Abstract
The p53 transcription factor modulates gene expression programs that induce cell cycle arrest, senescence, or apoptosis, thereby preventing tumorigenesis. However, the mechanisms by which these fates are selected are unclear. Our objective is to understand p53 target gene selection and, thus, enable its optimal manipulation for cancer therapy. We have generated targeted transgenic reporter mice in which EGFP expression is driven by p53 transcriptional activity at a response element from either the p21 or Puma promoter, which induces cell cycle arrest/senescence and apoptosis, respectively. We demonstrate that we could monitor p53 activity in vitro and in vivo and detect variations in p53 activity depending on the response element, tissue type, and stimulus, thereby validating our reporter system and illustrating its utility for preclinical drug studies. Our results also show that the sequence of the p53 response element itself is sufficient to strongly influence p53 target gene selection. Finally, we use our reporter system to provide evidence for p53 transcriptional activity during early embryogenesis, showing that p53 is active as early as embryonic day 3.5 and that p53 activity becomes restricted to embryonic tissue by embryonic day 6.5. The data from this study demonstrate that these reporter mice could serve as powerful tools to answer questions related to basic biology of the p53 pathway, as well as cancer therapy and drug discovery.
Keywords: reporter mouse models, embryonic development
The transcription factor p53 is crucial for tumor suppression, as evidenced by its frequent inactivation in many different cancers (1). p53 mediates changes in gene expression that culminate in cell cycle arrest, apoptosis, or senescence. However, the mechanisms that determine p53 target gene selection and how each of the three processes contributes to tumor suppression remain poorly understood (2). Understanding how p53 induces each pathway to modulate cell fate will allow us to exploit them for the optimal prevention and treatment of cancer.
p21 and Puma (p53 up-regulated modulator of apoptosis) are p53 target genes that are key executors of these cell fate decisions. p21 mediates cell cycle arrest and senescence (3–6), whereas Puma is crucial for p53-mediated apoptosis (7–13). Studies of mice lacking either gene have revealed crosstalk between the cell cycle arrest/senescence and apoptosis pathways. After irradiation, there is higher p21 expression in the small intestine of Puma-null mice than of wild-type mice, which correlates with increased proliferation and regeneration of cells in the intestinal crypts (14). In vitro experiments have also demonstrated that the relative expression of p21 and PUMA protein is key to deciding p53-induced cell fates (10). Therefore, studying the interplay between p21 and Puma is important for understanding the consequences of p53 activation.
We have, thus, used p21 and Puma as representative genes to study the activation of these different pathways by p53. Previous attempts to address this question involved assaying expression of endogenous p53 target genes (15), but such analysis is complicated by the contribution of transcription factors other than p53. Our current work avoids this issue by examining p53 transcriptional activity at the p21 and Puma promoters, specifically by using a p53 response element (RE) from either the p21 or Puma promoter to drive expression of enhanced green fluorescent protein (EGFP). We have used these two reporter constructs to generate reporter mice that allow us to directly compare p53 activity at the REs from the p21 and Puma promoters in vivo. Our data provide insight into the mechanics of p53 target gene selection, as well as the role of p53 during embryonic development. We anticipate that our reporter system may be used to answer a wide variety of basic biology questions, as well as for preclinical applications, including drug discovery and optimization of therapeutic regimens.
Results
Generation of p53 Reporter Mice.
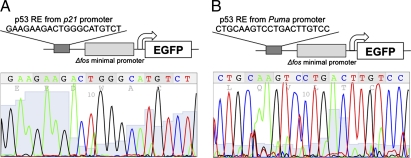
To create reporters to study p53 activity at different promoters, we cloned the ORF encoding EGFP downstream of the Δfos minimal promoter and a single p53 response element (RE) from either the p21 or the Puma promoter (Fig. 1). These reporter constructs are denoted as “p21p53RE-EGFP” and “Pumap53RE-EGFP,” respectively. The p21p53RE-EGFP reporter incorporates the p53 binding site from the p21 promoter previously referred to as the “3′ site” (16) (Fig. 1A). The Pumap53RE-EGFP reporter includes the p53 binding site commonly denoted as “BS2,” which is required for p53-mediated transcription of the Puma gene (7, 8) (Fig. 1B). EGFP expression is driven solely by p53 binding to these REs and activating transcription, thus serving as a direct indicator of p53 transcriptional activity.
Fig. 1.
Generation of reporter mice that express EGFP under the control of p53. Schematic (Upper) and chromatogram (Lower) of the p21p53RE-EGFP reporter (A) and Pumap53RE-EGFP reporter (B) constructs used for targeted transgenesis.
