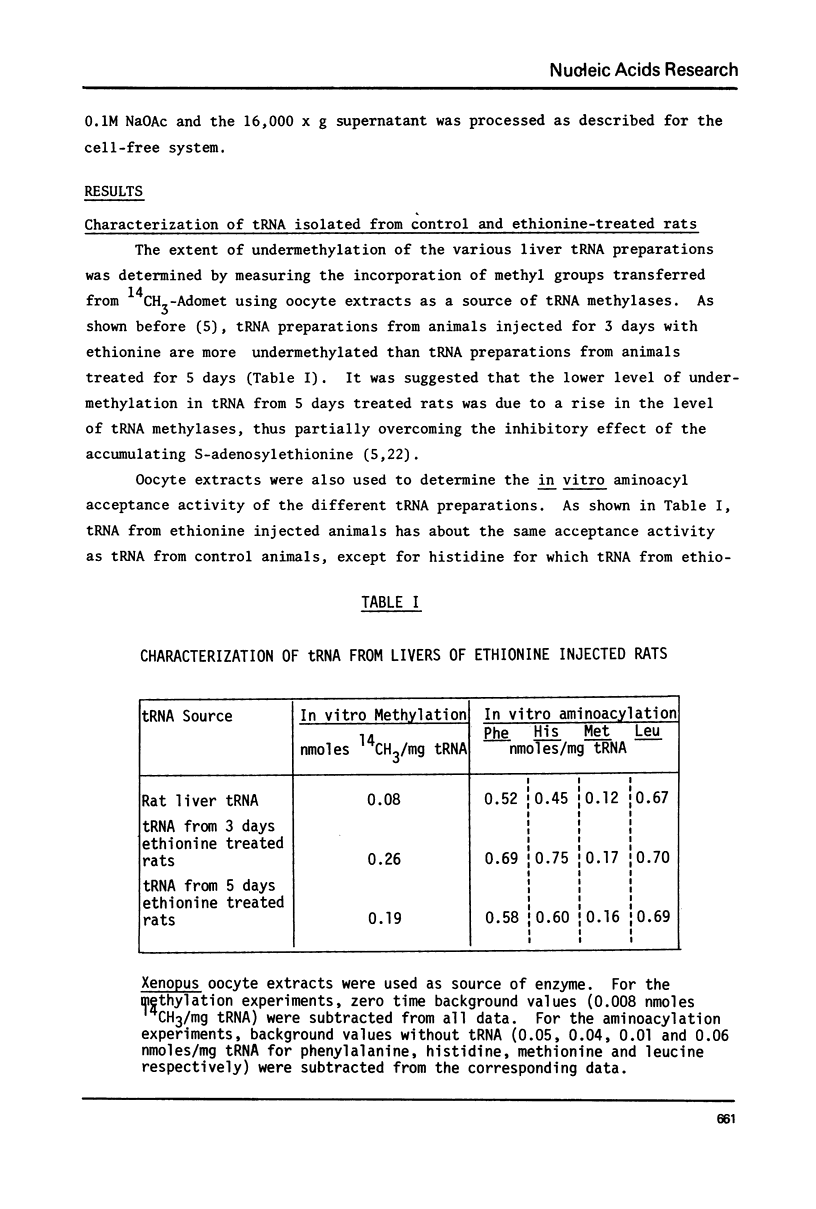
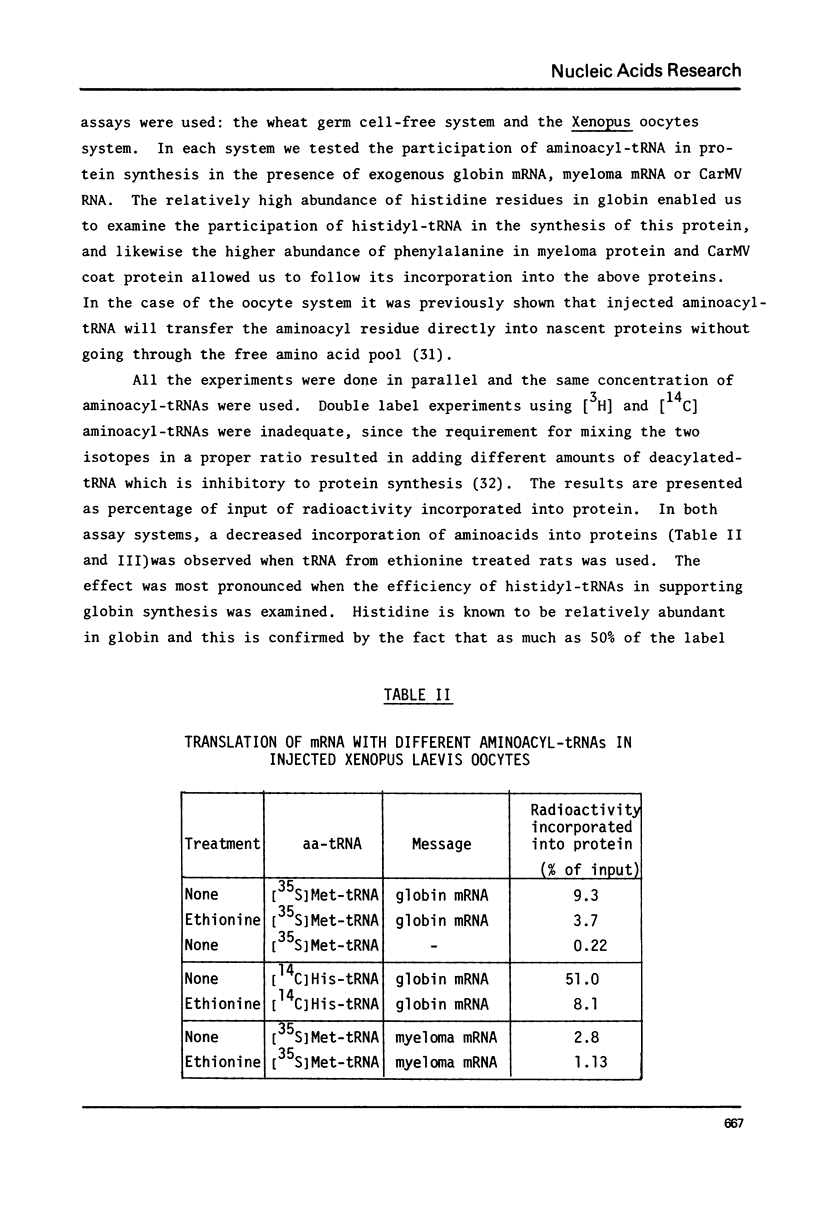
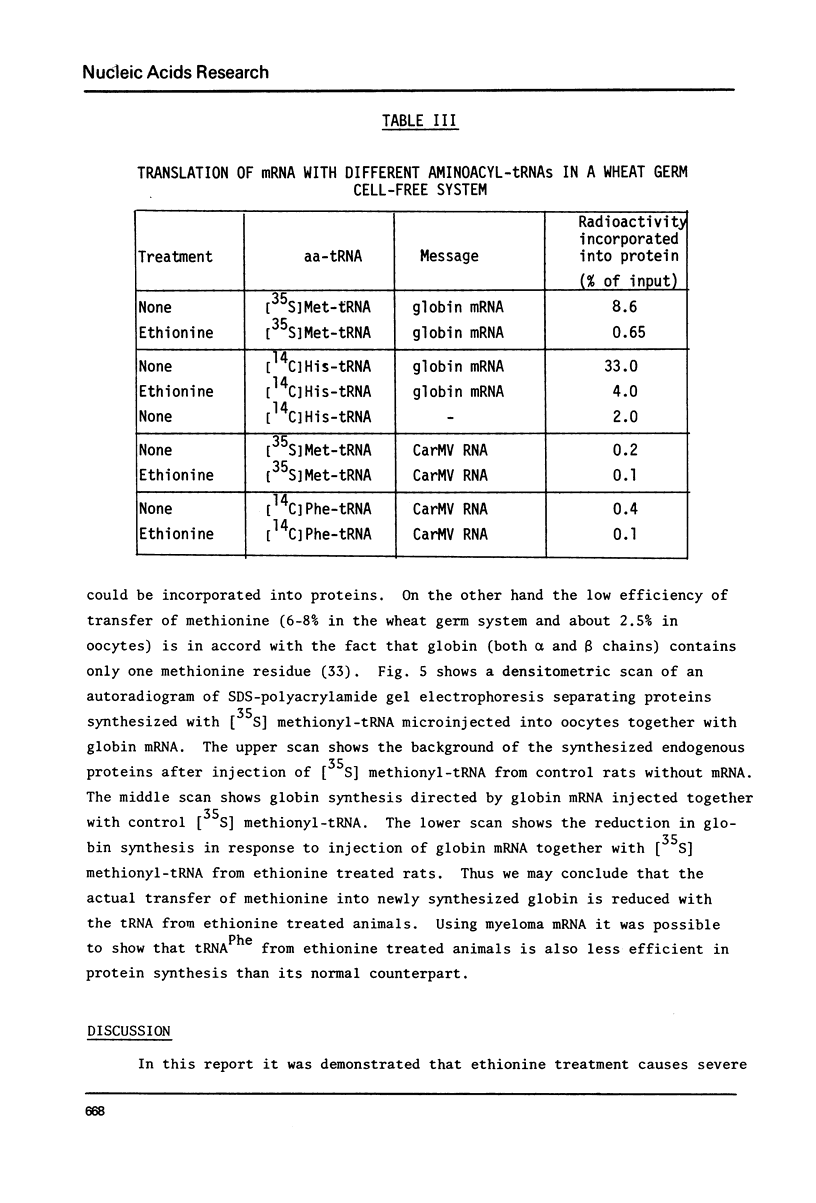
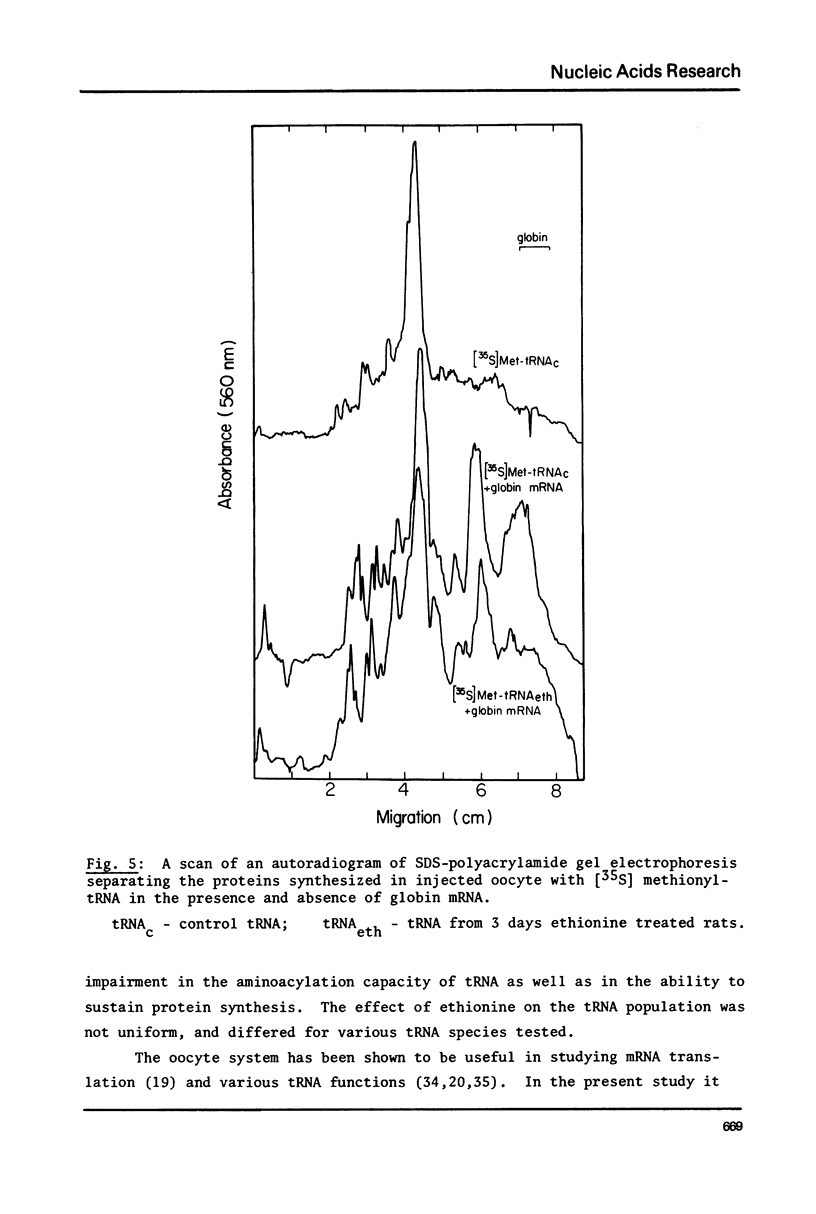
Abstract
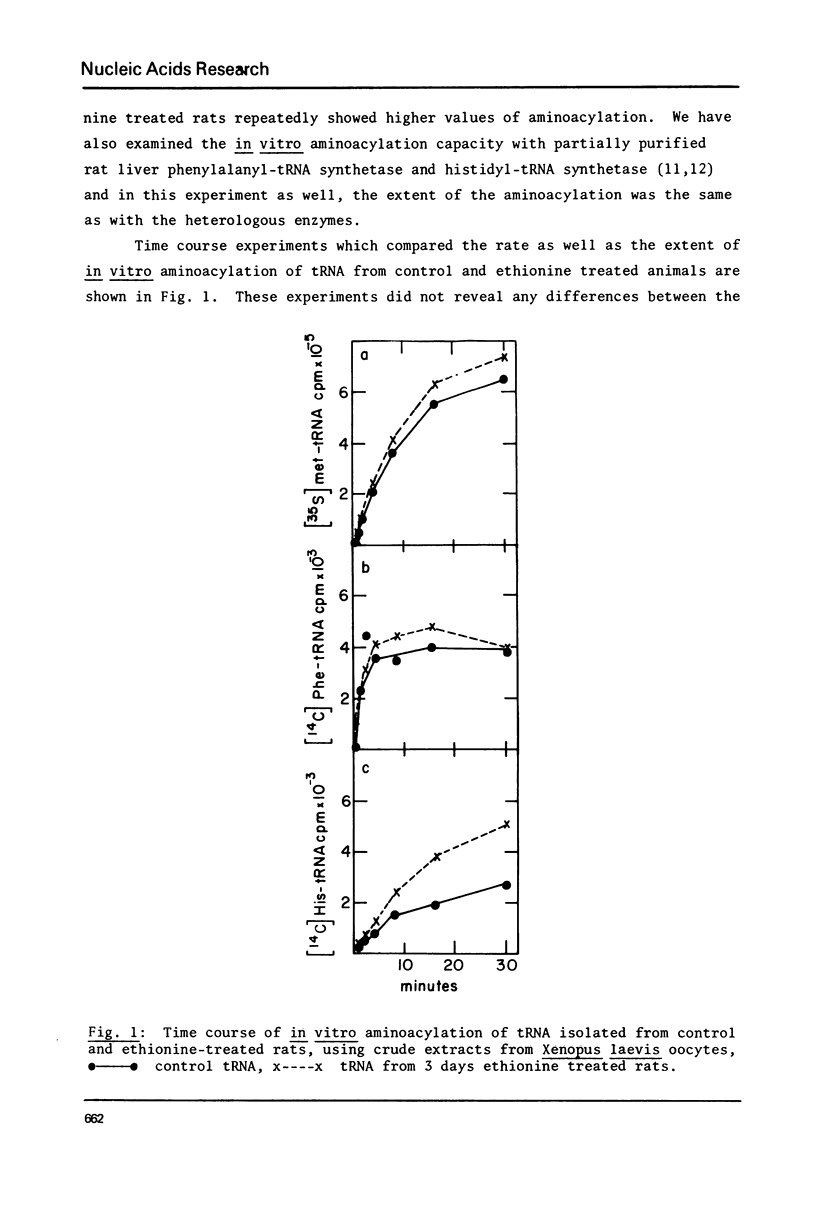
