as tumorigenic and metastatic (24, 25). To evaluate whether expression of APN by tumor cells contributes to cancer progression, we generated B16F10 and LLC APN-shRNA cell clones and found decreased production of APN and reduced enzymatic activity in the shRNA cell clones by Western blotting and enzymatic activity assays (SI Appendix, Fig. S1 B and C). Proliferation assays showed similar rates of cell division between control-shRNA and APN-shRNA cell clones at different time points (SI Appendix, Fig. S1D), thus ruling out cell multiplication rate as a major factor in subsequent tumor growth studies.
Inhibition of APN Expression Reduces Primary Tumor Growth.
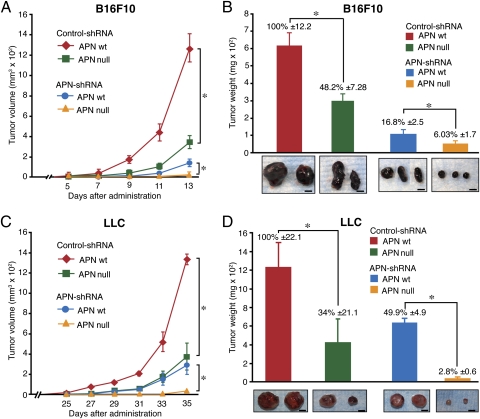
To investigate the role of alternative APN tissue sources, we injected B16F10 cells expressing control-shRNA and APN-shRNA subcutaneously into WT and APN-null mice. After 11 d, the B16F10 control-shRNA tumors showed a marked growth in WT mice, whereas, in contrast, these tumors showed reduced tumor volume and weight in the APN-null mice (Fig. 1 A and B), revealing the importance of nonmalignant sources of APN in tumorigenesis. Furthermore, tumors derived from the B16F10 APN-shRNA clones showed a significant (P < 0.02) reduction in tumor growth in WT mice, illustrating a crucial role for tumor-derived APN in tumor progression. The largest inhibitory effect on tumor growth was observed in the APN-shRNA tumor cells administrated to the APN-null mice, with almost no growth even at 2 wk after administration (Fig. 1 A and B), suggesting a synergistic effect of tumor and host-derived APN. Notably, both host and tumor cells lack the expression of APN before tumor establishment (i.e., “prevention” setting). We repeated these experiments with LLC cells as an alternative tumor model, which gave essentially the same results (Fig. 1 C and D). Thus, APN expressed by both the tumor microenvironment and the malignant cells cooperate to promote tumor growth.
Fig. 1.
Loss of APN expression by tumor or host nonmalignant cells impairs tumor growth. Control and APN shRNA B16F10 or LLC cells were injected into the right flank of WT or APN-null mice (n = 5/group), and tumor growth was followed. (A and C) Average tumor volume (mm3) was measured every 2 d (*P < 0.02). (B and D) Tumor weights were evaluated after 13 d (B16F10) or 35 d (LLC). Bars represent mean tumor weight ± SEM (*P < 0.02). Photographs show images of representative tumors. (Scale bar, 5 mm.)
We next performed a series of control experiments to exclude the possibility of genome integration and site-dependent, off-target effects of lentivirus-delivered shRNA. Namely, for reintroduction of APN expression in tumor knockdown lines, we generated an APN reconstitution (APN-r) cDNA construct by introducing three silent mutations (+225 C/T, +228 G/A, and +234 G/A) in the shRNA-binding region (SI Appendix, Fig. S2A). The APN-r cDNA or mock vector (negative control) was transfected into B16F10 and LLC APN-shRNA cell clones. Western blots and enzymatic activity assays demonstrated the reversal of functional APN expression in shRNA/APN-r B16F10 and LLC cells without any detectable effect on cell proliferation (SI Appendix, Fig. S2 B–D). To ascertain that tumor growth modulation depended on APN activity in the graft models, we administered shRNA/APN-r B16F10–expressing cell clones into WT and APN-null mice. We detected a significant (P < 0.02) restoration of tumor growth in the WT mice, whereas APN-null animals had a 10-fold lower tumor weight (SI Appendix, Fig. S3 A and B). Similarly, tumor growth was increased for shRNA/APN-r LLC grafts (SI Appendix, Fig. S3 D and E). By dissecting tumor cohorts of comparable sizes (∼250 mm3) from individual groups and measuring enzymatic activity, we confirmed restoration of APN activity in tumor cells expressing APN-r in each model (SI Appendix, Fig. S3 C and F). These results confirm that APN expression by both cancer cells and the host nonmalignant stromal cells plays an important role in sustaining tumor growth.
