Abstract
Epidermal growth factor receptor (EGFR) controls a wide range of developmental events, from body axes specification in insects to cardiac development in humans. During Drosophila oogenesis, a gradient of EGFR activation patterns the follicular epithelium. Multiple transcriptional targets of EGFR in this tissue have been identified, but their regulatory elements are essentially unknown. We report the regulatory elements of broad (br) and pipe (pip), two important targets of EGFR signaling in Drosophila oogenesis. br is expressed in a complex pattern that prefigures the formation of respiratory eggshell appendages. We found that this pattern is generated by dynamic activities of two regulatory elements, which display different responses to Pointed, Capicua, and Mirror, transcription factors involved in the EGFR-mediated gene expression. One of these elements is active in a pattern similar to pip, a gene repressed by EGFR and essential for establishing the dorsoventral polarity of the embryo. We demonstrate that this similarity of expression depends on a common sequence motif that binds Mirror in vitro and is essential for transcriptional repression in vivo.
Keywords: gene regulation, patterning, follicle cells
Epidermal growth factor receptor (EGFR) controls multiple developmental processes, including body axes specification in insects (1), vulval patterning in nematodes (2), skin pigmentation in fish (3), and cardiac development in humans (4). Drosophila oogenesis is one of the most extensively studied models of EGFR signaling in development. In this case, a gradient of EGFR activation is established by Gurken (GRK), a ligand that is secreted from the oocyte and signals through receptors in the follicular epithelium (5). Acting in concert with other pathways, EGFR controls region-specific expression of multiple genes involved in patterning of the Drosophila eggshell, a complex structure that holds inductive cues necessary for body axes specification during embryogenesis (6). Previous studies identified several transcription factors coordinating EGFR-mediated gene expression in the follicle cells (7–11). However, the regulatory regions of the EGFR-target genes are essentially unknown, a fact that complicates rigorous evaluation of proposed mechanisms (12–14). Here, we report the regulatory regions of broad (br) and pipe (pip), two important targets of EGFR signaling in oogenesis.
br encodes a Zn-finger transcription factor involved in multiple aspects of tissue morphogenesis in Drosophila and other insects. During oogenesis, br is expressed in a dynamic pattern that foreshadows the formation of two respiratory eggshell appendages (15–17). We demonstrate that this pattern is generated by two regulatory regions, which have different spatiotemporal activities and display differential sensitivity to transcription factors acting downstream of EGFR. Specifically, Pointed (PNT), an ETS-family transcription factor that mediates EGFR-dependent repression of br (8, 10, 12, 18), affects only one of the identified regulatory elements. On the other hand, Mirror (MIRR), an Iroquois transcription factor, which is essential for br regulation (7, 8), controls both of these regions, activating one and repressing the other.
Earlier studies established that EGFR cell-autonomously represses pip, a gene essential for transmitting the dorsoventral (DV) polarity of the egg to the embryo (19, 20). A gradient of EGFR activation by GRK generates a pattern in which pip is repressed in the follicle cells exposed to high and intermediate levels of EGFR signaling. We noticed that this pattern is similar to the activity of one of the identified regulatory regions of br. Based on this similarity, we identified a common sequence motif in the regulatory regions of both genes. This motif binds MIRR in vitro and is essential for pip repression in vivo. Thus, we identified a key regulatory element in the patterning event that ultimately controls germ layer specification in the embryo.
The article is organized as follows: First, we describe unbiased reporter studies that identified the two regulatory elements of br, which we call brE and brL. Second, we present the results of genetic mosaic experiments that revealed how these elements respond to transcription factors acting downstream of EGFR in the follicle cells. Third, based on sequence comparison analysis, we propose that similar expression patterns of brE and pip depend on a common sequence motif. Fourth, we support this hypothesis by protein/DNA binding studies and transcriptional reporter assays.
Results
br Is Regulated by Two Distinct Enhancers.
During the intermediate stages of oogenesis, br is expressed in all oocyte-associated follicle cells (12, 16, 18). Subsequently, anterior expression is lost in cells of the dorsal midline, which are exposed to the highest level of EGFR activation (Fig. 1 B–B′′ and H, Bottom). At the same time, levels of br begin to increase in two lateral groups of follicle cells and decrease in the rest of the follicular epithelium, establishing a pattern with two br expression domains. This two-domain pattern foreshadows the formation of two respiratory eggshell appendages.