Reporter mice were generated by targeted transgenesis, using homologous recombination to integrate a single copy of the reporter construct into the locus of the housekeeping gene hypoxanthine phosphoribosyltransferase (Hprt), which has been identified as a permissive locus suitable for gene targeting (17–19). The reporters are integrated into the same genetic location and subject to the same epigenetic regulation (17, 20), allowing us to use EGFP expression in these reporter mice to directly compare p53 activity at REs from the p21 and Puma promoters.
EGFP Expression Correlates with the Concentration-Dependent Induction of p53 Activity and Endogenous p21 and Puma Expression.
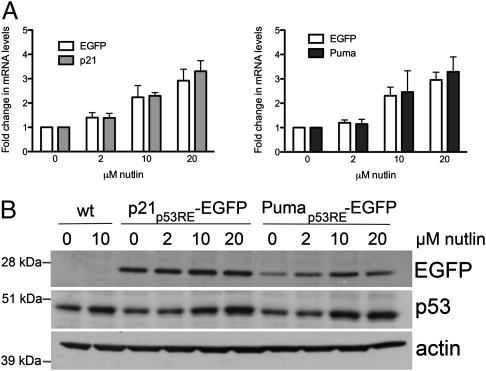
We first characterized EGFP reporter expression in vitro, using early passage primary mouse embryonic fibroblasts (MEFs) derived from reporter mice. To show that EGFP expression correlates with the induction of p53 transcriptional activity, we treated MEFs with increasing amounts of nutlin, a small molecule that increases p53 protein levels by inhibiting its degradation (21). Egfp mRNA levels in reporter MEFs increased upon nutlin treatment in a concentration-dependent manner, correlating with increases in the mRNA levels of the endogenous p21 and Puma genes (Fig. 2A). We confirmed that nutlin treatment induced an increase in both endogenous p53 protein and EGFP protein from both reporters (Fig. 2B). These data verify that EGFP expression from our reporter constructs serves as an indicator of p53 transcriptional activity and complement the published in vitro validation data obtained from embryonic stem cells (20).
Fig. 2.
EGFP expression is an indicator of p53 activity. A, RT-qPCR analysis of mRNA levels of the Egfp reporter or of endogenous p21 and Puma in p21p53RE-EGFP (Left) and Pumap53RE-EGFP (Right) MEFs treated with DMSO or increasing amounts of nutlin for 8 h. The increase in Egfp mRNA levels was dependent on nutlin concentration (P < 0.0001; two-way ANOVA) and correlated with the increase in p21 and Puma mRNA levels (P < 0.05; t test). Error bars indicate SD of three independent experiments. B, Western blots showing a concentration-dependent increase in EGFP and p53 protein levels after overnight treatment with nutlin. Actin served as a loading control.
Induction of EGFP Expression Is p53-Dependent.
To demonstrate that EGFP expression is p53-dependent, we crossed the reporter mice with p53 knockout mice. We isolated MEFs that carried either the p21p53RE-EGFP or Pumap53RE-EGFP reporter on a wild-type or p53-null background. We then characterized changes in EGFP expression after overnight treatment with nutlin or with the chemotherapeutic drug doxorubicin.
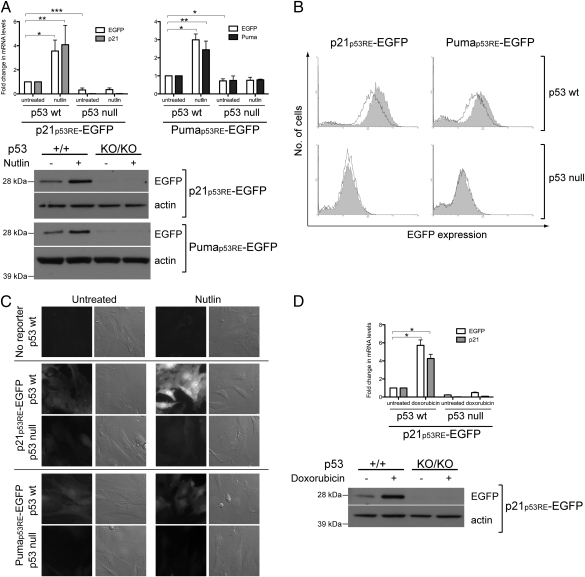
Nutlin treatment increased Egfp, p21, and Puma mRNA levels significantly in p53 wild-type but not in p53-null reporter MEFs (Fig. 3A, Upper). In contrast, Egfp mRNA levels were reduced in p53-null MEFs and remained unchanged upon nutlin treatment. Western blot analysis verified that EGFP protein increased in p53 wild-type reporter MEFs after nutlin treatment but was barely detectable in p53-null reporter MEFs (Fig. 3A, Lower). Furthermore, flow cytometric analysis for EGFP expression in p53 wild-type reporter MEFs showed that nutlin treatment shifted the histograms along the x axis in a positive direction (Fig. 3B, Top row), increasing the fluorescence intensity 1.7- and 1.5-fold in p21p53RE-EGFP and Pumap53RE-EGFP MEFs respectively, and indicating an increase in EGFP expression. However, the histograms corresponding to EGFP expression in p53-null reporter MEFs remained unchanged (Fig. 3B, Bottom row). Therefore, EGFP induction by nutlin treatment is p53-dependent.