Inhibition of Tumor Angiogenesis Is Associated with Loss of APN Enzymatic Activity.
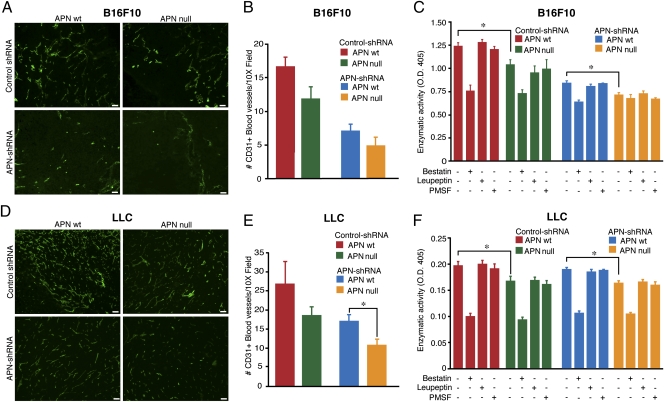
To evaluate whether decreased vascularization resulting from APN deficiency accounts for the observed retardation of tumor growth, we subsequently analyzed tumor vasculature by using antibodies against the endothelial marker CD31 (PECAM-1). We have optimized the analysis for a “middle”-size tumor (∼250 mm3), which avoided or minimized necrosis while still having angiogenic and proteolytic attributes. Immunofluorescence on tumor sections demonstrated that vascular density was decreased in mice lacking APN in either the host or the B16F10 tumor cells. Quantification studies demonstrated that, although lack of host APN had a relatively modest effect, APN knockdown in tumor cells was more severe, with an approximately threefold reduction in microvascular density (Fig. 2B). The combined APN loss in both host and tumor cells produced a more dramatic vascular defect (Fig. 2A), with virtually no CD31-positive blood vessels detected in tumors in the absence of APN in both host and tumor cells. Although large, tortuous blood vessels were numerous when both malignant and host cells expressed APN, loss of APN in either compartment reduced not only blood vessel density but also blood vessel size (SI Appendix, Fig. S4A). A clear difference of blood vessel density was observed in the LLC model (Fig. 2 D and E and SI Appendix, Fig. S4B). Anti-APN immunofluorescence was reduced, as expected, in tumors when the APN gene was deleted or knocked down in malignant cells (SI Appendix, Fig. S4 A and B). Finally, APN detection was located in tumor cells and was associated almost only with CD31-positive endothelial cells in control-shRNA tumors (SI Appendix, Fig. S5 A and B).
Fig. 2.
Reduction of blood vessel density in tumors correlates with reduction of APN activity. (A and D) Immunofluorescence analysis of sections from B16F10 or LLC tumors (as indicated) collected at the study end point was performed with anti-CD31 and secondary FITC-conjugated antibodies (green). (Scale bar, 100 μm.) (B and E) Quantification of blood vessel numbers by manual counting (16 randomly chosen 10× fields per group) in APN WT and APN knockout (null) mice injected with APN knockdown (shRNA) or control-shRNA. (C and F) Enzymatic activity of APN in protein extracts derived from APN WT and APN-null mice carrying control and APN-shRNA B16F10 tumors. Soluble tumor protein extracts were incubated with l-leucine-p-nitroanilide substrate in the presence (+) or absence (−) of enzymatic inhibitors (bestatin, leupeptin, and PMSF). The bars represent mean values of enzymatic activity ± SEM from triplicates (*P < 0.03).
Extracellular proteases participate in angiogenesis by degrading extracellular matrix proteins (ECM) and/or by producing peptides with angiogenic properties; therefore, the enzymatic activity is thought to be central for tumor growth and metastasis. To study this aspect, we surgically dissected tumors derived from B16F10 and LLC cells when they reached a volume of 250 mm3 and performed enzymatic activity assays for APN. We found a significant (P < 0.03) reduction in substrate cleavage in control-shRNA tumors obtained from the APN-null mice compared with WT mice (Fig. 2 C and F), indicating that APN expressed by host cells is enzymatically active within the tumor microenvironment and is sufficient to support angiogenesis, at least partially. Likewise, APN expression by malignant cells only in the APN-null background was enough for this residual APN activity to support baseline angiogenesis. When both tumor and host cells lacked APN, enzymatic activity was decreased to the minimal background levels observed upon its blockade with the aminopeptidase inhibitor bestatin (Fig. 2 C and F). Thus, APN expressed both by tumor malignant cells and by nonmalignant microenvironment stromal cells contributes to APN's net extracellular enzymatic activity, which is required for efficient neovascularization and tumor growth.