Fig. 1.
br expression is regulated by two cis-regulatory modules. (A) Schematic of the genomic locus of br with genomic fragments used to generate transgenic reporter constructs depicted as bars. Gray bars indicate fragments with no enhancer activity during oogenesis and black bars denote fragments which activate patterned reporter gene expression (br4 and br6). Fragments brL and brE, used in all subsequent experiments, are shown in green and red, respectively. (B–E′′) BR protein expression compared with the expression of brE-lacZ and brL-EGFP reporters in egg chambers at stages 9, 10A, and 10B (lateral views, dorsal side up). Samples were stained with anti-BR antibody (magenta), anti–β-Gal antibody (red), anti-GFP antibody (green), and DAPI (blue) to visualize nuclei. Panels E–E′′ are merged images of brE-lacZ and brL-GFP reporter staining. (B–B′′) At stage 9, uniform BR expression (B) is mediated by brE (C), but the brL reporter is silent (D). (B′–D′) At stage 10A, BR is cleared from the dorsal midline domain (B′). This pattern is formed by the combination of loss of brE-reporter expression in a wide dorsal domain (C′) and brL activating reporter expression in two distinct dorsolateral patches within the clearance of brE. (D′). At stage 10B, higher levels of BR are visible in the patches (B′′) produced by increasing activity of brL (D′′). (F–G′′) Reporter expression controlled by direct fusion of brL and brE fragments (brLE) fully recapitulates the dynamics of BR expression. Double immunostaining for brLE reporter expression (brLE-LacZ, anti–β-Gal antibody) and BR protein (αBR antibody). Nuclei stained by DAPI. Magnification, 20×. (H) Summary of the spatial and temporal contribution of the two CRMs to the dynamic changes of BR expression as derived from the profiling shown in B–G′′ and Fig. S1 (dorsal view). At any time point of egg shell development, expression of BR (magenta) is the sum of the expression activated by brE (red) and brL (green).
Because EGFR is a key regulator of br expression in follicle cells, it is possible that dynamic changes of br expression, from uniform to the two-domain patterns, reflect previously reported dynamic changes of EGFR activation (21–23). In the simplest case, patterns of br expression could be generated by a single cis-regulatory module (CRM), which responds to changes in the spatial pattern of EGFR signaling. Alternatively, br expression dynamics can reflect activities of two or more distinct CRMs. To explore these possibilities, we undertook an unbiased reporter analysis to identify cis-regulatory regions that account for br expression during oogenesis.
In the first round of experiments, six partially overlapping fragments covering ∼35 kb upstream of the br coding sequence were used to generate lacZ reporter constructs and assayed for transcriptional activity in transgenic flies (Fig. 1A). We found that two of these fragments, br4 and br6, are active in the follicle cells. Similar to the early phase of the endogenous pattern of br, the br6 region is first active in all oocyte associated follicle cells and then repressed in the dorsal region of the follicular epithelium. On the other hand, the br4 region is active at later stages of oogenesis, in a pattern that is similar to the later, two-domain pattern of br. These fragments were shortened and used for a more detailed analysis of the transcriptional activity. We called the identified fragments brL (br-Late) and brE (br-Early), respectively, based on their temporal expression during oogenesis.
To compare the activity of the reporters simultaneously, GFP versions of the reporter constructs were generated and brE-lacZ was combined with brL-GFP in the same fly (Fig. 1 B–E). At early stages of oogenesis, the expression driven by brE-lacZ is uniform; later, at stage 10A, reporter activity disappears in a dorsal region of the follicular epithelium, which corresponds to high and intermediate levels of EGFR activation by GRK (Fig. 1 C–C′′). As the dorsal domain of the early pattern starts to disappear, brL activates GFP-reporter expression in two patches, corresponding to the late pattern of BR protein (Fig. 1 D–D′′ and E–E′′).
To follow the activity patterns of the brL and brE regions with higher temporal resolution, we compared the transcript dynamics of both reporters to the spatiotemporal pattern of br mRNA (Fig. S1). Two differences became apparent from this analysis: First, the transcript driven by brE disappears from the dorsal domain before the expression in the roof domain is initiated by brL at stage 10A (Fig. S1 A–A′′′). Second, the early phase of expression regulated by brE ceases completely by stage 10B, but the lacZ transcript driven by brL persists until later stages of oogenesis (Fig. S1 C–C′′′). Apparently, the stability of the reporter protein masks temporal and spatial separation between the two phases of br expression driven by different enhancers.
Based on these observations, we conclude that the spatial pattern of br is generated by the dynamic superposition of two regulatory regions. To test whether these regions act independently, we produced a head-to-tail fusion of the two fragments (brLE) and analyzed the activity of the construct in transgenic flies. The brLE-lacZ reporter recapitulates remarkably well the expression of BR protein, including the difference in expression levels between the roof and ventral domains (Fig. 1 F–G′′ and Fig. S1 E–E′′′). Thus, the two regions can be viewed as independent modules with additive regulatory properties (Fig. 1H).
brE and brL Regions Are Differentially Regulated by PNT, MIRR, and Capicua.
EGFR-dependent control of br relies on three key transcription factors: Capicua (CIC), PNT, and MIRR. CIC, an HMG-box transcriptional repressor, is degraded in a wide dorsal region of the follicular epithelium (7, 24). This process results in derepression of MIRR, an Iroquois transcription factor, which is necessary for inducing high levels of br and subsequent formation of respiratory eggshell appendages (7). In the midline follicle cells, corresponding to the high levels of EGFR activation, br is repressed by PNT, an ETS-transcription factor (8, 10). As a result, br is expressed at the intermediate levels of EGFR signaling: above the level necessary for derepression of MIRR and below the level required for induction of PNT (18). This model was established on the basis of genetic mosaic experiments, which used the spatial pattern of BR protein to analyze the effects of removal or ectopic expression of CIC, PNT, and MIRR. We revisited this model, using transcriptional reporters for br expression.
