Abstract
N-methyl-d-aspartate receptors (NMDARs) mediate critical CNS functions, whereas excessive activity contributes to neuronal damage. At physiological glycine concentrations, NMDAR currents recorded from cultured rodent hippocampal neurons exhibited strong desensitization in the continued presence of NMDA, thus protecting neurons from calcium overload. Reducing copper availability by specific chelators (bathocuproine disulfonate, cuprizone) induced nondesensitizing NMDAR currents even at physiologically low glycine concentrations. This effect was mimicked by, and was not additive with, genetic ablation of cellular prion protein (PrPC), a key copper-binding protein in the CNS. Acute ablation of PrPC by enzymatically cleaving its cell-surface GPI anchor yielded similar effects. Biochemical studies and electrophysiological measurements revealed that PrPC interacts with the NMDAR complex in a copper-dependent manner to allosterically reduce glycine affinity for the receptor. Synthetic human Aβ1–42 (10 nM–5 μM) produced an identical effect that could be mitigated by addition of excess copper ions or NMDAR blockers. Taken together, Aβ1–42, copper chelators, or PrPC inactivation all enhance the activity of glycine at the NMDAR, giving rise to pathologically large nondesensitizing steady-state NMDAR currents and neurotoxicity. We propose a physiological role for PrPC, one that limits excessive NMDAR activity that might otherwise promote neuronal damage. In addition, we provide a unifying molecular mechanism whereby toxic species of Aβ1–42 might mediate neuronal and synaptic injury, at least in part, by disrupting the normal copper-mediated, PrPC-dependent inhibition of excessive activity of this highly calcium-permeable glutamate receptor.
Keywords: NMDA receptor, Alzheimer's disease, Aβ oligomer, 5XFAD mouse, Cu
The N-methyl-d-aspartate receptor (NMDAR) is a key ionotropic glutamate receptor in the mammalian CNS, playing a critical role in a range of functions including development, memory, and learning (1, 2). Although the primary activator of these receptors is glutamate, they typically also require the binding of the coagonist glycine (or d-serine) to the NR1 subunit of the receptor complex. Glycine binding results in enhanced peak current amplitude and slowing of receptor desensitization (3), an essential intrinsic mechanism that terminates receptor activity during prolonged agonist exposure to protect neurons from toxic calcium entry (3, 4). Indeed, excessive NMDAR activity has been implicated in the pathophysiology of several neurodegenerative disorders such as Alzheimer's disease (AD), but the underlying molecular mechanisms are poorly understood (5–7). One fundamental feature of AD pathogenesis is an excessive production and accumulation of Aβ peptides in the brain. Although the most characteristic histopathologic feature includes neurofibrillary tangles within neurons and deposition of congophilic β-amyloid plaques in the cortex (8, 9), recent work suggests that soluble oligomeric Aβ peptides are the neurotoxic species, rather than the large amyloid deposits (10). However, the precise mechanisms of Aβ neurotoxicity remain unclear (11), nor is the relationship between Aβ and NMDARs clearly elucidated. Lauren and colleagues (12) recently reported that Aβ1–42 oligomers are high-affinity ligands of cellular prion protein (PrPC) and that PrPc is required to mediate the effect of these toxic species. This observation was recently confirmed by Barry and coworkers (13) who showed that PrPC was required for Aβ oligomer-mediated suppression of in vivo long-term potentiation (LTP). Along these lines, Gimbel and colleagues (14) reported that memory impairment observed in a transgenic mouse model of Alzheimer's disease was abolished upon deletion of PrPC. Finally, Collinge and coworkers (15) showed that low-molecular-weight, Aβ-derived diffusible ligand prepared from human Alzheimer's brain disrupts hippocampal synaptic plasticity in a PrPC-dependent manner. Interestingly, their observed disruption of LTP induction is reminiscent of the effect of tonic activation of NMDARs (16). Data from our laboratory have revealed that PrPC forms a signaling complex with NMDARs (17). Furthermore, our work showed that knockout of PrPC in mouse hippocampal neurons enhances NMDAR currents by slowing their deactivation kinetics, perhaps due to an increase in receptors containing the NR2D subunit. Given that Aβ can regulate NMDA receptors (18), we studied the mechanistic relationship between Aβ, NMDA receptors, and PrPC.
Results
Aβ1–42 Induces Steady-State NMDAR Current.