Functional APN Promotes Experimental Lung Metastasis.
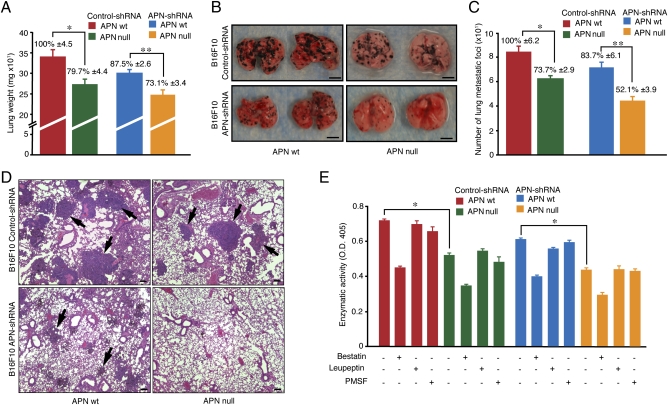
Metastasis is a complex multistep process during which malignant cells disseminate to distant organs through the systemic circulation (21, 22). Expression of APN has been previously associated with tumor invasion and metastasis (26, 27). However, the relative effects of cancer cell-expressed and host-expressed APN on tumor cell dissemination have not been previously compared. Through enzymatic activity assays and immunofluorescence, we demonstrated that normal lungs express functional APN (SI Appendix, Fig. S6 A and B), a result suggesting that lungs might be an appropriate organ to test the effects of host and tumor APN on metastasis. We evaluated the formation of pulmonary metastases in the lungs of APN-null mice after i.v. administration of either B16F10 shRNA or APN-positive cells. After 19 d, there was a significant (P < 0.01) difference in lung weight between control and APN-shRNA cell clones and WT and APN-null mice (Fig. 3A), indicating a clear difference in metastasis burden. Moreover, macroscopic analysis confirmed that APN knockdown cells formed fewer lung metastatic foci than control cells of WT mice, compared with the more significant (P < 0.006) reduction in metastatic colony density observed in APN-null mice (Fig. 3 B and C). Hematoxylin and eosin (H&E) staining served to validate the identity of malignant colonies in the lungs of mice that had received tumor cells intravenously. (Fig. 3D). Importantly, histological analysis confirmed that tumor cells generated not only fewer but also much smaller metastatic foci in the APN-null mice, with B16F10 APN-shRNA further reducing the metastatic burden. Finally, analysis of homogenized lung tissue demonstrated that APN enzymatic activity in the lungs of WT and APN-null mice also correlated with the metastatic burden (Fig. 3E), indicating that APN was active.
Fig. 3.
Disruption of APN expression in tumor and/or nonmalignant stromal cells affects formation of lung metastases. B16F10 melanoma cells (control and APN-shRNA) were injected into a tail vein of WT and APN-null mice. Lungs (n = 5) were dissected 3 wk later. (A) Lung weights are shown as means ± SEM (*P < 0.01; **P < 0.006). (B) Representative photographs of lungs. (Scale bar, 5 mm.) (C) Metastatic foci were counted on lungs of WT and APN-null mice (*P < 0.01; **P < 0.002). (D) Formalin-fixed lung sections were stained with H&E. Black arrows indicate lung metastatic foci. (Scale bar, 100 μm.) (E) Enzymatic activity of APN was evaluated in lungs with metastasis. Bars represent means ± SEM (*P < 0.0001).
To validate the prometastatic function of APN in an independent model, we administered LLC cells expressing control-shRNA or APN-shRNA intravenously into WT and APN-null mice. After 8 wk, the lungs were removed, weighed, fixed, and stained with H&E. Consistent with the B16F10 melanoma model, enzymatically active APN expressed by either host or malignant cells contributed to the formation of metastases (Fig. S6 C–E). Collectively, these findings indicate a functional role for APN enzymatic activity in lung metastases.