Previous results, reproduced in our study (Fig. 2 A and B), established that genetic removal of PNT leads to ectopic expression of BR protein in the midline follicle cells (8, 12). Because neither brE nor brL are normally expressed in these cells at stage 10B of oogenesis, one can expect that PNT represses either one or both of the regulatory regions. We found that removal of PNT in this area generates ectopic activity of brL (Fig. 2B), but has no effect on the brE activity (Fig. 2A). Thus, PNT controls the BR pattern by regulating only brL, the late regulatory element of br.
Fig. 2.
Effectors of EGFR signaling regulate brE and brL differentially. (A–F′′′ and G) Immunostaining for GFP (green, A–F), β-Gal (red, A′–F′), and BR (gray, A′′′–F′′′) of stage 10B egg chambers carrying either brE-LacZ or brL-LacZ reporter (A–F). Mutant clones are marked by the loss of GFP. Yellow lines have been added to mark clone boundaries relevant for this analysis. G schematically summarizes the results of the mosaic experiments and illustrate the effects of clonal inactivation of pnt, mirr, and cic on the expression of brE, brL, and BR (clones represented within dotted back lines) (A–B′′′) pntΔ86 clones in the dorsal midline domain did not affect brE (29 of 30 clones), but produced ectopic expression of brL (10 of 11 clones). White arrowheads mark the position of the dorsal midline; dorsal views are shown. (C–D′′′) mirr1825 clones in dorsal follicle cells produced ectopic expression of brE (C and C′) (22 of 23 clones), and loss of brL expression in the appendage forming cells (D and D′) (26 of 28 clones). (C′′ and D′′) GFP and β-Gal merged images show enlarged views of the anterior domain (clone areas 1 in both C and D). Dorsal views are shown. (E–F′′′) cicfetU6 clones in ventral anterior follicle cells produced loss of brE (109 of 115 clones; clone 2 in E), and ectopic expression of brL (11 of 11 clones; clone 2 in F), but dorsal anterior clones did not produce misexpression (clone 1 in E, and clone 1 in F). These effects were restricted to the anterior half of the egg chamber, as shown in clones spanning the anterior-posterior prepatterning boundary (clone 3 in E, and clone 2 in F; see also schematic in G). brL expression in cic clones was still suppressed in the anteriormost two to three rows of cells (arrowhead in F′′), potentially by the Dpp pathway. (E′′ and F′′) GFP and β-Gal merged images show enlarger views of the ventral anterior domain (lower left section in E and F, respectively). Lateral views with dorsal side up are shown; magnification, 20×. (H) Summary of the effects of EGFR signaling on the two CRMs of br. PNT acts solely on brL to repress its expression at dorsal most cells. MIRR, which becomes activated by EGFR signaling through the repression of CIC, represses brE and activates brL.
High levels of br expression in two dorsolateral groups of the follicle cells that contribute to the formation of the future eggshell appendages depend on MIRR (Fig. S2) (7). MIRR expression is de-repressed by EGFR, resulting in a dorsal pattern that complements the activity of the brE region and contains the domain where the brL region is active. Using genetic mosaic experiments, we found that loss of mirr has opposite effects on the transcriptional activity of the brE and brL elements. Specifically, mirr clones induced in the dorsal follicle cells led to ectopic expression of brE (Fig. 2C). On the other hand, the same genetic perturbation led to loss of brL activity in the cells that correspond to the future dorsal appendages (Fig. 2D). These observations reveal that MIRR controls br through two different regulatory regions, one of which is activated and the other repressed by this transcription factor (Fig. 2G).
Following a similar strategy, we found that the effects of CIC on the activity of the brE and brL regions are consistent with the previously proposed model where CIC down-regulation is required for mirr expression in the anterior half of the oocyte-associated follicle cells (7). When cic mutant clones are located in the ventral-anterior region of the follicular epithelium, the activity of the brE region is repressed, whereas the brL region is activated ectopically (Fig. 2 E and F). Thus, in addition to displaying different activity patterns, the two regulatory regions of br are differentially controlled by CIC, MIRR, and PNT (Fig. 2H). Based on these results, we propose that the wild-type pattern of br is generated as follows: the early phase of br repression in the dorsal part of the follicular epithelium cells requires MIRR, but is independent of PNT. On the other hand, the late pattern of br is activated by MIRR and split in the dorsal midline by PNT.
Comparative Sequence Analysis Identifies a cis-Element Essential for pip Repression.
The spatial pattern of brE activity is very similar to the expression pattern of another EGFR target in dorsal follicle cells, pipe (pip) (14, 25, 26). This similarity of expression patterns suggested that transcriptional control of pip and brE elements depends on the same mode of regulation involving similar cis-regulatory sequences. As a first step toward testing this hypothesis, we used computational analysis to compare the sequences of the brE region and pipA, an 8-kb fragment that was shown to recapitulate the wild-type pattern of pip expression (19). Specifically, we compared a 1-kb long subfragment of brE, which is sufficient to generate the early expression pattern of br (Fig. S3), with either the intronic part of pipA (5 kb) or the 3-kb subfragment located upstream of the transcriptional start of the pip gene.