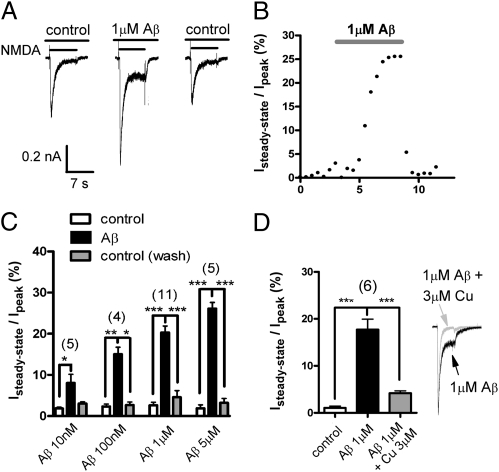
To determine whether Aβ can directly regulate NMDAR function, we acutely applied Aβ1–42 to cultured hippocampal pyramidal neurons and examined its effect on NMDAR receptor function using whole-cell voltage clamp. Synapses are among the very first structures to be compromised during the early stages of AD (10, 19). Thus, NMDAR-dependent currents were elicited in the presence of 300 nM glycine, a concentration chosen to mirror the submicromolar levels of this coagonist estimated to be present under physiological conditions in the synapse (20, 21). Under our experimental conditions, application of 500 μM NMDA evoked robust inward currents that exhibited near-complete current decay after several seconds in both rat (Fig. 1A) and mouse (see below) hippocampal neurons. In contrast, although Aβ1–42 itself exhibited no effect, NMDA currents elicited in the presence of 1 μM unfractionated Aβ1–42 (which, like the intact brain, contains a mixture of monomers and oligomers) showed a substantial steady-state component that persisted for many seconds. The effects of Aβ1–42 on NMDAR current kinetics developed slowly over several minutes, were rapidly reversed upon washout, and could be observed at concentrations as low as 10 nM (Fig. 1 B and C). Application of 100 nM scrambled Aβ1–42 did not significantly alter NMDA currents (Isteady state = 5.6 ± 2.9% of Ipeak, P = 0.29 vs. control, n = 7). These robust effects of Aβ1–42 appear to contrast with recent findings from cultured cortical neurons (22) where no alteration of NMDA currents was observed as a result of chronic treatment with Aβ. However, as is often the case with NMDAR activity measurements, this prior study used high concentrations of the coagonist glycine (20 μM). Glycine and the other classical NMDAR coagonist d-serine are effective chelators of copper ions, and exogenous copper is known to decrease NMDAR-mediated currents (23–25). Given that these coagonists slow NMDAR desensitization (3) and that Aβ1–42 also binds copper ions with picomolar or higher affinity (26, 27), we hypothesized that Aβ1–42 might modulate NMDAR kinetics by altering copper regulation. Consistent with such a mechanism, addition of 3 μM copper to 1 μM Aβ1–42 to yield an excess of copper restored the normal decay of NMDAR currents (Fig. 1D). Our findings thus suggest that Aβ1–42 might produce a pathological enhancement of NMDAR-mediated current by altering copper availability at the receptor complex.
Fig. 1.
Soluble Aβ1–42 increased steady-state NMDAR current. (A) Representative currents from a rat hippocampal neuron in response to 500 μM NMDA exhibited virtually complete desensitization in 300 nM glycine. Aβ1–42 peptide (1 μM) induced a pronounced steady-state current, which was reversible. (B) Representative time course of the effects of Aβ1–42 on the steady-state NMDA current. (C) Bar graph showing mean ± SEM steady-state current as a percentage of peak in control neurons (white bars) and after exposure to unfractionated Aβ1–42 (10 nM–5 μM; black bars). The induction of steady-state current by Aβ1–42 was completely reversible (gray bars). (D) Addition of excess copper ions almost completely reversed the effect of Aβ1–42, suggesting that the effect is mediated by alteration of copper ion availability at the receptor. *P < 0.05; **P < 0.01; ***P < 0.001.
Copper Ions Modulate NMDAR Kinetics.
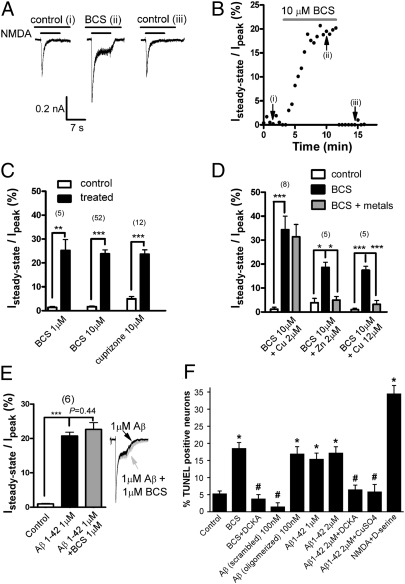
To separate copper-dependent effects from other possible actions of Aβ1–42, we performed analogous experiments using the selective copper ion chelator bathocuproine disulfonate (BCS) (Materials and Methods) (28). Application of BCS (1–10 μM) closely mimicked the effects of Aβ1–42 on NMDAR kinetics, as did application of another copper chelator, cuprizone (Fig. 2 A–C). Although both BCS and cuprizone are considered selective copper chelators, it was important to rule out the possibility that the observed effects were due to chelation of other metals such as zinc, a known regulator of NMDARs (29). We therefore took advantage of the known NMDAR-blocking effects of micromolar zinc (30). Application of 2 μM zinc to cells bathed in 10 μM BCS markedly reduced the steady-state current (Fig. 2D), indicating that BCS did not appreciably chelate zinc ions. In contrast, application of 2 μM copper in the presence of excess BCS had no effect under these conditions, confirming the selectivity of BCS for copper. Raising copper levels to 12 μM, to yield an excess of this metal ion, resulted in near-complete elimination of the steady-state current. Together, these data confirm that the observed effects of BCS are due to selective chelation of copper rather than zinc and establish ambient copper ions as a potent regulator of NMDAR function. The effects of BCS were not additive with those of 1 μM Aβ1–42, further supporting the notion that this peptide's effects on NMDAR kinetics were also due to alterations in copper ion availability (Fig. 2E). Finally, internal dialysis of neurons with either 10 μM BCS (Isteady state= 6.3 ± 0.8% of Ipeak, n = 3) or 1 μM Aβ1–42 (Isteady state= 4.1 ± 0.8% of Ipeak, n = 3) had no significant effect on NMDAR kinetics, indicating that copper-dependent regulation of the receptor involves a location accessible from the extracellular space.