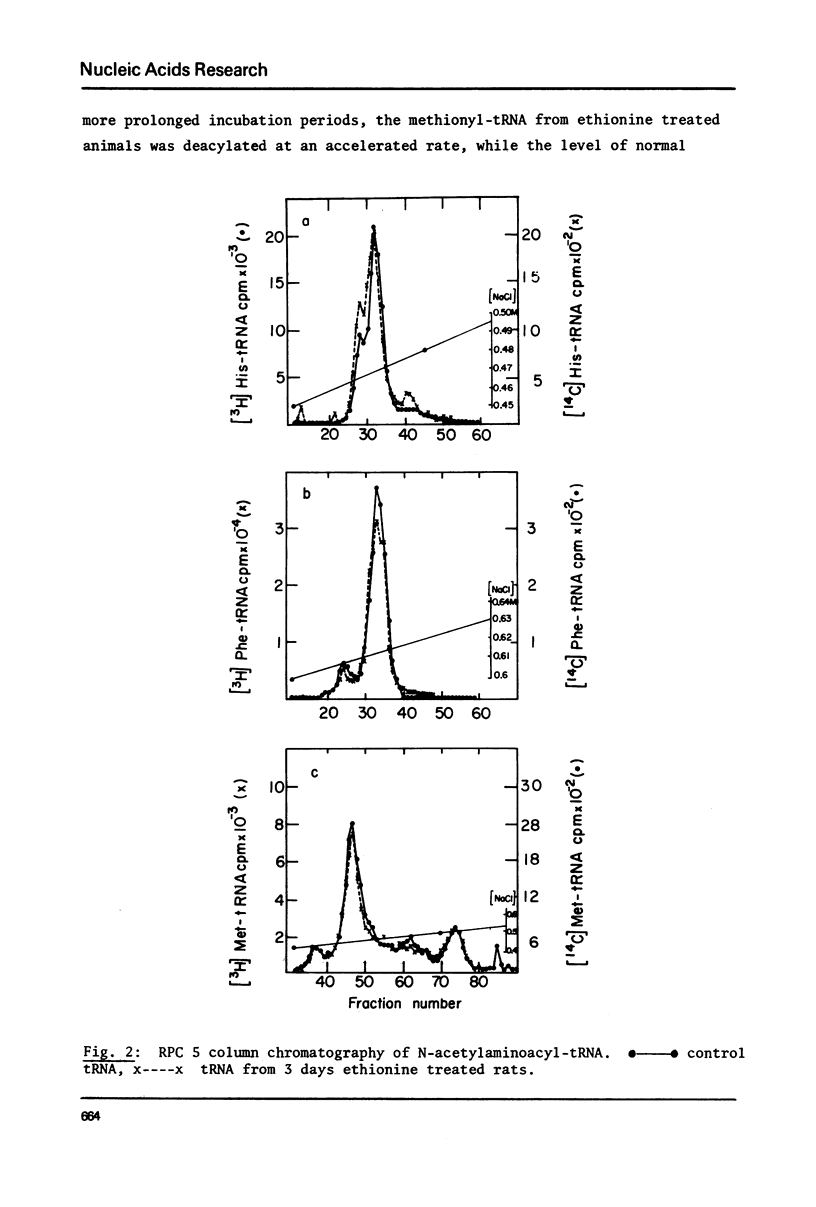
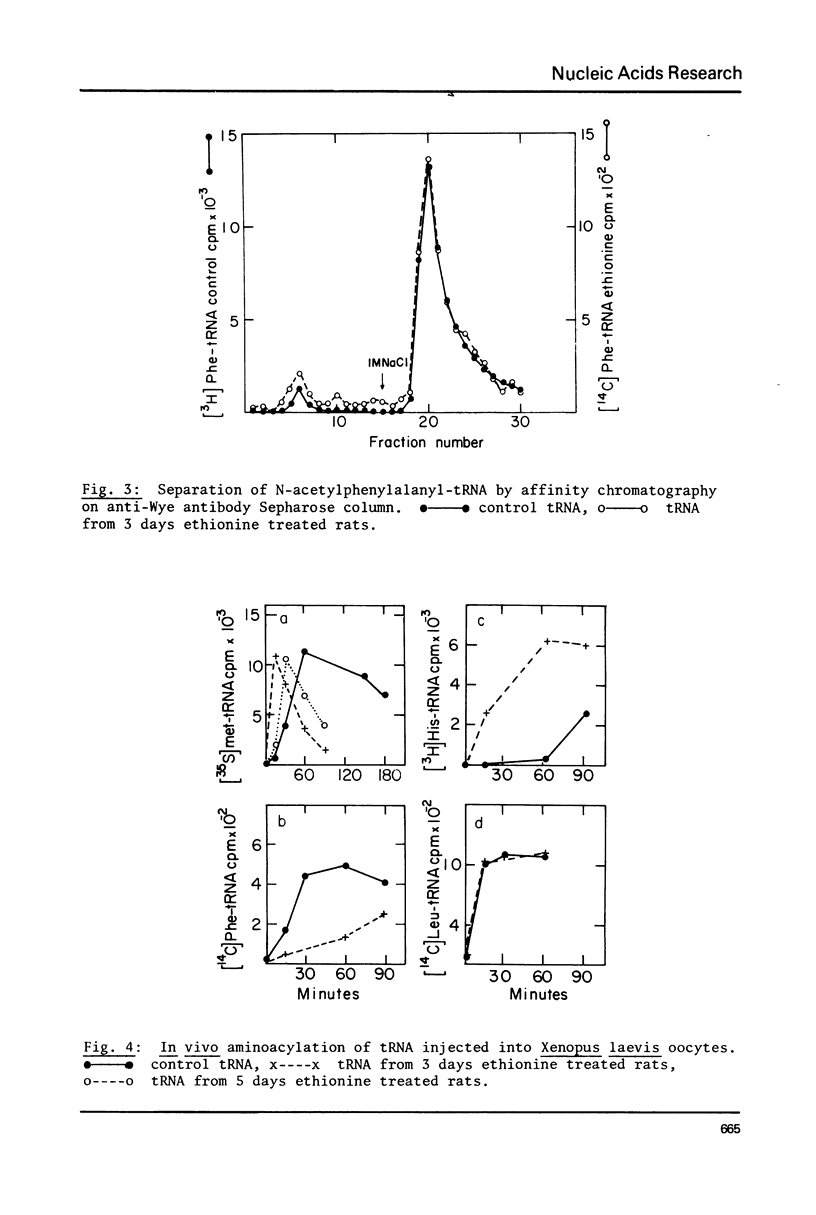
Treatment of rats with ethionine was found to cause severe impairment in the aminoacylation capacity of tRNA. This effect was only observed when assayed in injected oocytes, while invitro assays of aminoacylation failed to detect differences between normal tRNA and tRNA from ethionine treated animals. The effect of ethionine on the tRNA population was not uniform and differed for various amino acid specific tRNAs. Thus liver tRNA from ethionine treated rats showed a decreased capacity for phenylalanine aminoacylation, while no change was found in the case of leucine. On the other hand, the level of histidine aminoacylation was higher for tRNA from ethionine treated animals. An even more complex response was observed with methionine aminoacylation where tRNA from ethionine treated animals showed an initially faster rate than control tRNA. With more prolonged incubation periods, the methionyl-tRNA from ethionine treated animals was deacylated at an accelerated rate while the level of normal methionyl-tRNA remained almost constant.