We reasoned that if a common regulatory motif exists, it should be conserved. Thus, our comparative analysis included sequence information from other available Drosophila genomes (see Materials and Methods for details). Consistent with our hypothesis, we identified a number of highly conserved DNA segments that are present in either brE or in the two pip fragments from different Drosophila species. One of these fragments, an A/T-rich 50-bp DNA sequence, is present in both brE element and in the 3-kb fragment of pipA (Fig. 3B). In the rest of the article, we focus on the role of this motif in the EGFR-mediated regulation of pip.
Fig. 3.
A short DNA sequence mediates dorsal repression of pip. (A) Genomic locus of pip with exons indicated in dark blue and intronic sequences in light blue pip_up and pip_down fragments used to generate reporter constructs are shown in red and gray, respectively. pipA (black bar) is the previously reported regulatory region of pip. The position of the identified MRE in pip_up is indicated by the arrowhead. (B) Sequence logo of the evolutionarily conserved motif present in both brE and pip_up. (C–D′) Reporter activity of stage 10 egg chambers (lateral view, dorsal up) from flies carrying either a pip_up-lacZ transgene (C) or a version of pip_up-lacZ lacking the 50-bp motif pip_upΔMRE (D) assessed by β-Gal immunostaining. Nuclei were stained by DAPI (C′ and D′); magnification, 20×. Although pip_up activates reporter expression in a pattern indistinguishable to that of endogenous pip, deletion of the motif results in ectopic expression of the reporter in dorsal follicle cells.
The identified motif enabled us to narrow down the regulatory sequence of pip. We established that the 3-kb subfragment of the pipA region (pip_up), containing solely the region of pipA upstream to the transcriptional start of the pip gene, and including the identified 50-bp sequence, captures all aspects of pip expression (Fig. 3C). In contrast, the 5-kb intronic fragment of pipA failed to produce any expression pattern in follicle cells. Thus, cis-regulatory information controlling pip expression resides within the 3-kb fragment centered on the identified 50-bp sequence. To directly test the role of this sequence for pip regulation, we deleted it from the pip_up region and tested its transcriptional activity in vivo. Reporter expression under the control of this fragment is drastically expanded, and is detected even in dorsal-most cells (Fig. 3D). Thus, the identified 50-bp sequence is essential for restricting the pip expression to the ventral follicle cells.
Repression of the Identified Regulatory Element Depends on Direct Binding of MIRR.
As was shown in Fig. 2C, restriction of brE activity to the ventral follicle cells depends on repression of this regulatory region by MIRR. Based on the similarity of brE and pip expression patterns, the presence of a common sequence motif, and the earlier studies of pip regulation, we hypothesized that MIRR also represses pip. To test this hypothesis, we expressed MIRR in marked clones of follicle cells and examined the effect on the spatial pattern of our pip_up reporter. Indeed, and similar to the effects on brE (Fig. 4A), ectopic MIRR fully repressed expression of pip_up (Fig. 4 B and C). This effect was evident both in clones abutting the endogenous domain of mirr (Fig. 4B) and in the ventral clones within the endogenous pip expression domain (Fig. 4C). Importantly, similar to the previously reported effects of the EGFR signaling (14, 25, 26), repression of pip by ectopic MIRR was strictly cell autonomous. These results demonstrate that MIRR can cell-autonomously repress pip.
Fig. 4.
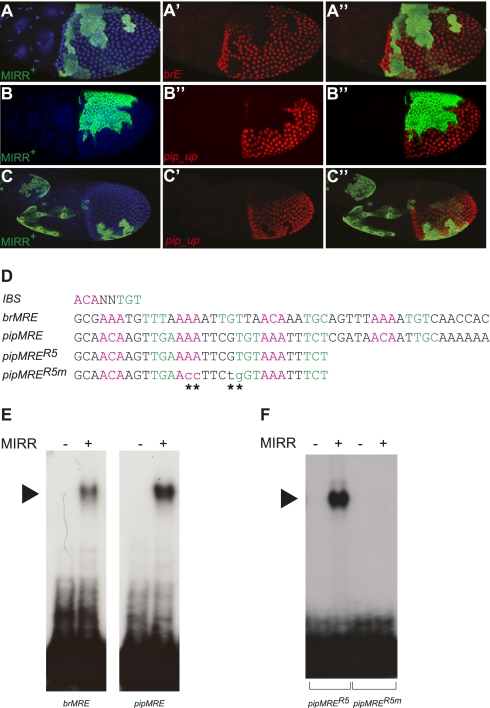
MIRR represses pip through direct binding to a MRE. (A–C) Lateral view of stage 10 egg chambers expressing MIRR in clones marked by coexpression of GFP (nuclei are shown in blue; magnification, 20×). Ectopic MIRR expression abolishes both brE expression (in 54 of 59 clones; A′) and pip_up expression (in 53 of 53 clones; B′ and C′, overlay in B′′ and C′′) in a cell-autonomous and position-independent manner. (D) Sequences of the Mirror response elements of br (brMRE) and pip (pipMRE). Nucleotide in green and magenta correspond to the two halves of the palindrome comprising the Iro binding site (IBS; ACANNTGT). pipMRER5 is a subfragment of pipMRE still able to bind to MIRR. pipMRER5m carries four nucleotide exchanges at positions that are conserved between the brMRE and the pipMRE (indicated by the asterisks). (E) EMSA with extracts expressing HA-tagged MIRR (+) or a control protein (−) and radioactively labeled brMRE or pipMRE probes. MIRR/DNA-complexes are indicated with the black arrowhead. (F) EMSA with control (−) or MIRR (+) expressing extracts and radioactively labeled pipMRER5 or pipMRER5m. Note that the four point mutations in pipMRER5m completely abolish MIRR binding.