Fig. 3.
Induction of EGFP expression is p53-dependent. A, RT-qPCR (Upper) and Western blot (Lower) analysis of p21p53RE-EGFP and Pumap53RE-EGFP MEFs after overnight treatment with 10 μM nutlin, showing EGFP induction only in p53 wild-type (p53+/+) but not p53-null (p53KO/KO) MEFs. Changes in mRNA levels were determined relative to those in the respective untreated p53+/+ reporter MEFs. Error bars indicate SD of four independent experiments. *P < 0.02; **P < 0.05; ***P < 0.0005 (t test). B, Flow cytometric analysis of MEFs derived from p21p53RE-EGFP (Left) and Pumap53RE-EGFP (Right) mice that were either p53 wild-type or p53-null. The bold lines and shaded histograms correspond to untreated and nutlin-treated MEFs, respectively. Data shown are representative of three independent experiments. C, Fluorescence imaging of unfixed p21p53RE-EGFP and Pumap53RE-EGFP MEFs that were either p53 wild-type or p53-null, as well as of p53 wild-type MEFs lacking either reporter. The MEFs were treated with DMSO or 10 μM nutlin overnight. Bright-field images are included to illustrate the presence of nonfluorescent MEFs. D, RT-qPCR (Upper) and Western blot (Lower) analysis of p21p53RE-EGFP MEFs after overnight treatment with 400 nM doxorubicin showing EGFP induction only in p53 wild-type (p53+/+) but not p53-null (p53KO/KO) MEFs. Changes in mRNA levels were determined relative to those in untreated p53+/+ reporter MEFs. Error bars indicate SD of three independent experiments. *P < 0.01 (t test).
Similar observations of EGFP levels were made upon fluorescence imaging of unfixed MEFs (Fig. 3C). EGFP fluorescence was detected in p53 wild-type reporter MEFs but not in p53 wild-type MEFs that do not carry either reporter. Upon nutlin treatment, fluorescence increased in p53 wild-type reporter MEFs but not p53-null reporter MEFs. Remarkably, p53 wild-type p21p53RE-EGFP MEFs showed higher levels of fluorescence than p53 wild-type Pumap53RE-EGFP MEFs. Indeed, Egfp mRNA levels were higher in p21p53RE-EGFP MEFs than Pumap53RE-EGFP MEFs (Fig. S1).
We also treated the primary MEFs with 400 nM doxorubicin to induce DNA damage (22) and, thus, activate p53. After overnight treatment, Egfp mRNA and protein were significantly induced in p53 wild-type but not p53-null p21p53RE-EGFP MEFs, with a corresponding increase in p21 mRNA in the p53 wild-type MEFs (Fig. 3D). However, doxorubicin did not significantly increase Egfp mRNA levels in Pumap53RE-EGFP MEFs (fold change of 2.3 ± 0.886 but P > 0.05; t test), showing that the two reporters responded differentially to identical induction conditions. A time course experiment showed that Egfp induction occurred just 2 h after doxorubicin addition, whereas Puma was induced at 4–8 h (Fig. S2A). Therefore, Egfp and Puma expression were induced much earlier than our original 16-h time point.
To show that EGFP induction corresponds to biological outcome, we performed a time-course experiment to assay apoptosis induced by nutlin and doxorubicin (Fig. S2B). Doxorubicin induced a prominent increase in caspase activity only after 24 h, whereas nutlin induced smaller but significant increases after 8 and 16 h. The kinetics of Puma induction and caspase activation are thus different for the two drugs but show similar trends to the respective Egfp inductions. These results suggest that EGFP expression is linked to biological outcome. Taken together, these data verify the p53 dependence of EGFP induction and also indicate that we could detect differences in EGFP expression in p21p53RE-EGFP and Pumap53RE-EGFP MEFs grown under identical conditions.
EGFP Expression, Like p53 Activity, Is Dependent on the Response Element, Tissue, and Stimulus.
The reporter dependence of EGFP expression in MEFs was confirmed by flow cytometric analysis of untreated reporter MEFs. There was more EGFP expression in Pumap53RE-EGFP MEFs than in MEFs lacking either reporter, and EGFP expression in p21p53RE-EGFP MEFs was even higher (Fig. 4A). This result suggests that p53 activity at REs within the p21 and Puma promoters can differ even in the same cell type subjected to the same growth conditions in vitro, and our reporter system is sensitive enough to discern these subtle differences.