Discussion
In this report, we generated B16F10 and LLC cancer cell lines stably expressing shRNA that efficiently abrogated APN translation, allowing us to dissect possible roles of alternative enzyme sources in WT and APN-null mice. We excluded the likelihood that the lentivirus carrier of the shRNA was generating artifacts in the tumor cell clones by showing that tumorigenicity was reestablished by reintroduction of APN resistant to shRNA degradation. Our data demonstrate the main point that both host cells and cancer cells serve as sources of APN that promotes local tumor growth and tumor metastasis to a distant site. Our study is based on syngeneic tumor models in APN-null mice showing that nonmalignant stromal cells in the local microenvironment and malignant tumor cells cooperate in establishing the APN activity levels that regulate tumor angiogenesis, growth, and metastatic cell survival at a secondary site.
Our results add a relevant contribution to the body of evidence for the role of APN in genesis of pathological vasculature (28). We have previously reported that APN is expressed in tumor blood vessels and is necessary for pathological angiogenesis (15, 18, 19, 23), a function also confirmed by others (8–14). Reduction in angiogenesis likely accounts for the tumor growth defect resulting from enzyme inactivation. APN is expressed not only by endothelial cells, but also by pericytes and myeloid and mesenchymal stromal cells (29–34), all of which are central components of the tumor microenvironment.
Our future goals will include the identification (i) of various cell components with functional APN activity and (ii) of native substrates that might promote tumor growth and metastasis. In addition, one might be able to explore the genetic expression of either alternative aminopeptidase family members or other proteases that might functionally compensate for the lack of APN enzymatic activity in the host and tumor cell compartment.
Previous reports have shown that APN cleaves ECM proteins (such as entactin and type IV collagen, among others) during cell invasion (35, 36). In addition, to promote cell survival, tumors secrete growth factors to sustain proliferation and stimulate angiogenesis. For example, VEGF induces the early expression of APN in endothelial cells and fibroblast or stromal cells, so that both tumor and nonmalignant components might cooperate in complex tumorigenic and/or angiogenic paracrine-type mechanisms (17).
Relating our work to the clinical importance of APN, analysis of human lung carcinoma tissue revealed that stromal fibroblasts are the most numerous of the APN-positive cells, and their APN expression correlated positively with increased angiogenesis and poor prognosis; similar results were obtained in patients with pancreatic carcinoma (13, 37–39). These observations suggest that tumor cells might secrete growth factors to induce a sustained APN expression in normal lung cells, which thus provide a favorable local environment (i.e., a “niche”) for metastases. Previous reports have shown that tumor cells lacking APN expression have decreased invasive and metastatic capacity (26, 27). Thus, an impaired APN expression in both host and malignant cells might conceivably affect mechanisms to support tumor colony growth in the lungs. Given that the lungs have a dual (bronchial and pulmonary) vasculature with different ligand-receptor targeting attributes (40), the regulation of metastasis through APN may be even more complex. The importance of APN expression by malignant cells in primary tumors and metastases is also supported by clinical studies (9). Recently, APN has been described as a surface marker for tumor-initiating cells in human liver cancer cell lines and in clinical samples (41). Whether APN functions as a marker of tumor-initiating cells in the systems studied in this work remains to be determined.
Previously, bestatin, an APN inhibitor, has been shown to suppress tumor growth in experimental xenograft models (18, 42–44), further suggesting that APN enzymatic activity is important for tumorigenesis. However, bestatin has multipharmacological functions (45), and its activity on other aminopeptidases could also account for tumor growth inhibition. Here, by inactivating APN specifically, we demonstrate unequivocally that activity of APN by both host and grafted malignant cells contributes to cancer progression in a genetically engineered mouse model. Identification of APN substrates implicated in tumor growth and metastasis is another required next step. A number of cytokines, growth factors, and extracellular matrix molecules implicated in angiogenesis have been reported as putative APN substrates (46–55), and testing their possible roles in the models presented here will be the focus of future studies.
To add a further level of complexity when one analyzes tumor versus stroma-derived APN, two functional aspects must be taken into account: (i) differential activity of the various APN isoforms (56, 57) and (ii) accessibility to a circulating ligand (17). Specifically, APN is a heavily glycosylated protein with at least five diverse molecular isoforms corresponding to differences in oligosaccharide composition (56, 57) and/or site-specific expression and accessibility (17), which have thus far been detected in various myeloid cells, epithelia, and tumor-associated vascular endothelium (57, 58). In the work presented here, we did observe a clear correlation between tumor-derived and host-derived APN. Whether such a synergistic effect is quantitative, qualitative, or both remains an open question to be addressed in future studies, but it is certainly conceivable—perhaps even likely—that a complex CD13-mediated interplay in the tumor microenvironment exists. If so, one could speculate that functionally different or site-specific isoforms might be differentially targeted either by ligand motifs [such as asparagine–glycine–arginine (NGR)] (5,18,19) or by certain anti-CD13 monoclonal antibodies (58). Previous studies from our laboratory have demonstrated reduction of tumor growth by targeting an isoform of APN expressed in the tumor vasculature with NGR coupled to doxorubicin in murine models (18–20). These preclinical studies have been followed with phase II/III clinical trials based on NGR genetically fused to tumor necrosis factor α for the treatment of hepatocarcinomas, pleural mesotheliomas, and colorectal cancer (59–64). The APN-null mouse-based model that we have established here will be useful for further investigation of the role of distinct APN isoforms expressed by individual populations of cells in cancer and of their potential relevance for additional medical applications.