MIRR belongs to the group of Iroquois (Iro) transcription factors, a highly conserved family of DNA-binding homeodomain proteins involved in a variety of developmental processes. In the few cases analyzed so far, Iro proteins have been assigned transcriptional repressor activity (27), and based on this, we hypothesized that MIRR physically interacts with the identified sequences in br and pip to repress transcription. Indeed, recombinant MIRR readily interacted with both sequences (referred to as MIRR response elements, MRE) in electrophoretic mobility-shift assays (Fig. 4 D and E). Moreover, nucleotide exchanges in positions that are conserved between the two MREs abolished MIRR-complex assembly on pipMRE (Fig. 4 D and F). Thus, binding of MIRR to the identified MRE is specific and depends on the identified sequence motif.
Previous studies suggested that MIRR directly represses target genes through an Iro binding site (IBS), a short sequence identified in an in vitro selection assay (Fig. 4D) (27). The IBS motif comprises a palindrome to which MIRR can bind as a homodimer, probably with each MIRR molecule making contact to one half of the palindrome. Although neither of our MREs contains a perfect IBS, both MREs contain multiple sites that deviate from the reported sequence at single nucleotide positions and at the spacing between the two halves of the palindrome (Fig. 4D). Moreover the mutations in MRE that abolish MIRR-complex formation (Fig. 4F) overlap with these sites, suggesting that MIRR binding to the MRE involves multiple, diversified IBS motifs.
Discussion
Current models of pattern formation in Drosophila oogenesis involve multiple components, signaling pathways, and network motifs (5, 9, 11–13, 28–30). Critical tests of these models require direct analysis of the cis-regulatory sequences of genes comprising the network. As a first step in this direction, we identified the regulatory elements of br, a gene that plays a key role in eggshell patterning and morphogenesis. We established that the dynamic pattern of br is generated by superposition of the activities of two distinct regulatory regions, which drive br expression in nonoverlapping regions of space and display differential sensitivity to three transcription factors that act downstream of EGFR (Fig. 1 B–G′′).
It was shown that loss of MIRR induces ectopic br expression in the dorsal midline follicle cells, but leads to a complete loss of br in the lateral cells, which form dorsal appendages (Fig. 2 C′′′ and D′′′) (8). This region-specific effect can be now explained, and is fully consistent with our finding that MIRR represses the brE and activates brL regions, respectively. Previous studies suggest that MIRR functions as a dedicated repressor (27). Based on this theory, we speculate that the activating effect of MIRR on the expression of the brL region is indirect and involves intermediate factors. On the other hand, our results strongly suggest that MIRR represses the brE region directly (Fig. 4E).
In contrast to the brL region, which generates br expression in a two-domain pattern that is necessary for the formation of two eggshell appendages, the function of the brE region is unclear. At the same time, this regulatory region was instrumental in our identification of a critical cis-element that controls the expression of pip, a gene which must be repressed in the dorsal follicle cells for proper induction of the DV polarity of the embryo (20). The regulatory regions of both br and pip contain a sequence essential for their transcriptional restriction to the ventral follicle cells (Fig. 3B). Moreover, our data suggest that the identified sequence is a direct sensor of MIRR (Fig. 4E), which is derepressed by EGFR. Thus, we uphold the earlier proposal that MIRR connects the EGFR-mediated patterning of the follicle cells to the DV patterning of the embryo (9). In the emerging transcriptional cascade, EGFR signaling down-regulates CIC, which derepresses MIRR, which in turn represses pip.
Work by the Ruohola-Baker group demonstrated that MIRR can repress pip, but suggested that this effect requires a relay mechanism (9). Our results, based on marked mirr overexpression clones, demonstrate that the effect is cell-autonomous (Fig. 4 B–C′′). Studies by the Roth group argue against MIRR-dependent pip repression, based on the fact that mirr mutant clones did not induce ectopic expression of pip (14). These results may be because of the fact that the mirr allele used in that study is not a complete null and has residual activity sufficient for pip repression. We argue that our data, demonstrating pip derepression by deletion of a sequence that binds MIRR, provide a strong support for MIRR-dependent repression of pip. Thus, our findings close a long-standing gap in the chain of events that convert EGFR signaling to pipe repression, a key step in transmitting the DV polarity from the egg to the embryo.