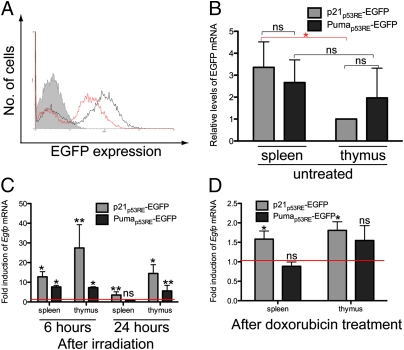
Fig. 4.
EGFP expression and p53 activity are dependent on the response element, tissue, and stimulus. A, Flow cytometric analysis of MEFs derived from p21p53RE-EGFP (black histogram) and Pumap53RE-EGFP (red histogram) mice or from mice lacking either reporter (shaded histogram). Graphs shown are representative of three independent experiments. B, RT-qPCR analysis of spleen and thymus tissue from untreated p21p53RE-EGFP and Pumap53RE-EGFP mice. Egfp mRNA levels were normalized to that in the untreated p21p53RE-EGFP thymus. Within either tissue, Egfp expression was not significantly affected by the reporter construct [not significant (ns): P > 0.1; t test]. There was a significant tissue-specific difference in Egfp expression from the p21p53RE-EGFP reporter (*P < 0.01; t test) but not the Pumap53RE-EGFP reporter (ns: P > 0.1; t test). Error bars indicate SD of six independent experiments. C, RT-qPCR analysis of spleen and thymus tissue from age-matched p21p53RE-EGFP and Pumap53RE-EGFP mice, harvested 6 or 24 h after treatment with 8-Gy ionizing radiation. Changes in Egfp mRNA levels were determined relative to basal levels in the respective tissues from nonirradiated mice, which are represented by the red line (i.e., fold induction of 1). At 24 h, radiation-induced Egfp expression was dependent on both tissue type (P < 0.01; two-way ANOVA) and reporter construct (P < 0.01; two-way ANOVA). Error bars indicate SD of at least three independent experiments. *P < 0.01; **P < 0.05; ns (P > 0.5) (t test). D, RT-qPCR analysis of spleen and thymus tissue from age-matched p21p53RE-EGFP and Pumap53RE-EGFP mice, harvested 24 h after injection with 0.01 mg/g doxorubicin. Changes in Egfp mRNA levels were determined relative to basal levels in the respective tissues from control mice, which are represented by the red line (i.e., fold induction of 1). Doxorubicin-induced Egfp expression was dependent on both tissue type (P < 0.02; two-way ANOVA) and reporter construct (P < 0.02; two-way ANOVA). Error bars indicate SD of three independent experiments. *P < 0.05; ns (P > 0.1) (t test).
To determine whether we could detect differences between the reporters in vivo, we first assayed Egfp mRNA levels in the spleen and thymus of untreated reporter mice. Egfp mRNA levels in each tissue were normalized to that in the thymus of the p21p53RE-EGFP mouse, which has the lowest Egfp mRNA levels of the samples tested (Fig. 4B). In the absence of p53 induction, there was no reporter-specific difference in Egfp expression within either tissue, nor was there a significant tissue-specific difference in Egfp expression from the Pumap53RE-EGFP reporter. There was, however, higher Egfp expression from the p21p53RE-EGFP reporter in the spleen than in the thymus (Fig. 4B).
We then induced p53 activity in vivo by subjecting age-matched reporter mice to total body irradiation, which is a well-characterized means for inducing genotoxic damage (23). We harvested the spleens and thymi, which are radiosensitive tissues with a robust p53 response (15), and determined Egfp mRNA levels relative to that in the respective tissues from untreated mice. Six hours after irradiation, increased Egfp mRNA levels were detected in the spleen and thymus of both p21p53RE-EGFP and Pumap53RE-EGFP mice. Twenty-four hours after irradiation, Egfp mRNA levels were still elevated except in the spleen of Pumap53RE-EGFP mice (Fig. 4C). Similarly, p21 and Puma mRNA levels were increased in both tissues 6 and 24 h after irradiation, whereas Puma induction was detected at 6 h but not at 24 h (Fig. S3 A and B). Statistical analysis revealed that EGFP expression after ionizing irradiation was dependent on both the reporter construct and tissue type. These data illustrate that we could detect and quantify reporter- and tissue-specific differences in p53 activity in vivo and that endogenous gene induction generally follows a similar trend to that of EGFP induction.