Materials and Methods
Tumor Models.
APN heterozygous mice (23) were backcrossed (N4) onto a C57BL/6 genetic background by the use of speed congenics (Genetic Services and MD Anderson Cancer Center). Male 8- to 12-wk-old WT and APN-null mice were injected either subcutaneously or intravenously with 105 B16F10 or LLC cells. Tumor size was measured with a caliper, and ellipsoid volume was calculated as 1/2 × length × width × height (65) every 2–3 d until the mice were killed. The lungs were removed, rinsed with phosphate buffer saline (PBS), and weighed. Black metastatic foci were counted under a binocular dissecting microscope. LLC cells (1 × 105) were injected subcutaneously and intravenously into APN-null and WT mice; animals were killed at 35 and 54 days, respectively. The Institutional Animal Care and Use Committee of the University of Texas M. D. Anderson Cancer Center approved all animal experiments.
Retroviral Transduction.
The lentiviral plasmid encoding APN-short hairpin and vectors pMD2.VSVG, pRSV-Rev, pCCLsin.PPT.hPGK.GFP.Wpre, and pMDLg/pRRE (gift from L. Naldini, “Vita Salute San Raffaele” University, Milan, Italy) were mixed with lipofectamine 2000 (Invitrogen) and added to confluent HEK-293FT cells (Invitrogen). Viral particles in the supernatant were collected 24, 48, and 72 h after transfection, filtered, and centrifuged at 50,000 × g for 4 h at 20 °C. Purified lentiviral particles were superimposed on cells overnight and replaced with complete media for 24 h. Cells were selected with 10 μg/mL of puromycin (Sigma) for 7 d.
Reconstitution of APN Expression.
We used the endotoxin-free Maxiprep kit (Sigma) to purify the APN reconstitution (APN-r) cDNA and mock-expressing vectors. B16F10 and LLC APN-shRNA cell lines were lipofectamine-transfected with APN-r and mock expression vectors. After 3 wk of neomycin selection at 5 mg/mL, single clones expressing APN-r in the B16F10 APN-shRNA and LLC APN-shRNA cells were isolated. APN expression was confirmed by Western blot.
APN Enzymatic Activity Assay.
APN enzymatic activity was measured spectrophotometrically with l-leucine-p-nitroanilide (Peptide Institute) as a substrate. Confluent monolayers of B16F10 and LLC cells were grown in 24-well plates. Tissues were prepared as described (19). The enzymatic inhibitor bestatin (Sigma) was incubated for 30 min at 37 °C before substrate addition. Next, cells were washed with PBS, 1.6 mM of the substrate was added in a final volume of 400 μL, and cells were incubated for 2 h at 37 °C. APN enzymatic activity was estimated by the absorbance at 405 nm in a microplate reader (SpectraMax M5; Molecular Devices).
Statistical Analysis.
All data are presented as means ± SD. Statistical differences between groups were measured by Student's t tests with P < 0.05 deemed as statistically significant.
Supplementary Material
Acknowledgments
We thank C. Sun and L. Bitner for technical assistance. This work was supported by grants from the National Institutes of Health, National Cancer Institute, and Department of Defense (to W.A. and R.P.) and by awards from AngelWorks, the Gilson-Longenbaugh Foundation, and the Marcus Foundation (to W.A. and R.P.). R.R. received support from the Odyssey Scholar Program at the University of Texas MD Anderson Cancer Center.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120790109/-/DCSupplemental.