EGFR-dependent patterning of the follicle cells and the resulting effects for patterning of the embryo represent canonical examples of inductive effects in development. Indeed, genetic connection between EGFR signaling and pipe repression are found in essentially all textbooks of development. However, as discussed above, the identity of transcription factors involved in pipe regulation remained controversial and the cis-regulatory sequences responsible for pipe repression were unknown. Our results, which established MIRR as a direct repressor or pipe and identified the regulatory element responding to MIRR, clearly change this status. Thus, our results provide a significant addition to a very important model of inductive signaling. We arrived at the regulatory element of pipe using an approach that harnesses both conventional and modern techniques of gene regulation research and can be extended to other transcriptional targets of EGFR pathway in the follicle cells. Finally, we note that most of the available information on the transcriptional effects of EGFR signaling is related to gene activation (mediated by PNT) or derepression (mediated by CIC). Our work reveals a mechanism for EGFR-dependent gene repression, mediated by MIRR. Given the central role played by the EGFR signaling in development, the identified regulatory sequences can shed light on other EGFR-dependent pattern formation events.
Materials and Methods
Fly Stocks and Clonal Analysis.
The following Drosophila stocks were used: UAS-Mirr12 (31), FRT82B pntΔ86 (Gift from T. Schüpbach, Princeton University, Princeton, NJ), FRT82B cicfetU6 (32), and mirr1825 (33). The mirr1825 allele was recombined onto a FRT80B containing chromosome. The mirr1825 allele did not complement the mirre48 allele (34), and mutant clones in the follicle cells produced loss-of-dorsal-appendage phenotypes (Fig. S2). The FLP/FRT recombinant technique was used to generate loss-of-function clones (35). The ywhsflp122;; FRT82B,ubi-GFP and ywhsflp122;; ubi-GFP,FRT80B stocks were used to generate mutant clones marked by the loss of GFP, and the ywhsflp122 and act5C-FRTCD2FRT-Gal4, UAS-GFP stocks were used for ectopic expression of MIRR in clones coexpressing GFP. Recombination was induced by subjecting flies to a 37 °C heat shock for 2 h (loss-of function clones) 3 and 4 d before dissection or to a single 10-min heat shock at 37 °C (gain of MIRR clones) 3 d before dissection.
Transgenic Reporter Analysis.
Genomic fragments from the br locus were amplified by PCR and fused to a minimal hsp70-promoter upstream of a nuclear GFP or lacZ in the reporter vectors pnEGFPattB-GW and pnlacZattB-GW, respectively. The reporter vectors were generated from pUASTattB (36), by replacing the UAST-MCS with MCS-hsp70-nuclearEGFP (pH-Stinger-nuclear EGFP) or MCS-hsp70-lacZ (pH-Pelican-lacZ) supplemented with a nuclear localization signal (37). Subsequently reporter vectors were equipped with a Gateway attP1-ccdB-CmR-attP2 cassette (Invitrogen). All constructs were inserted by PhiC31/attB-mediated integration into chromosomal position 68A4 of the P2-line (38) or into 22A3 of the VK37 line (39). Genomic fragments from the pip locus were cloned into placZattB by conventional techniques. The primer information is available upon request.
Immunostaining, in Situ Hybridization, and Microscopy.
The activity of the reporters was assayed by fluorescent immunostaining or FISH. A detailed description of immunostaining and FISH assays is provided in the SI Materials and Methods.
Bioinformatics.
Genomic DNA fragments from Drosophila species (Drosophila erecta, Drosophila pseudoobscura, Drosophila yakuba, Drosophila ananassae) corresponding to the ∼1-kb subfragment of brE (present study) and the ∼8 kb pipA (19) of Drosophila melanogaster were retrieved from the University of California at Santa Cruz genome browser. The pipA sequence was further split into two: a fragment upstream to the transcriptional start of the gene (∼3 kb) and an intronic fragment (∼5 kb), and each fragment was individually compared separately to brE sequences using the MEME platform (Multiple Expectation maximization for Motif Elicitation, hosted at http://meme.nbcr.net), an algorithm that searches for statistically significant, repeated and ungapped sequence patterns in a group of sequences (40).
Protein-DNA Interaction Studies.
HA-tagged full-length MIRR protein was produced using a TNT T7 Quick Coupled Transcription/Translation System (Promega) and plasmid pFTX11-HAmirr (generous gift of H. McNeill, Samuel Lunenfeld Research Institute, Toronto, Canada) as a template. 32P-labeled probes were generated by annealing and filling in overlapping oligonucleotides with 5′-AATT overhangs in the presence of [a-32P]dATP. EMSA reactions were performed as described elsewhere (41). Briefly, binding reactions were carried out in 20 μL 20 mM Hepes (pH 7.9), 100 mM KCl, 20% glycerol, 0.3% BSA, 0.01% Nonidet P-40 containing 1 μg dIdC, 20 kcpm probe, and extracts expressing either MIRR or a control protein. After incubation for 40 min at 4 °C, reactions were analyzed by nondenaturing 4% polyacrylamide gel electrophoresis and autoradiography.