Fig. 2.
Copper chelators induced steady-state NMDAR current. (A) Chelating copper with bathocuproine disulfonate (BCS) reversibly induced steady-state NMDAR current in rat hippocampal neurons. (B) Representative time course of development of the steady-state current after addition of BCS. (C) Mean ± SEM steady-state current as a percentage of peak. There was minimal steady-state current in the absence of copper chelators (white bars), whereas copper chelators induced a substantial steady-state component. (D) Specificity of BCS was determined by replacing metal ions. Addition of 2 μM copper to 10 μM BCS had no effect as the chelator remained substantially in excess. In contrast, addition of 2 μM zinc completely abolished the steady-state current, indicating that BCS does not appreciably bind zinc at the concentrations used. Adding excess copper to BCS predictably abolished the steady-state current. (E) Effect of BCS was not additive with that of Aβ1–42. (Inset) Representative traces scaled to normalized peak currents. (F) Disrupting copper homeostasis by exposure of neurons to BCS (10 μM) induced significant neuronal death as measured by TUNEL labeling. Aβ1–42 was equally toxic, but scrambled Aβ1–42 (subjected to the same oligomerization procedure as normal sequence peptide) was not. NMDAR block with 5,7-dichlorokynurenic acid (DCKA, 100 μM) or replenishment with CuSO4 (4 μM) protected neurons from injury. Neurons exposed to NMDA and d-serine (both 500 μM) served as positive controls. Values are mean ± SEM. *P < 10−5; #P > 0.95 vs. control.
Akin to inactivation of voltage-gated calcium channels, decay of NMDAR-mediated current prevents toxic calcium overload of neurons. Hence, one might expect that an increase in steady-state NMDAR-mediated current due to copper chelation by BCS or Aβ1–42 would be neurotoxic. As shown in Fig. 2F, this is indeed the case. Exposure of hippocampal cultures to BCS or Aβ1–42 resulted in a similar degree of neuronal cell death, which was significantly abrogated by NMDAR block with the selective antagonist 5,7-dichlorokynurenic acid (100 μM) or by addition of excess copper ions.
Cellular Prion Protein Modulates NMDAR Kinetics.
We next explored the physiological mechanism by which ambient copper might act on the NMDAR. The CNS expresses a broad spectrum of copper-binding proteins (31). One attractive candidate is PrPC, a widely expressed cuproprotein (32) that is anchored to the extracellular surface of cell membranes and that is especially abundant at synapses (33). Moreover, the expression of PrPC has been shown to influence synaptic transmission, although the mechanism remains unclear (34, 35). PrPC contains a number of octarepeat regions (36) that can bind several copper ions with affinities spanning many orders of magnitude (37). Given that PrPC can directly interact with Aβ1–42 (12) and also regulate NMDAR activity (17), our current findings raised the possibility that PrPC may function to modulate NMDARs in a copper-dependent manner. To test this hypothesis, we performed whole-cell recordings from hippocampal pyramidal cells cultured from PrPC knockout mice. When activated by NMDA and 300 nM glycine, these neurons exhibited a steady-state current (Fig. 3A) that could not be further augmented by addition of either 10 μM BCS or 100 nM Aβ1–42 (Fig. 3B). Experiments involving recombinant NMDARs composed of NR1/NR2B subunits expressed in Xenopus oocytes in the absence and the presence of recombinant PrPC yielded results similar to those obtained with hippocampal neurons (Fig. S1). Finally, we acutely interfered with PrPC function in rat hippocampal neurons by enzymatically cleaving the GPI anchor on PrPC using phosphatidylinositol-specific phospholipase C (PI-PLC), which would cause the protein to be removed from its extracellular location. Under these conditions, we observed a steady-state current that was similar in magnitude to that observed in PrPC-null mouse neurons or to wild-type neurons treated with copper chelators (Fig. 3C). These observations indicate that the steady-state currents seen in PrPC-null mice were indeed due to the absence of PrPC rather than a compensatory mechanism. In contrast, application of PI-PLC did not produce additional effects in PrPC-null mouse neurons (Fig. 3C), indicating that if other proteins were removed from the cell surface by cleavage of their GPI anchors, they did not affect the NMDAR-mediated currents that we recorded. Moreover, the steady-state current observed in PrPC-null neurons could be eliminated upon application of 2 μM exogenous copper (Fig. 3B), indicating that copper has the ability to regulate NMDAR current kinetics even in the absence of PrPC, perhaps by acting directly at the receptor complex. This result, together with the fact that tissue culture media contain ∼100 nM to low-micromolar copper, might explain why neuronal cultures from PrPC-null mice remain healthy (17), whereas cell death is observed under conditions where copper ions are deliberately chelated to near-zero concentrations (Fig. 2F). Similarly, resting copper levels might be sufficiently elevated in brains of PrP-null mice to explain why these animals do not exhibit significant early neurodegeneration.
Fig. 3.