References
- 1.Dixon J, et al. Expression of aminopeptidase-n (CD 13) in normal tissues and malignant neoplasms of epithelial and lymphoid origin. J Clin Pathol. 1994;47:43–47. doi: 10.1136/jcp.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stange T, Kettmann U, Holzhausen HJ. Immunoelectron microscopic single and double labelling of aminopeptidase N (CD 13) and dipeptidyl peptidase IV (CD 26) Acta Histochem. 1996;98:323–331. doi: 10.1016/S0065-1281(96)80025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mina-Osorio P. The moonlighting enzyme CD13: Old and new functions to target. Trends Mol Med. 2009;9:449–456. doi: 10.1016/j.molmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Favaloro EJ, Moraitis N, Bradstock K, Koutts J. Co-expression of haemopoietic antigens on vascular endothelial cells: A detailed phenotypic analysis. Br J Haematol. 1990;74:385–394. doi: 10.1111/j.1365-2141.1990.tb06324.x. [DOI] [PubMed] [Google Scholar]
- 5.Ashmun RA, Look AT. Metalloprotease activity of CD13/aminopeptidase N on the surface of human myeloid cells. Blood. 1990;75:462–469. [PubMed] [Google Scholar]
- 6.Razak K, Newland AC. Induction of CD13 expression on fresh myeloid leukaemia: Correlation of CD13 expression with aminopeptidase-N activity. Leuk Res. 1992;16:625–630. doi: 10.1016/0145-2126(92)90012-v. [DOI] [PubMed] [Google Scholar]
- 7.Shipp MA, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: Cutting is the key! Blood. 1993;82:1052–1070. [PubMed] [Google Scholar]
- 8.Bogenrieder T, et al. Expression and localization of aminopeptidase A, aminopeptidase N, and dipeptidyl peptidase IV in benign and malignant human prostate tissue. Prostate. 1997;33:225–232. doi: 10.1002/(sici)1097-0045(19971201)33:4<225::aid-pros1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 9.Kehlen A, Lendeckel U, Dralle H, Langner J, Hoang-Vu C. Biological significance of aminopeptidase N/CD13 in thyroid carcinomas. Cancer Res. 2003;63:8500–8506. [PubMed] [Google Scholar]
- 10.Röcken C, Licht J, Roessner A, Carl-McGrath S. Canalicular immunostaining of aminopeptidase N (CD13) as a diagnostic marker for hepatocellular carcinoma. J Clin Pathol. 2005;58:1069–1075. doi: 10.1136/jcp.2005.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terauchi M, et al. Inhibition of APN/CD13 leads to suppressed progressive potential in ovarian carcinoma cells. BMC Cancer. 2007;7:140. doi: 10.1186/1471-2407-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teranishi J, et al. Evaluation of role of angiotensin III and aminopeptidases in prostate cancer cells. Prostate. 2008;68:1666–1673. doi: 10.1002/pros.20835. [DOI] [PubMed] [Google Scholar]
- 13.Ito S, et al. Stromal aminopeptidase N expression: Correlation with angiogenesis in non-small-cell lung cancer. Gen Thorac Cardiovasc Surg. 2009;57:591–598. doi: 10.1007/s11748-009-0445-x. [DOI] [PubMed] [Google Scholar]
- 14.Di Matteo P, et al. Enhanced expression of CD13 in vessels of inflammatory and neoplastic tissues. J Histochem Cytochem. 2011;59:47–59. doi: 10.1369/jhc.2010.956644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhagwat SV, et al. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood. 2001;97:652–659. doi: 10.1182/blood.v97.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Ozawa MG, et al. Beyond receptor expression levels: The relevance of target accessibility in ligand-directed pharmacodelivery systems. Trends Cardiovasc Med. 2008;18:126–132. doi: 10.1016/j.tcm.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Pasqualini R, et al. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60:722–727. [PMC free article] [PubMed] [Google Scholar]
- 19.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 20.Khakoo AY, Sidman RL, Pasqualini R, Arap W. Does the renin-angiotensin system participate in regulation of human vasculogenesis and angiogenesis? Cancer Res. 2008;68:9112–9115. doi: 10.1158/0008-5472.CAN-08-0851. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 22.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangel R, et al. Impaired angiogenesis in aminopeptidase N-null mice. Proc Natl Acad Sci USA. 2007;104:4588–4593. doi: 10.1073/pnas.0611653104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolson GL, Brunson KW, Fidler IJ. Specificity of arrest, survival, and growth of selected metastatic variant cell lines. Cancer Res. 1978;38:4105–4111. [PubMed] [Google Scholar]