Given that p53 target gene selection is stimulus-specific (24), we tested whether our reporter system could detect differences in p53 transcriptional activity upon treatment with different genotoxic agents. We injected reporter mice with 0.01 mg/g doxorubicin and measured the induction of Egfp mRNA in each tissue. Twenty-four hours after doxorubicin injection, Egfp mRNA levels remained significantly elevated in the spleen and thymus of p21p53RE-EGFP mice but not in the corresponding tissues of Pumap53RE-EGFP mice (Fig. 4D). Indeed, we detected increased p21 mRNA in the spleen and no induction of Puma (Fig. S3C). EGFP expression after doxorubicin treatment was significantly affected by both reporter and tissue type. These data show that different p53-activating signals stimulate p53 activity differentially at the p21 and Puma promoters and that our reporter system provides us with a sensitive assay to characterize these responses.
p53 is Transcriptionally Active During Early Embryonic Development.
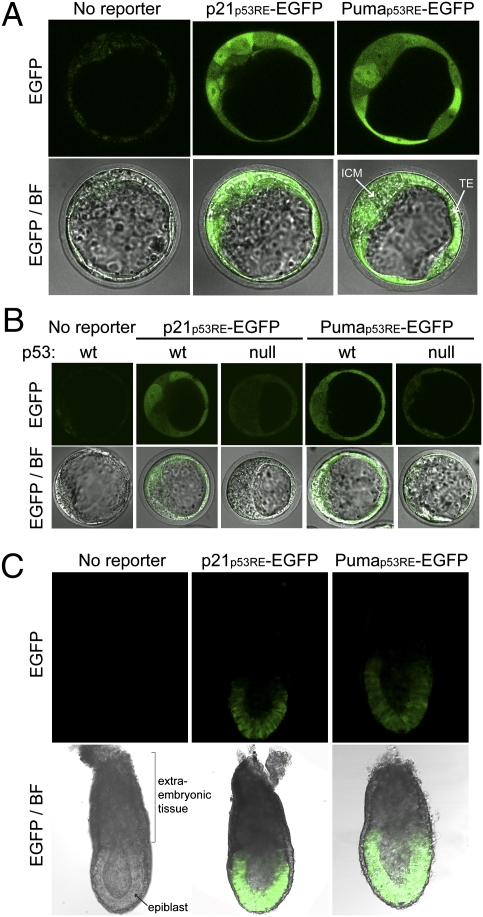
p53 is best known as a protector of genomic integrity, but it is also involved in a wide variety of other cellular processes, including stem cell biology and embryonic development (25). p53 function in these systems has important implications for its role in tumorigenesis but remains controversial. We took advantage of our EGFP reporter system to directly assay p53 activity in early stage embryos. We isolated them from mice homozygous for p21p53RE-EGFP or Pumap53RE-EGFP and examined them immediately by confocal microscopy. At embryonic day (E)3.5, EGFP fluorescence was detected in all blastocyst cells, including the inner cell mass (ICM), and trophectoderm (TE) (Fig. 5A). This fluorescence was reduced in p53-null reporter blastocysts and absent in blastocysts from mice lacking either reporter (Fig. 5 A and B). EGFP fluorescence was also detected in E6.5 postimplantation reporter embryos but only in the epiblasts and not in the extraembryonic tissues (Fig. 5C). These results show that p53 is indeed transcriptionally active during early embryogenesis, particularly in the cells that give rise to the embryo proper.
Fig. 5.
p53 is transcriptionally active in early embryonic development. Fluorescence microscopy of freshly harvested E3.5 blastocysts (A and B) and E6.5 embryos (C). ICM, inner cell mass; TE, trophectoderm.
Discussion
To study p53 target gene selection, we used targeted transgenesis to generate two strains of EGFP reporter mice to examine p53 activity at REs from the p21 and Puma promoters. The controlled and stable integration of the reporter constructs into the mouse genome gives these mice advantages over previously published transgenic (26–28) and xenograft (29) mouse models. In addition, the use of EGFP as a reporter enables us to exploit advances in microscope technology and to avoid complications arising from endogenous gene expression, such as the induction of endogenous β–galactosidase by treatments like ionizing radiation (30). These reporter mice are genetically identical except for the sequence of the p53 RE within the reporter construct; we know of no other reporter system that permits such a direct comparison of p53 activity at different target gene REs in vivo.
The in vitro and in vivo data obtained from our reporter mice are consistent with previous reports that the p53 response is dependent on promoter, cell type, and stimulus (15, 26, 28, 31–35). In vitro experiments have shown that p53 binds similarly to the p21 3′ site and the Puma BS2 (36, 37) REs used in our reporter constructs; therefore, binding affinity is unlikely to explain the differences in p53 activity that we observed. It was recently shown that DNA-bound p53 can adopt a variety of quaternary structures, suggesting a model in which posttranslational modifications, protein cofactors, and DNA sequence can modulate p53–DNA interaction and, thus, target gene selection (38). Indeed, experiments with knock-in mice have shown that phosphorylation has a cell type- and stimulus-specific effect on p53 function (39) and that p53 acetylation is essential for its transactivation of genes such as p21 and Puma (40). Our data suggest that the primary DNA sequence of the p53 RE is sufficient to confer selectivity in terms of target gene activation, highlighting the importance of posttranslational modifications and cofactor binding in p53 target gene selection.