In addition to the aminoacylation reaction, the participation of aminoacyl-tRNA in protein synthesis was severely impaired. In this case, both the injected oocyte system and the cell-free wheat germ assay revealed these differences which were manifested with various mRNA and viral RNA preparations.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende C. C., Allende J. E., Firtel R. A. The degradation of ribonucleic acids injected into Xenopus laevis oocytes. Cell. 1974 Jul;2(3):189–196. doi: 10.1016/0092-8674(74)90093-2. [DOI] [PubMed] [Google Scholar]
- Briscoe W. T., Griffin A. C., McBride C., Bowen J. M. The distribution and properties of aspartyl transfer RNA in human and animal tumors. Cancer Res. 1975 Sep;35(9):2586–2593. [PubMed] [Google Scholar]
- Cornelis P., Classen E., Claessen J. Reversed phase chromatography of isoaccepting tRNA's from healthy and crown gall tissues from Nicotiana tabacum. Nucleic Acids Res. 1975 Jul;2(7):1153–1161. doi: 10.1093/nar/2.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Irving C. C. Inhibition of DNA methylation by S-adenosylethionine with the production of methyl-deficient DNA in regenerating rat liver. Cancer Res. 1977 Jan;37(1):222–225. [PubMed] [Google Scholar]
- Ehresmann B., Imbault P., Weil J. H. Spectrophotometric determination of protein concentration in cell extracts containing tRNA's and rRNA's. Anal Biochem. 1973 Aug;54(2):454–463. doi: 10.1016/0003-2697(73)90374-6. [DOI] [PubMed] [Google Scholar]
- Gatica M., Allende J. E. Aminoacyl transfer from phenylalanyl-tRNA microinjected into Xenopus laevis oocytes. Biochem Biophys Res Commun. 1977 Nov 21;79(2):352–356. doi: 10.1016/0006-291x(77)90164-4. [DOI] [PubMed] [Google Scholar]
- Gatica M., Tarragó A., Allende C. C., Allende J. E. Aminoacylation of transfer RNA microinjected into Xenopus laevis oocytes. Nature. 1975 Aug 21;256(5519):675–678. doi: 10.1038/256675a0. [DOI] [PubMed] [Google Scholar]
- Grunberger D., Weinstein I. B., Mushinski J. F. Deficiency of the Y base in a hepatoma phenylalanine tRNA. Nature. 1975 Jan 3;253(5486):66–67. doi: 10.1038/253066a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Chapeville F. The behaviour of acetylphenylalanyl soluble ribonucleic acid in polyphenylalanine synthesis. Biochim Biophys Acta. 1966 Jan 18;114(1):135–148. doi: 10.1016/0005-2787(66)90261-9. [DOI] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Burny A., Hubert E., Leclercq M., Cleuter Y., Chantrenne H., Soreq H., Littauer U. Z. Degradation of deadenylated rabbit alpha-globin mRNA in Xenopus oocytes is associated with its translation. Nature. 1977 Mar 31;266(5601):473–474. doi: 10.1038/266473a0. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Alterations in SVT2 cell transfer RNAs in response to cell density and serum type. Biochim Biophys Acta. 1975 Mar 10;383(2):131–139. doi: 10.1016/0005-2787(75)90254-3. [DOI] [PubMed] [Google Scholar]