Prion protein influenced NMDAR kinetics and partially mediated the effect of Aβ1–42. (A) Representative traces from hippocampal neurons showing large steady-state currents only in the PrPC-nulls; copper chelation with BCS had no additional effect. (B) Mean ± SEM steady-state current as a percentage of peak recorded from PrP knockout neurons. Mere absence of PrPC resulted in an ∼20% steady-state current, similar in magnitude to BCS-treated neurons (cf. Fig. 2). Neither BCS nor Aβ1–42 was additive. **P < 0.01; ***P < 0.001. (C) Acute cleavage of the PrPC GPI anchor by PI-PLC induced substantial steady-state current in WT rat neurons, which was not additive in PrP-null neurons. (D) Mean ± SEM steady-state current as a percentage of peak from rat neurons showing that oligomeric Aβ1–42 was particularly effective at inducing steady-state NMDAR current. *P < 0.05; **P < 0.01. (E) Quantification of the steady-state current recorded in 300 nM glycine from cultured 5XFAD mouse neurons vs. WT littermates (*P < 0.05, t test). (Inset) Representative current trace from a 5XFAD neuron.
In light of our data implicating PrPC in copper regulation of NMDARs, it is important to note that, although both monomeric and oligomeric species of Aβ1–42 interact with copper (26, 38), PrPC selectively interacts with Aβ1–42 oligomers (12). Soluble Aβ1–42 normally used in our experiments contained a mixture of monomers and oligomers, and this is further complicated by the fact that copper ions are known to promote oligomerization (39). To determine which of the Aβ species mediated the observed modulation of NMDAR kinetics, we varied the relative proportion of monomeric and oligomeric Aβ1–42 species using established protocols (40) (SI Materials and Methods and Fig. S2). We found that 100 nM of oligomer-enriched Aβ1–42 triggered a steady-state current that was notably more pronounced than the steady-state current evoked by either 100 nM or 1 μM of the mainly monomeric form (Fig. 3D).
To determine the effect of natural Aβ species on NMDAR current activity, we cultured hippocampal neurons from hemizygous 5XFAD mice and their control littermates. 5XFAD mice generate large quantities of natural Aβ1–42 and are considered a suitable animal model of AD (41). As shown in Fig. 3E, neurons cultured from these mice exhibited a significantly greater tonic steady-state current that is consistent with an increase in naturally produced ambient Aβ1–42 levels.
Taken together, our data indicate that PrPC plays an essential role in copper-dependent effects on NMDAR kinetics in neurons. Moreover, the potent effects of Aβ on NMDAR currents might be mediated either by direct chelation of copper by the peptide or by interference with the normal PrPC-dependent regulation of the receptor; either mechanism results in excessive NMDAR-mediated currents and neuronal damage.
PrPC and Copper Regulate Glycine Affinity.
Next, we sought mechanistic insight into the molecular mode of action of copper and PrPC on NMDAR kinetics. Our observations in the presence of Aβ oligomers, copper chelators, or upon inactivation of PrPC, all strongly parallel the effects of increasing concentrations of the coagonist glycine (4). Conversely, at a fixed concentration of glycine, an increase in glycine affinity for the receptor would be expected to produce a similar enhancement of the steady-state current. This led us to hypothesize that PrPC, in its copper-bound form, reduces glycine affinity for the receptor complex, enhancing desensitization and reducing steady-state current. We therefore compared the glycine dose dependence of NMDAR current decay in WT and PrPC-null mouse neurons. As shown in Fig. 4A, in neurons lacking PrPC, glycine was more potent at inducing steady-state NMDAR currents over a concentration range considered physiological (20, 21), consistent with the notion that PrPC normally reduces the apparent affinity of glycine for the receptor. Chelation of ambient copper with BCS (Fig. 4B) produced a similar shift in glycine dose dependence as that observed with PrPC knockout, suggesting that the effects of PrPC on reducing glycine affinity require copper binding. A direct modulatory effect of PrPC on NMDARs was further suggested by our observation that PrPC coimmunoprecipitated in a copper-dependent manner with NR1—the obligatory NMDAR subunit that contains the glycine-binding site (Fig. 4C). In contrast, interactions between mGluR1 receptors and PrPC (42) were not affected by chelating copper (Fig. S3). Together, these data represent a unique mechanism whereby PrPC suppresses NMDAR activity by regulating glycine interactions with the receptor complex in a manner strongly dependent on copper ions.
Fig. 4.
PrPC reduced the affinity of the NMDAR for glycine. (A) Glycine dose dependence of the percentage of steady-state current in wild-type and PrP-null mouse neurons. The data were fitted with a modified Hill equation. Note the leftward shift in the glycine dose dependence and the greater maximal effect in PrP-null mouse neurons. *P < 0.05 wild type vs. PrP-null. (B) Representative traces from rat hippocampal neurons obtained in the absence or presence of 10 μM BCS and corresponding bar chart depicting the size of steady-state current. Consistent with the data in A, BCS had little effect at low glycine (0.1 μM) compared with control, but, in contrast, induced a significant steady-state current at 0.3 and 1 μM glycine, indicating a shift to a higher apparent glycine affinity in the absence of copper. *P < 0.05; ***P < 0.001. (C) Coimmunoprecipitation of NR1 subunits and PrPC from rat brain homogenate under control conditions after addition of 10 μM CuSO4 or after chelation of copper with BCS. NR1 and PrPC coimmunoprecipitated, with the strength of this association being dependent on ambient copper levels. The blot was probed with an NR1 antibody (expected molecular weight for NR1 is ∼120 kDa) and is a representative example of four separate experiments; equal amounts of lysate were used for each condition. The empty lanes reflect a bead-only control (“Beads”) and a coimmunoprecipitation control experiment from PrP-null mouse brain. The bar graph is a quantification of band intensities normalized to that observed under control conditions.