- 25.van Lamsweerde AL, Henry N, Vaes G. Metastatic heterogeneity of cells from Lewis lung carcinoma. Cancer Res. 1983;43:5314–5320. [PubMed] [Google Scholar]
- 26.Fujii H, et al. Human melanoma invasion and metastasis enhancement by high expression of aminopeptidase N/CD13. Clin Exp Metastasis. 1995;13:337–344. doi: 10.1007/BF00121910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fontijn D, et al. CD13/aminopeptidase N overexpression by basic fibroblast growth factor mediates enhanced invasiveness of 1F6 human melanoma cells. Br J Cancer. 2006;94:1627–1636. doi: 10.1038/sj.bjc.6603157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luan Y, Xu W. The structure and main functions of aminopeptidase N. Curr Med Chem. 2007;14:639–647. doi: 10.2174/092986707780059571. [DOI] [PubMed] [Google Scholar]
- 29.Alliot F, Rutin J, Leenen PJM, Pessac B. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J Neurosci Res. 1999;58:367–378. [PubMed] [Google Scholar]
- 30.Cowburn AS, et al. Aminopeptidase N (CD13) regulates tumor necrosis factor-alpha-induced apoptosis in human neutrophils. J Biol Chem. 2006;281:12458–12467. doi: 10.1074/jbc.M511277200. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 32.Mund JA, Case J. The role of circulating endothelial progenitor cells in tumor angiogenesis. Curr Stem Cell Res Ther. 2011;6:115–121. doi: 10.2174/157488811795495468. [DOI] [PubMed] [Google Scholar]
- 33.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 35.Menrad A, Speicher D, Wacker J, Herlyn M. Biochemical and functional characterization of aminopeptidase N expressed by human melanoma cells. Cancer Res. 1993;53:1450–1455. [PubMed] [Google Scholar]
- 36.Saiki I, et al. Role of aminopeptidase N (CD13) in tumor-cell invasion and extracellular matrix degradation. Int J Cancer. 1993;54:137–143. doi: 10.1002/ijc.2910540122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ichimura E, Yamada M, Nishikawa K, Abe F, Nakajima T. Immunohistochemical expression of aminopeptidase N (CD13) in human lung squamous cell carcinomas, with special reference to Bestatin adjuvant therapy. Pathol Int. 2006;56:296–300. doi: 10.1111/j.1440-1827.2006.01963.x. [DOI] [PubMed] [Google Scholar]
- 38.Tokuhara T, et al. Clinical significance of aminopeptidase N in non-small cell lung cancer. Clin Cancer Res. 2006;12:3971–3978. doi: 10.1158/1078-0432.CCR-06-0338. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda N, et al. Clinical significance of aminopeptidase N/CD13 expression in human pancreatic carcinoma. Clin Cancer Res. 2003;9:1503–1508. [PubMed] [Google Scholar]
- 40.Oh Y, et al. Phenotypic diversity of the lung vasculature in experimental models of metastases. Chest. 2005;128(6, Suppl):596S–600S. doi: 10.1378/chest.128.6_suppl.596S. [DOI] [PubMed] [Google Scholar]
- 41.Haraguchi N, et al. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010;120:3326–3339. doi: 10.1172/JCI42550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoi K, et al. Aminopeptidase inhibitor ubenimex (bestatin) inhibits the growth of human choriocarcinoma in nude mice through its direct cytostatic activity. Anticancer Res. 1995;15(5B):2081–2087. [PubMed] [Google Scholar]
- 43.Imamura N, Kimura A. Effect of ubenimex (Bestatin) on the cell growth and phenotype of HL-60 and HL-60R cell lines: Up- and down-regulation of CD13/aminopeptidase N. Leuk Lymphoma. 2000;37:663–667. doi: 10.3109/10428190009058523. [DOI] [PubMed] [Google Scholar]
- 44.Tsukamoto H, et al. Aminopeptidase N (APN)/CD13 inhibitor, Ubenimex, enhances radiation sensitivity in human cervical cancer. BMC Cancer. 2008;8:74. doi: 10.1186/1471-2407-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathé G. Bestatin, an aminopeptidase inhibitor with a multi-pharmacological function. Biomed Pharmacother. 1991;45:49–54. doi: 10.1016/0753-3322(91)90122-a. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, Wellner D, Scheinberg DA. Substance P and bradykinin are natural inhibitors of CD13/aminopeptidase N. Biochem Biophys Res Commun. 1995;208:664–674. doi: 10.1006/bbrc.1995.1390. [DOI] [PubMed] [Google Scholar]