Our use of EGFP as a reporter for p53 activity permits us to image unfixed tissue, an advantage that we exploited to investigate the unresolved contribution of p53 to early embryonic development. Experiments with embryonic stem (ES) cells have yielded conflicting data, either that p53 is transcriptionally inactive in ES cells (41–43) or that p53 is indeed active and responsive to stress (20, 44, 45). We observed distinct and specific EGFP fluorescence in the ICM of E3.5 mouse blastocysts, indicating that p53 is indeed transcriptionally active in the cells from which ES cells are derived. We also examined E6.5 embryos, which consist of both embryonic and extraembryonic cell types and are a younger gestational age than those studied in previous reports (26, 28, 46, 47). At this stage, the embryonic tissue is called the epiblast and comprises pluripotent cells that are proliferating rapidly. p53 activity was readily detected in the epiblasts of E6.5 embryos even in the absence of exogenous damage, but it was notably absent in the extraembryonic tissue. Therefore, p53 is active and poised for its role in developmental processes such as differentiation and self-renewal. Previous reports indicate that high p53 mRNA and protein levels do not necessarily correlate with p53 activity (26, 46). The use of EGFP levels as a direct measurement of p53 activity in our reporter mice could provide an important approach for the study of p53 function during embryogenesis.
Our reporter system may also provide useful drug discovery tools, given that the induction of cell cycle arrest and apoptosis by p53 are common assays in drug development. Restoring wild-type p53 activity may be an anticancer strategy, as shown by genetic (48–50) and xenograft (21, 51, 52) experiments. Our reporter mice provide a renewable source of various primary cells in which p53 activity can be sensitively assayed and may be used in vitro to screen for p53-inducting molecules. The reporter mice may then be used to characterize the p53 response to specific chemotherapeutic drugs. The information obtained on pharmacokinetics, pharmacodynamics, and therapeutic index will be invaluable for the optimization of therapeutic regimens.
In vivo studies have shown that acute genotoxic stress induces two waves of p53 activation: an immediate response that results in widespread apoptosis and pathology but that is not required for tumor suppression; and a delayed response that is indeed crucial for cancer prevention (53). Recent reports show that p53-dependent tumor suppression is activated only when there are high levels of oncogene activity (54, 55). By studying when, where, and how p53 induces cell cycle arrest/senescence and apoptosis, we can manipulate them for cancer prevention or therapy to minimize toxic side effects and maximize efficacy.
Materials and Methods
Mice.
All mouse experiments were approved by the A*STAR Institutional Animal Care and Use Committee (IACUC) and performed in compliance with IACUC regulations. The reporter mice were generated at the HPRT Targeted Transgenesis Core Facility, run by the Canadian Genetic Diseases Network (17, 18). The chimeric mice produced were backcrossed to C57BL/6 albino mice for at least seven generations to generate near congenic (>99%) mice. The reporter constructs were further verified by sequencing genomic DNA from primary MEFs derived from each reporter mouse strain. The Trp53 knockout mouse strain was generated by crossing Trp53 conditional knockout mice (56) with mice expressing Cre under the control of the β-actin promoter (57). To obtain reporter mice with varying p53 genotypes, Trp53-knockout males were mated with p21p53RE-EGFP or Pumap53RE-EGFP reporter-homozygous females. The progeny were then intercrossed to obtain mice homozygous for each reporter. Males were used for experiments at 8–12 wk of age. Age-matched p21p53RE-EGFP and Pumap53RE-EGFP mice were subject to a single dose of 8 Gy total body irradiation or a single i.p. injection of 0.01 mg/g doxorubicin dissolved in PBS (28). The spleens and thymi were harvested and immediately immersed in RNAlater (Invitrogen) in preparation for subsequent RT–quantitative (q)PCR analysis.
Mouse Embryos.
To obtain embryos at the desired stages of development, matings were set up at the end of the day, and the females were assessed for copulation plugs the following morning. E3.5 blastocysts were obtained using standard protocols (58). In brief, female mice were superovulated with pregnant mare serum gonadotropin and human CG (5 IU each; Sigma) and then set up in timed matings, following which E3.5 blastocysts were recovered by flushing the uterine horns and oviducts with M2 media (Millipore).
Tissue Culture.