Discussion
Copper is a critically important transition metal involved in numerous metabolic pathways (31, 43). Under our conditions, we identify copper as an endogenous, PrPC-dependent regulator of NMDARs that prevents excessive activity of the receptor by enhancing desensitization, thus limiting potentially toxic steady-state NMDAR-mediated currents. At physiological levels of synaptic glycine (20, 21), ambient levels of copper greatly limited steady-state NMDAR currents, thus preventing calcium overload and neurotoxicity. In contrast, when even nanomolar amounts of oligomerized Aβ1–42 peptide were added, pathological nondesensitizing currents were observed in the presence of physiologically relevant concentrations of glycine due to interference with the normal copper-dependent regulation of NMDARs. Such dysregulated NMDAR currents might contribute to neurotoxicity in AD. Copper-dependent regulation of the NMDAR is also known to affect LTP (44), a correlate of learning and memory, and is thus additionally relevant to AD and related disorders. The pathophysiological importance of these copper-dependent phenomena may have been overlooked previously because most investigations of NMDAR activity are conducted in relatively high (10–50 μM) concentrations of glycine or d-serine where NMDAR desensitization is already dramatically reduced.
In the present study, both copper chelators and Aβ1–42 produced similar effects on NMDAR current decay, and their effects were not additive. This, together with the reversal of the steady-state current upon readdition of copper, and the very high affinity of Aβ peptide for copper ions (26, 27), suggests that Aβ1–42 might mediate its functional effects on NMDAR activity by disturbing copper homeostasis near the receptor. The corollary would be that under conditions of excess production/liberation of Aβ as in AD, the homeostasis of ambient copper ions is altered, in turn causing an increase in NMDAR current producing potentially toxic calcium influx. With time, synaptic elements would be damaged by chronic calcium overload, leading to synaptic loss, which is one of the earliest manifestations of AD (9, 19). This might also offer a mechanistic explanation for a recent report in a mouse model of AD showing increased spontaneous calcium transients in cortical neurons that could be blocked by NMDAR antagonists (45).
It is thought that free copper is virtually absent in biological fluids, instead existing bound to various amino acids and proteins (34). Our data show that one such copper binding protein, PrPC, is a key regulator of NMDAR activity. We propose that PrPC, in its copper-loaded state, binds to the NMDAR complex (Fig. 4C) to allosterically reduce its glycine affinity, thereby increasing desensitization. When copper is chelated (i.e., by BCS or monomeric Aβ1–42) or when PrPC is absent or functionally compromised (by GPI anchor cleavage or binding to Aβ oligomers, for example), glycine affinity is enhanced, reducing receptor desensitization and producing pathologically large, steady-state currents that contribute to neuronal damage (Fig. 5).
Fig. 5.
Proposed model linking copper-dependent effects of PrPC and Aβ1–42 to NMDAR activity. (A) NMDARs are closely associated with PrPC protein (as shown in Fig. 4C) (17). Under physiological conditions, copper-bound PrPC reduces glycine affinity for the receptor complex, thus enhancing NMDAR desensitization and limiting calcium flux through the receptor. (B) At high-glycine concentrations, receptor occupancy increases even though affinity remains low, augmenting the proportion of steady-state current. (C) In the absence of endogenous PrPC, the glycine affinity is enhanced, leading to steady-state currents. (D) Under pathological conditions of excessive Aβ production, the peptide acts as a high-affinity copper chelator, preventing copper ions from normally binding to PrPC; this effect might alter the ability of PrPC to normally regulate NMDAR desensitization and/or cause dissociation of the two proteins (Fig. 4C), mimicking the genetic or biochemical ablation of PrPC. The result is an increased glycine affinity, leading to prolonged steady-state current and pathological calcium influx. (E) Aβ oligomers act as high-affinity ligands for PrPC protein (12), and this interaction might also disturb PrPC-dependent modulation of NMDAR kinetics.
We have previously reported that PrPC can be coimmunoprecipitated with NR2D subunits (17). Our finding that PrPC and NR1 subunits (which are common to all subtypes of NMDARs) can be coimmunoprecipitated is consistent with the existence of an NMDAR–PrPC-signaling complex. This assay does not allow us to discern whether PrPC interacts with the NMDAR complex via NR1 or NR2 subunits. However, given that the NR1 subunit contains the glycine-binding site and that PrPC regulates glycine affinity, a direct interaction with NR1 is plausible.