Note Added in Proof.
While this manuscript was under revision, Technau et al. (42) described a 31-bp DNA motif required for EGFR mediated repression of pip. In agreement with our findings, the 31-bp motif is contained within pipMRE.
Supplementary Material
Acknowledgments
We thank Trudi Schüpbach, Helen McNeill, Laura Nilson, and Wu-Min Deng for providing fly stocks and plasmids; Jana Dautzenberg for technical assistance; the Life Imaging Center of SFB592 for technical support; and Mark Norman and Robin Vuilleumier for comments on the manuscript. This work was supported in part by National Science Foundation Grants DMS-1119714 and DMS-0718604 (to S.Y.S.) and Human Frontiers Science Program Grant RGP0052/2009-C (to S.Y.S.). Research in the G.P. laboratory was funded by a grant of the Deutsche Forschungsgemeinschaft Collaborative Research Centre SFB592, the research training program GRK 1104, and the Excellence Initiative of the German Federal and State Governments (EXC294).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115190109/-/DCSupplemental.
References
- 1.Lynch JA, Peel AD, Drechsler A, Averof M, Roth S. EGF signaling and the origin of axial polarity among the insects. Curr Biol. 2010;20:1042–1047. doi: 10.1016/j.cub.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moghal N, Sternberg PW. The epidermal growth factor system in Caenorhabditis elegans. Exp Cell Res. 2003;284:150–159. doi: 10.1016/s0014-4827(02)00097-6. [DOI] [PubMed] [Google Scholar]
- 3.Budi EH, Patterson LB, Parichy DM. Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development. 2008;135:2603–2614. doi: 10.1242/dev.019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanchez-Soria P, Camenisch TD. ErbB signaling in cardiac development and disease. Semin Cell Dev Biol. 2010;21:929–935. doi: 10.1016/j.semcdb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung LS, Schüpbach T, Shvartsman SY. Pattern formation by receptor tyrosine kinases: Analysis of the Gurken gradient in Drosophila oogenesis. Curr Opin Genet Dev. 2011;21:719–725. doi: 10.1016/j.gde.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg CA. The Drosophila shell game: Patterning genes and morphological change. Trends Genet. 2005;21:346–355. doi: 10.1016/j.tig.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Atkey MR, Lachance JF, Walczak M, Rebello T, Nilson LA. Capicua regulates follicle cell fate in the Drosophila ovary through repression of mirror. Development. 2006;133:2115–2123. doi: 10.1242/dev.02369. [DOI] [PubMed] [Google Scholar]
- 8.Boisclair Lachance JF, Fregoso Lomas M, Eleiche A, Bouchard Kerr P, Nilson LA. Graded Egfr activity patterns the Drosophila eggshell independently of autocrine feedback. Development. 2009;136:2893–2902. doi: 10.1242/dev.036103. [DOI] [PubMed] [Google Scholar]
- 9.Jordan KC, et al. The homeobox gene mirror links EGF signalling to embryonic dorso-ventral axis formation through notch activation. Nat Genet. 2000;24:429–433. doi: 10.1038/74294. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto AM, et al. Pointed, an ETS domain transcription factor, negatively regulates the EGF receptor pathway in Drosophila oogenesis. Development. 1996;122:3745–3754. doi: 10.1242/dev.122.12.3745. [DOI] [PubMed] [Google Scholar]
- 11.Boyle MJ, Berg CA. Control in time and space: Tramtrack69 cooperates with Notch and Ecdysone to repress ectopic fate and shape changes during Drosophila egg chamber maturation. Development. 2009;136:4187–4197. doi: 10.1242/dev.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lembong J, Yakoby N, Shvartsman SY. Pattern formation by dynamically interacting network motifs. Proc Natl Acad Sci USA. 2009;106:3213–3218. doi: 10.1073/pnas.0810728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward EJ, Zhou XF, Riddiford LM, Berg CA, Ruohola-Baker H. Border of Notch activity establishes a boundary between the two dorsal appendage tube cell types. Dev Biol. 2006;297:461–470. doi: 10.1016/j.ydbio.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Peri F, Technau M, Roth S. Mechanisms of Gurken-dependent pipe regulation and the robustness of dorsoventral patterning in Drosophila. Development. 2002;129:2965–2975. doi: 10.1242/dev.129.12.2965. [DOI] [PubMed] [Google Scholar]
- 15.Dorman JB, James KE, Fraser SE, Kiehart DP, Berg CA. bullwinkle is required for epithelial morphogenesis during Drosophila oogenesis. Dev Biol. 2004;267:320–341. doi: 10.1016/j.ydbio.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Deng WM, Bownes M. Two signalling pathways specify localised expression of the Broad-Complex in Drosophila eggshell patterning and morphogenesis. Development. 1997;124:4639–4647. doi: 10.1242/dev.124.22.4639. [DOI] [PubMed] [Google Scholar]