- Kerr S. J. Interaction of normal and tumor transfer RNA methyltransferases with ethionine-induced methyl-deficient rat liver transfer RNA. Cancer Res. 1975 Nov;35(11 Pt 1):2969–2973. [PubMed] [Google Scholar]
- Kuchino Y., Sharma O. K., Borek E. Lysine transfer RNA2 is the major target for L-ethionine in the rat. Biochemistry. 1978 Jan 10;17(1):144–147. doi: 10.1021/bi00594a021. [DOI] [PubMed] [Google Scholar]
- Moore B. G., Smith R. C. S-Adenosylethionine as an inhibitor of tRNA methylation. Can J Biochem. 1969 May;47(5):561–565. doi: 10.1139/o69-088. [DOI] [PubMed] [Google Scholar]
- Mukerjee H., Goldfeder A. Transfer RNA species in tumors of different growth rates. Cancer Res. 1976 Sep;36(9 PT1):3330–3338. [PubMed] [Google Scholar]
- Münch H. J., Thiebe R. Biosynthesis of the nucleoside Y in yeast tRNAPhe: incorporation of the 3-amino-3-carboxypropyl-group from methionine. FEBS Lett. 1975 Mar 1;51(1):257–258. doi: 10.1016/0014-5793(75)80900-8. [DOI] [PubMed] [Google Scholar]
- Okada N., Shindo-Okada N., Nishimura S. Isolation of mammalian tRNAAsp and tRNATyr by lectin-Sepharose affinity column chromatography. Nucleic Acids Res. 1977 Feb;4(2):415–423. doi: 10.1093/nar/4.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B. A., Stankiewicz A. F., Chen C. Y. Chromatographic behavior of several mammalian tRNAs on acylated dihydroxyl-borate cellulose and Aminex A-28. Nucleic Acids Res. 1977 Jul;4(7):2191–2204. doi: 10.1093/nar/4.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen L. Ethylation in vivo of purines in rat-liver RNA by L-ethionine. Biochem Biophys Res Commun. 1968 Nov 25;33(4):546–550. doi: 10.1016/0006-291x(68)90329-x. [DOI] [PubMed] [Google Scholar]
- Salomon R., Giveon D., Kimhi Y., Littauer U. Z. Abundance of tRNAPhe lacking the peroxy Y-base in mouse neuroblastoma. Biochemistry. 1976 Nov 30;15(24):5258–5262. doi: 10.1021/bi00669a010. [DOI] [PubMed] [Google Scholar]
- Solari A., Gatica M., Allende J. E. In vivo repair of the 3'terminus of transfer RNA injected into amphibian oocytes. Nucleic Acids Res. 1977 Jun;4(6):1873–1880. doi: 10.1093/nar/4.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. T., Cass K. H., Stellwagen E. Blue dextran-sepharose: an affinity column for the dinucleotide fold in proteins. Proc Natl Acad Sci U S A. 1975 Feb;72(2):669–672. doi: 10.1073/pnas.72.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainfan E., Tscherne J. S., Maschio F. A., Balis M. E. Time dependence of ethionine-induced changes in rat liver transfer RNA methylation. Cancer Res. 1977 Mar;37(3):865–869. [PubMed] [Google Scholar]
- Wilson J. E. Applications of blue dextran and Cibacron Blue F3GA in purification and structural studies of nucleotide-requiring enzymes. Biochem Biophys Res Commun. 1976 Oct 4;72(3):816–823. doi: 10.1016/s0006-291x(76)80206-9. [DOI] [PubMed] [Google Scholar]