It has been shown that PrPC is a conduit for mediating neurotoxic effects of various β-sheet rich aggregates including Aβ, and that toxicity is prevented by NMDA receptor antagonists (46). As noted earlier, Strittmatter and colleagues (12) reported that Aβ1–42 oligomers are high-affinity ligands of PrPC, which in turn is required for Aβ-mediated neurotoxicity and suppression of LTP (13–15). Together, these findings are consistent with the mechanism of Aβ-mediated suppression of NMDAR desensitization that we report here. Other groups, however, have disputed the above findings, reporting that PrPC is not required for Aβ-induced interference with synaptic activity (47–49). It is possible that the discordant results might arise from different amounts of copper and/or glycine present in the various preparations used in these studies. Indeed, our data show that copper, at sufficiently high concentrations, is able to regulate NMDAR function in the absence of PrPC (Fig. 3B), perhaps as a result of direct interactions with the receptor or due to formation of copper–glycine complexes that might render this coagonist ineffective. Depending on the combination of copper and glycine concentrations in any given preparation, Aβ may thus affect NMDA receptor currents independently of PrPC through direct copper chelation, i.e., by virtue of the extremely high affinity of Aβ for this metal (26, 27). Altogether, the discordant findings with regard to the necessity for PrPC in Aβ-mediated neurotoxicity can be reconciled by our model that indicates that Aβ can induce pathologically large, steady-state NMDAR currents under conditions of physiological glycine and copper concentrations via interactions with PrPC (Fig. 5). Notably, the electrophysiological effects of excess Aβ1–42 produced by cultured 5XFAD neurons were indistinguishable from those of copper chelation, synthetic Aβ1–42 in its various forms, or PrPC ablation (Figs. 1–3). Brains of these mice contain various Aβ1–42 complexes ranging from low-n oligomers to higher-molecular-weight Aβ-derived diffusible ligands (50, 51). On the basis of our own data and the reported interactions of larger Aβ aggregates with PrPC (12, 15), it is likely that various species of Aβ contributed to inducing the significant steady-state NMDAR currents. Importantly, however, our results show that naturally overproduced Aβ1–42 closely recapitulated what we observed with synthetic peptide.
In summary, the copper-dependent mechanism that we describe here might explain, at least in part, the neurodegeneration observed in AD. This, in turn, could pave the way for the design of effective therapeutics aimed at targeting such a mechanism; state-dependent inhibition or suppression of steady-state NMDAR current might therefore be a promising approach. In contrast and by extension, copper chelator therapy might have unexpected deleterious effects.
Materials and Methods
Experimental procedures pertaining to biochemistry, Xenopus oocyte recordings, TUNEL assays, and data analysis are presented in SI Materials and Methods.
Neuronal Primary Culture.
Pregnant Sprague–Dawley rats were purchased from Charles River and maintained in compliance with the University of Calgary and Sanford-Burnham Medical Research Institute Animal Care and Use Policies. Wild-type and PrP knockout mice (Zuerich 1 strain outbred to a pure C57 genetic background by Frank Jirik's laboratory, University of Calgary, Calgary, AB, Canada) were prepared as described (17). 5XFAD mice were purchased from The Jackson Laboratory. Rat and mouse hippocampal neurons were prepared from P0-2 pups as described by us previously in detail (17). Total copper in culture media was measured at ∼100 nM.
Electrophysiology of Hippocampal Neurons.
Unless stated otherwise, chemicals were obtained from Sigma-Aldrich. Whole-cell voltage-clamp recordings were performed on hippocampal pyramidal neurons after 10–15 d in culture at room temperature using an Axopatch 200B amplifier (Axon Instruments). The holding potential was −60 mV throughout. The external solution contained 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 25 mM Hepes, and 33 mM d-glucose, pH adjusted to 7.4 with NaOH. Total measured copper was ∼50 nM before addition of any exogenous copper. To obtain NMDA currents, the external solution was supplemented with 0.5 μM TTX (Tocris Bioscience), 100 μM picrotoxin, 15 μM 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt, and different concentrations of glycine as indicated. The internal pipette solution was composed of 140 mM CsCl, 11 mM EGTA, 1 mM CaCl2, 2 mM MgCl2 and 10 mM Hepes, pH adjusted to 7.3 with CsOH. The internal solution was supplemented with 4 mM K2ATP and 0.6 mM GTP, which were added directly to the internal solution immediately before use. Aβ1–42 sodium salt was purchased from Anaspec (catalog no. 60883) or rPeptide. Drug delivery was controlled by a rapid microperfusion system (AutoMate Scientific), which was composed of a ValveLink 8.2 controller, an eight-channel mini Lee valve, an eight-channel perfusion pencil, a 250-μm diameter removable tip, and a pressurized superfusion device to achieve fast switching of solution. The perfusion tip was positioned a few hundred micrometers from the cell and kept as constant as possible throughout the experiments. The solution was perfused with a pressure of 1.6–1.8 psi. The solution exchange was computer controlled for timing and initiation using a Digidata 1320A interface (Molecular Devices). NMDAR-mediated currents were evoked by application of NMDA (500 μM, Tocris Bioscience) for 7 s at 30-s intervals. In a typical experiment, a stable baseline current was obtained by applying external recording solution from a pair of channels. After reaching a stable state, the solution was switched to one that contained NMDA with or without other compounds. The steady-state current (Isteady-state) was determined as the nondesensitizing current amplitude at the end of a 7-s NMDA application. Solution could be rapidly washed out by switching back to a channel that contained the external recording solution. Trace copper was chelated using the canonical Cu2+-selective chelator cuprizone (52) or BCS (28). Although the latter has been reported to preferentially bind Cu+ over Cu2+ (53), BCS also interacts with Cu2+ with high affinity to form bis [Cu(BCS)2]2- complexes (54, 55), or the protonated form of BCS [H⋅BCS]− associates with Cu2+ to form complex species such as [Cu2+ (BCS) (H2O)x] and a proton (56).