Primary MEFs were prepared from E13.5 embryos and cultured in DMEM containing 10% FBS and antibiotics. Only early passage primary MEFs from male embryos were used for experiments. The MEFs were treated with the indicated amounts of nutlin-3a (Calbiochem), doxorubicin (Sigma), or vehicle control for 8 h or overnight (16 h) before being harvested for RNA or protein analysis. Caspase activation was assayed using the Caspase-Glo 3/7 Assay (Promega).
RNA Analysis.
RNA from MEFs was prepared using the RNeasy kit (QIAGEN). RNA from mouse tissues was prepared by TRIzol (Invitrogen) extraction, digested with RNase-free DNase (QIAGEN), and further purified using the RNeasy kit (QIAGEN). Reverse transcription was performed with MultiScribe Reverse Transcriptase (Applied Biosystems). Real-time PCRs were performed using iQ SYBR Green Supermix (Bio-Rad) on the Bio-Rad CFX96 RealTime System.
Western Blot.
MEFs were harvested and lysed in RIPA buffer [20 mM Tris-HCl (pH 7.5), 2 mM EDTA, 150 mM NaCl, 0.25% SDS, 1% Nonidet P-40, 1% deoxycholate] containing protease inhibitor mixture (Calbiochem). Protein concentrations were measured by BCA assay (Pierce) before Western blotting with these antibodies: EGFP (Invitrogen; A11122), actin [AC-15 (Abcam); ab6276], p21 [F-5 (Santa Cruz Biotechnology); sc-6246], and p53 (CM5 rabbit polyclonal antibody; a gift from Borivoj Vojtesek, Masaryk Memorial Cancer Institute, Czech Republic).
Flow Cytometry.
MEFs were harvested by trypsinization and resuspended in PBS containing 1% FBS. The same number of cells were gated and analyzed in each experiment. Flow cytometric data were acquired using a Becton-Dickinson LSR II and were analyzed using WinMDI software.
Microscopy.
Images of MEFs were captured with a Nikon Ti widefield fluorescence microscope equipped with a QuantEM CCD camera. Embryo images were captured with an Olympus FV1000 inverted confocal microscope. All images were taken with 60× objective lenses. Image analysis was performed with ImageJ 1.45d software (National Institutes of Health).
Statistical Analysis.
One-sample t tests and two-way ANOVA were performed using GraphPad Prism software.
Supplementary Material
Acknowledgments
We thank Nancy Jenkins for support and useful discussions; Graham Wright for microscopy advice; Sebastien Teissier for advice on flow cytometry; and Keith Rogers, Susan Rogers, and the Histopathology Laboratory for technical help. We also thank Borivoj Vojtesek for the p53 CM5 antibody. This work was funded by the Agency for Science, Technology and Research (A*STAR).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114173109/-/DCSupplemental.
References
- 1.Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: Functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 2.Jackson JG, Post SM, Lozano G. Regulation of tissue- and stimulus-specific cell fate decisions by p53 in vivo. J Pathol. 2011;223:127–136. doi: 10.1002/path.2783. [DOI] [PubMed] [Google Scholar]
- 3.Macleod KF, et al. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995;9:935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- 4.el-Deiry WS, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Y, et al. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 6.Jackson JG, Pereira-Smith OM. p53 is preferentially recruited to the promoters of growth arrest genes p21 and GADD45 during replicative senescence of normal human fibroblasts. Cancer Res. 2006;66:8356–8360. doi: 10.1158/0008-5472.CAN-06-1752. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 8.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 9.Han J, et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci USA. 2001;98:11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA. 2003;100:1931–1936. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villunger A, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 12.Hemann MT, et al. Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci USA. 2004;101:9333–9338. doi: 10.1073/pnas.0403286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeffers JR, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 14.Qiu W, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fei P, Bernhard EJ, El-Deiry WS. Tissue-specific induction of p53 targets in vivo. Cancer Res. 2002;62:7316–7327. [PubMed] [Google Scholar]
- 16.Resnick-Silverman L, St Clair S, Maurer M, Zhao K, Manfredi JJ. Identification of a novel class of genomic DNA-binding sites suggests a mechanism for selectivity in target gene activation by the tumor suppressor protein p53. Genes Dev. 1998;12:2102–2107. doi: 10.1101/gad.12.14.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bronson SK, et al. Single-copy transgenic mice with chosen-site integration. Proc Natl Acad Sci USA. 1996;93:9067–9072. doi: 10.1073/pnas.93.17.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palais G, et al. Targeted transgenesis at the HPRT locus: An efficient strategy to achieve tightly controlled in vivo conditional expression with the tet system. Physiol Genomics. 2009;37:140–146. doi: 10.1152/physiolgenomics.90328.2008. [DOI] [PubMed] [Google Scholar]