- 17.Tzolovsky G, Deng WM, Schlitt T, Bownes M. The function of the broad-complex during Drosophila melanogaster oogenesis. Genetics. 1999;153:1371–1383. doi: 10.1093/genetics/153.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yakoby N, Lembong J, Schüpbach T, Shvartsman SY. Drosophila eggshell is patterned by sequential action of feedforward and feedback loops. Development. 2008;135:343–351. doi: 10.1242/dev.008920. [DOI] [PubMed] [Google Scholar]
- 19.Sen J, Goltz JS, Stevens L, Stein D. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell. 1998;95:471–481. doi: 10.1016/s0092-8674(00)81615-3. [DOI] [PubMed] [Google Scholar]
- 20.Amiri A, Stein D. Dorsoventral patterning: A direct route from ovary to embryo. Curr Biol. 2002;12:R532–R534. doi: 10.1016/s0960-9822(02)01031-x. [DOI] [PubMed] [Google Scholar]
- 21.Peri F, Bökel C, Roth S. Local Gurken signaling and dynamic MAPK activation during Drosophila oogenesis. Mech Dev. 1999;81:75–88. doi: 10.1016/s0925-4773(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 22.Zartman JJ, Kanodia JS, Cheung LS, Shvartsman SY. Feedback control of the EGFR signaling gradient: Superposition of domain-splitting events in Drosophila oogenesis. Development. 2009;136:2903–2911. doi: 10.1242/dev.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wasserman JD, Freeman M. An autoregulatory cascade of EGF receptor signaling patterns the Drosophila egg. Cell. 1998;95:355–364. doi: 10.1016/s0092-8674(00)81767-5. [DOI] [PubMed] [Google Scholar]
- 24.Astigarraga S, et al. A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling. EMBO J. 2007;26:668–677. doi: 10.1038/sj.emboj.7601532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James KE, Dorman JB, Berg CA. Mosaic analyses reveal the function of Drosophila Ras in embryonic dorsoventral patterning and dorsal follicle cell morphogenesis. Development. 2002;129:2209–2222. doi: 10.1242/dev.129.9.2209. [DOI] [PubMed] [Google Scholar]
- 26.Pai LM, Barcelo G, Schüpbach T. D-cbl, a negative regulator of the Egfr pathway, is required for dorsoventral patterning in Drosophila oogenesis. Cell. 2000;103:51–61. doi: 10.1016/s0092-8674(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 27.Bilioni A, Craig G, Hill C, McNeill H. Iroquois transcription factors recognize a unique motif to mediate transcriptional repression in vivo. Proc Natl Acad Sci USA. 2005;102:14671–14676. doi: 10.1073/pnas.0502480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zartman JJ, et al. Pattern formation by a moving morphogen source. Phys Biol. 2011;8:045003. doi: 10.1088/1478-3975/8/4/045003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yakoby N, et al. A combinatorial code for pattern formation in Drosophila oogenesis. Dev Cell. 2008;15:725–737. doi: 10.1016/j.devcel.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shilo BZ. Regulating the dynamics of EGF receptor signaling in space and time. Development. 2005;132:4017–4027. doi: 10.1242/dev.02006. [DOI] [PubMed] [Google Scholar]
- 31.Yang CH, Simon MA, McNeill H. mirror controls planar polarity and equator formation through repression of fringe expression and through control of cell affinities. Development. 1999;126:5857–5866. doi: 10.1242/dev.126.24.5857. [DOI] [PubMed] [Google Scholar]
- 32.Goff DJ, Nilson LA, Morisato D. Establishment of dorsal-ventral polarity of the Drosophila egg requires capicua action in ovarian follicle cells. Development. 2001;128:4553–4562. doi: 10.1242/dev.128.22.4553. [DOI] [PubMed] [Google Scholar]
- 33.Collins RT, Cohen SM. A genetic screen in Drosophila for identifying novel components of the hedgehog signaling pathway. Genetics. 2005;170:173–184. doi: 10.1534/genetics.104.039420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNeill H, Yang CH, Brodsky M, Ungos J, Simon MA. mirror encodes a novel PBX-class homeoprotein that functions in the definition of the dorsal-ventral border in the Drosophila eye. Genes Dev. 1997;11:1073–1082. doi: 10.1101/gad.11.8.1073. [DOI] [PubMed] [Google Scholar]
- 35.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 36.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barolo S, Carver LAP, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques. 2000;29:726–732, 728, 730, 732. doi: 10.2144/00294bm10. [DOI] [PubMed] [Google Scholar]
- 38.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phi C31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: A BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 40.Bailey TL, et al. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server issue):W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pyrowolakis G, Hartmann B, Müller B, Basler K, Affolter M. A simple molecular complex mediates widespread BMP-induced repression during Drosophila development. Dev Cell. 2004;7:229–240. doi: 10.1016/j.devcel.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Technau GM, Knispel M, Roth S. Molecular mechanisms of EGF signaling-dependent regulation of pipe, a gene crucial for dorsoventral axis formation in Drosophila. Dev Genes Evol. 2011 doi: 10.1007/s00427-011-0384-2. 10.1007/s00427-011-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.