Supplementary Material
Acknowledgments
The authors thank Dr. Frank Jirik for providing PrP knockout mice, Dr. Clinton Doering and Karen Cummins for genotyping, Lorinda Butlin for Cu analysis, and Dr. Tobias Fürstenhaupt for assistance with EM. This work was supported in part by grants from PrioNet Canada and the Alberta Prion Research Institute (G.W.Z.); the Canadian Institutes of Health Research (P.K.S.); and National Institutes of Health Grants P01 HD29587 and P01 ES016738 (to S.A.L.). G.W.Z. and P.K.S. hold Canada Research Chair awards and are Scientists of the Alberta Heritage Foundation for Medical Research. P.X. holds a fellowship from the American Heart Association, and H.Y. holds an Alberta Heritage Foundation for Medical Research Fellowship.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110789109/-/DCSupplemental.
References
- 1.MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol. 2006;18(1-2):71–84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- 2.Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer ML, Vyklicky LJ, Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989;338:425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- 4.Lerma J, Zukin RS, Bennett MV. Glycine decreases desensitization of N-methyl-D-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci USA. 1990;87:2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: Excitatory times ahead. Lancet Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 7.Palop JJ, Mucke L. Amyloid-β-induced neuronal dysfunction in Alzheimer's disease: From synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68(1):1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 10.Walsh DM, Selkoe DJ. A β oligomers: A decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 11.Aguzzi A, O'Connor T. Protein aggregation diseases: Pathogenicity and therapeutic perspectives. Nat Rev Drug Discov. 2010;9:237–248. doi: 10.1038/nrd3050. [DOI] [PubMed] [Google Scholar]
- 12.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barry AE, et al. Alzheimer's disease brain-derived amyloid-β-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J Neurosci. 2011;31:7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimbel DA, et al. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freir DB, et al. Interaction between prion protein and toxic amyloid β assemblies can be therapeutically targeted at multiple sites. Nat Commun. 2011;2:336. doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zajaczkowski W, Frankiewicz T, Parsons CG, Danysz W. Uncompetitive NMDA receptor antagonists attenuate NMDA-induced impairment of passive avoidance learning and LTP. Neuropharmacology. 1997;36:961–971. doi: 10.1016/s0028-3908(97)00070-1. [DOI] [PubMed] [Google Scholar]
- 17.Khosravani H, et al. Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J Cell Biol. 2008;181:551–565. doi: 10.1083/jcb.200711002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 19.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 20.Supplisson S, Roux MJ. Why glycine transporters have different stoichiometries. FEBS Lett. 2002;529(1):93–101. doi: 10.1016/s0014-5793(02)03251-9. [DOI] [PubMed] [Google Scholar]
- 21.Yang CR, Svensson KA. Allosteric modulation of NMDA receptor via elevation of brain glycine and D-serine: The therapeutic potentials for schizophrenia. Pharmacol Ther. 2008;120:317–332. doi: 10.1016/j.pharmthera.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Gu Z, Liu W, Yan Z. β-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J Biol Chem. 2009;284:10639–10649. doi: 10.1074/jbc.M806508200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harada K, Fujii N. Optical resolution of aspartic acid by using copper complexes of optically active amino acids. Bull Chem Soc Jpn. 1983;56:653–654. [Google Scholar]
- 24.Martin RP, Mosoni L, Sarkar B. Ternary coordination complexes between glycine, copper (II), and glycine peptides in aqueous solution. J Biol Chem. 1971;246:5944–5951. [PubMed] [Google Scholar]
- 25.Vlachová V, Zemková H, Vyklický LJ., Jr Copper modulation of NMDA responses in mouse and rat cultured hippocampal neurons. Eur J Neurosci. 1996;8:2257–2264. doi: 10.1111/j.1460-9568.1996.tb01189.x. [DOI] [PubMed] [Google Scholar]
- 26.Atwood CS, et al. Characterization of copper interactions with Alzheimer amyloid beta peptides: Identification of an attomolar-affinity copper binding site on amyloid beta1-42. J Neurochem. 2000;75:1219–1233. doi: 10.1046/j.1471-4159.2000.0751219.x. [DOI] [PubMed] [Google Scholar]
- 27.Hung YH, Bush AI, Cherny RA. Copper in the brain and Alzheimer's disease. J Biol Inorg Chem. 2010;15(1):61–76. doi: 10.1007/s00775-009-0600-y. [DOI] [PubMed] [Google Scholar]
- 28.Mohindru A, Fisher JM, Rabinovitz M. Bathocuproine sulphonate: A tissue culture-compatible indicator of copper-mediated toxicity. Nature. 1983;303(5912):64–65. doi: 10.1038/303064a0. [DOI] [PubMed] [Google Scholar]
- 29.Peters S, Koh J, Choi DW. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987;236:589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- 30.Chen N, Moshaver A, Raymond LA. Differential sensitivity of recombinant N-methyl-D-aspartate receptor subtypes to zinc inhibition. Mol Pharmacol. 1997;51:1015–1023. doi: 10.1124/mol.51.6.1015. [DOI] [PubMed] [Google Scholar]