- 47.Fortin JP, et al. Endogenous aminopeptidase N decreases the potency of peptide agonists and antagonists of the kinin B1 receptors in the rabbit aorta. J Pharmacol Exp Ther. 2005;314:1169–1176. doi: 10.1124/jpet.105.088799. [DOI] [PubMed] [Google Scholar]
- 48.Danziger RS. Aminopeptidase N in arterial hypertension. Heart Fail Rev. 2008;13:293–298. doi: 10.1007/s10741-007-9061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann T, Faust J, Neubert K, Ansorge S. Dipeptidyl peptidase IV (CD 26) and aminopeptidase N (CD 13) catalyzed hydrolysis of cytokines and peptides with N-terminal cytokine sequences. FEBS Lett. 1993;336:61–64. doi: 10.1016/0014-5793(93)81609-4. [DOI] [PubMed] [Google Scholar]
- 50.Proost P, et al. Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood. 2007;110:37–44. doi: 10.1182/blood-2006-10-049072. [DOI] [PubMed] [Google Scholar]
- 51.Kanayama N, et al. Inactivation of interleukin-8 by aminopeptidase N (CD13) J Leukoc Biol. 1995;57:129–134. doi: 10.1002/jlb.57.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kehlen A, et al. Increased expression of interleukin-8 and aminopeptidase N by cell-cell contact: Interleukin-8 is resistant to degradation by aminopeptidase N/CD13. Eur Cytokine Netw. 2001;12:316–324. [PubMed] [Google Scholar]
- 53.Palter SF, Mulayim N, Senturk L, Arici A. Interleukin-8 in the human fallopian tube. J Clin Endocrinol Metab. 2001;86:2660–2667. doi: 10.1210/jcem.86.6.7584. [DOI] [PubMed] [Google Scholar]
- 54.Seli E, Senturk LM, Bahtiyar OM, Kayisli UA, Arici A. Expression of aminopeptidase N in human endometrium and regulation of its activity by estrogen. Fertil Steril. 2001;75:1172–1176. doi: 10.1016/s0015-0282(01)01779-4. [DOI] [PubMed] [Google Scholar]
- 55.Tagore DM, et al. Peptidase substrates via global peptide profiling. Nat Chem Biol. 2009;5:23–25. doi: 10.1038/nchembio.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Connell PJ, Gerkis V, d'Apice AJ. Variable O-glycosylation of CD13 (aminopeptidase N) J Biol Chem. 1991;266:4593–4597. [PubMed] [Google Scholar]
- 57.van Hensbergen Y, et al. Reduced growth, increased vascular area, and reduced response to cisplatin in CD13-overexpressing human ovarian cancer xenografts. Clin Cancer Res. 2004;10:1180–1191. doi: 10.1158/1078-0432.ccr-0482-3. [DOI] [PubMed] [Google Scholar]
- 58.Curnis F, et al. Differential binding of drugs containing the NGR motif to CD13 isoforms in tumor vessels, epithelia, and myeloid cells. Cancer Res. 2002;62:867–874. [PubMed] [Google Scholar]
- 59.Curnis F, et al. Enhancement of tumor necrosis factor α antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13) Nat Biotechnol. 2000;18:1185–1190. doi: 10.1038/81183. [DOI] [PubMed] [Google Scholar]
- 60.Zarovni N, Monaco L, Corti A. Inhibition of tumor growth by intramuscular injection of cDNA encoding tumor necrosis factor alpha coupled to NGR and RGD tumor-homing peptides. Hum Gene Ther. 2004;15:373–382. doi: 10.1089/104303404322959524. [DOI] [PubMed] [Google Scholar]
- 61.Corti A, Ponzoni M. Tumor vascular targeting with tumor necrosis factor alpha and chemotherapeutic drugs. Ann N Y Acad Sci. 2004;1028:104–112. doi: 10.1196/annals.1322.011. [DOI] [PubMed] [Google Scholar]
- 62.Corti A, Curnis F, Arap W, Pasqualini R. The neovasculature homing motif NGR: More than meets the eye. Blood. 2008;112:2628–2635. doi: 10.1182/blood-2008-04-150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corti A, Giovannini M, Belli C, Villa E. Immunomodulatory agents with antivascular activity in the treatment of non-small cell lung cancer: Focus on TLR9 agonist, IMiDS and NGR-TNF. J Oncol. 2010 doi: 10.1155/2010/732680. 732680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marcucci F, Corti A. How to improve exposure of tumor cells to drugs: Promoter drugs increase tumor uptake and penetration of effector drugs. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.09.007. in press. [DOI] [PubMed] [Google Scholar]
- 65.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.