- 31.Schlief ML, Gitlin JD. Copper homeostasis in the CNS: A novel link between the NMDA receptor and copper homeostasis in the hippocampus. Mol Neurobiol. 2006;33(2):81–90. doi: 10.1385/MN:33:2:81. [DOI] [PubMed] [Google Scholar]
- 32.Burns CS, et al. Copper coordination in the full-length, recombinant prion protein. Biochemistry. 2003;42:6794–6803. doi: 10.1021/bi027138+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown DR. Prion and prejudice: Normal protein and the synapse. Trends Neurosci. 2001;24(2):85–90. doi: 10.1016/s0166-2236(00)01689-1. [DOI] [PubMed] [Google Scholar]
- 34.Brown DR, et al. The cellular prion protein binds copper in vivo. Nature. 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- 35.Collinge J, et al. Prion protein is necessary for normal synaptic function. Nature. 1994;370:295–297. doi: 10.1038/370295a0. [DOI] [PubMed] [Google Scholar]
- 36.Aguzzi A, Baumann F, Bremer J. The prion's elusive reason for being. Annu Rev Neurosci. 2008;31:439–477. doi: 10.1146/annurev.neuro.31.060407.125620. [DOI] [PubMed] [Google Scholar]
- 37.Jackson GS, et al. Location and properties of metal-binding sites on the human prion protein. Proc Natl Acad Sci USA. 2001;98:8531–8535. doi: 10.1073/pnas.151038498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarell CJ, Syme CD, Rigby SE, Viles JH. Copper(II) binding to amyloid-β fibrils of Alzheimer's disease reveals a picomolar affinity: Stoichiometry and coordination geometry are independent of Abeta oligomeric form. Biochemistry. 2009;48:4388–4402. doi: 10.1021/bi900254n. [DOI] [PubMed] [Google Scholar]
- 39.Huang X, et al. Trace metal contamination initiates the apparent auto-aggregation, amyloidosis, and oligomerization of Alzheimer's Abeta peptides. J Biol Inorg Chem. 2004;9:954–960. doi: 10.1007/s00775-004-0602-8. [DOI] [PubMed] [Google Scholar]
- 40.Stine WBJ, Jr, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-β peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- 41.Oakley H, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: Potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beraldo FH, et al. Metabotropic glutamate receptors transduce signals for neurite outgrowth after binding of the prion protein to laminin γ1 chain. FASEB J. 2011;25:265–279. doi: 10.1096/fj.10-161653. [DOI] [PubMed] [Google Scholar]
- 43.Banci L, Bertini I, Cantini F, Ciofi-Baffoni S. Cellular copper distribution: A mechanistic systems biology approach. Cell Mol Life Sci. 2010;67:2563–2589. doi: 10.1007/s00018-010-0330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doreulee N, Yanovsky Y, Haas HL. Suppression of long-term potentiation in hippocampal slices by copper. Hippocampus. 1997;7:666–669. doi: 10.1002/(SICI)1098-1063(1997)7:6<666::AID-HIPO8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 45.Busche MA, et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 46.Resenberger UK, et al. The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. EMBO J. 2011;30:2057–2070. doi: 10.1038/emboj.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balducci C, et al. Synthetic amyloid-β oligomers impair long-term memory independently of cellular prion protein. Proc Natl Acad Sci USA. 2010;107:2295–2300. doi: 10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calella AM, et al. Prion protein and Abeta-related synaptic toxicity impairment. EMBO Mol Med. 2010;2:306–314. doi: 10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kessels HW, Nguyen LN, Nabavi S, Malinow R. The prion protein as a receptor for amyloid-beta. Nature. 2010;466:E3–E4, discussion E4–E5. doi: 10.1038/nature09217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohno M, et al. Temporal memory deficits in Alzheimer's mouse models: Rescue by genetic deletion of BACE1. Eur J Neurosci. 2006;23:251–260. doi: 10.1111/j.1460-9568.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- 51.Hong HS, et al. Candidate anti-A beta fluorene compounds selected from analogs of amyloid imaging agents. Neurobiol Aging. 2010;31:1690–1699. doi: 10.1016/j.neurobiolaging.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peterson RE, Bollier ME. Spectrophotometric determination of serum copper with biscyclohexanoneoxalyldihydrazone. Anal Chem. 1955;27:1195–1197. [Google Scholar]
- 53.Zhao J, Bertoglio BA, Devinney MJJ, Jr, Dineley KE, Kay AR. The interaction of biological and noxious transition metals with the zinc probes FluoZin-3 and Newport Green. Anal Biochem. 2009;384(1):34–41. doi: 10.1016/j.ab.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Shatti N, Lappin AG, Sykes AG. Influence of coordination number on copper(I)-copper(II) redox interconversions. 2. Fe(CN)64- reduction of a sterically constrained bis(substituted phenanthroline) complex of copper(II) in aqueous solution. Inorg Chem. 1981;20:1466–1469. [Google Scholar]
- 55.Sayre LM. Alzheimer's precursor protein and the use of bathocuproine for determining reduction of copper(II) Science. 1996;274:1933–1934. doi: 10.1126/science.274.5294.1933. [DOI] [PubMed] [Google Scholar]
- 56.Xiao Z, et al. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: Detection probes and affinity standards. J Biol Chem. 2011;286:11047–11055. doi: 10.1074/jbc.M110.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.