- 19.Jasin M, Moynahan ME, Richardson C. Targeted transgenesis. Proc Natl Acad Sci USA. 1996;93:8804–8808. doi: 10.1073/pnas.93.17.8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ménendez S, et al. MDM4 downregulates p53 transcriptional activity and response to stress during differentiation. Cell Cycle. 2011;10:1100–1108. doi: 10.4161/cc.10.7.15090. [DOI] [PubMed] [Google Scholar]
- 21.Vassilev LT, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 22.Attardi LD, de Vries A, Jacks T. Activation of the p53-dependent G1 checkpoint response in mouse embryo fibroblasts depends on the specific DNA damage inducer. Oncogene. 2004;23:973–980. doi: 10.1038/sj.onc.1207026. [DOI] [PubMed] [Google Scholar]
- 23.Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer. 2003;3:117–129. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- 24.Bunz F, et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spike BT, Wahl GM. p53, stem cells, and reprogramming: Tumor suppression beyond guarding the genome. Genes Cancer. 2011;2:404–419. doi: 10.1177/1947601911410224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottlieb E, et al. Transgenic mouse model for studying the transcriptional activity of the p53 protein: Age- and tissue-dependent changes in radiation-induced activation during embryogenesis. EMBO J. 1997;16:1381–1390. doi: 10.1093/emboj/16.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamstra DA, et al. Real-time evaluation of p53 oscillatory behavior in vivo using bioluminescent imaging. Cancer Res. 2006;66:7482–7489. doi: 10.1158/0008-5472.CAN-06-1405. [DOI] [PubMed] [Google Scholar]
- 28.Komarova EA, et al. Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J. 1997;16:1391–1400. doi: 10.1093/emboj/16.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, El-Deiry WS. Bioluminescent molecular imaging of endogenous and exogenous p53-mediated transcription in vitro and in vivo using an HCT116 human colon carcinoma xenograft model. Cancer Biol Ther. 2003;2:196–202. doi: 10.4161/cbt.2.2.347. [DOI] [PubMed] [Google Scholar]
- 30.Coates PJ, Lorimore SA, Rigat BA, Lane DP, Wright EG. Induction of endogenous beta-galactosidase by ionizing radiation complicates the analysis of p53-LacZ transgenic mice. Oncogene. 2001;20:7096–7097. doi: 10.1038/sj.onc.1204904. [DOI] [PubMed] [Google Scholar]
- 31.Bouvard V, et al. Tissue and cell-specific expression of the p53-target genes: bax, fas, mdm2 and waf1/p21, before and following ionising irradiation in mice. Oncogene. 2000;19:649–660. doi: 10.1038/sj.onc.1203366. [DOI] [PubMed] [Google Scholar]
- 32.Burns TF, Bernhard EJ, El-Deiry WS. Tissue specific expression of p53 target genes suggests a key role for KILLER/DR5 in p53-dependent apoptosis in vivo. Oncogene. 2001;20:4601–4612. doi: 10.1038/sj.onc.1204484. [DOI] [PubMed] [Google Scholar]
- 33.Coates PJ, Lorimore SA, Lindsay KJ, Wright EG. Tissue-specific p53 responses to ionizing radiation and their genetic modification: The key to tissue-specific tumour susceptibility? J Pathol. 2003;201:377–388. doi: 10.1002/path.1456. [DOI] [PubMed] [Google Scholar]
- 34.Murray-Zmijewski F, Slee EA, Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol. 2008;9:702–712. doi: 10.1038/nrm2451. [DOI] [PubMed] [Google Scholar]
- 35.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 36.Veprintsev DB, Fersht AR. Algorithm for prediction of tumour suppressor p53 affinity for binding sites in DNA. Nucleic Acids Res. 2008;36:1589–1598. doi: 10.1093/nar/gkm1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberg RL, Veprintsev DB, Bycroft M, Fersht AR. Comparative binding of p53 to its promoter and DNA recognition elements. J Mol Biol. 2005;348:589–596. doi: 10.1016/j.jmb.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Melero R, et al. Electron microscopy studies on the quaternary structure of p53 reveal different binding modes for p53 tetramers in complex with DNA. Proc Natl Acad Sci USA. 2011;108:557–562. doi: 10.1073/pnas.1015520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kenzelmann Broz D, Attardi LD. In vivo analysis of p53 tumor suppressor function using genetically engineered mouse models. Carcinogenesis. 2010;31:1311–1318. doi: 10.1093/carcin/bgp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133:612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aladjem MI, et al. ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr Biol. 1998;8:145–155. doi: 10.1016/s0960-9822(98)70061-2. [DOI] [PubMed] [Google Scholar]
- 42.Hong Y, Stambrook PJ. Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation. Proc Natl Acad Sci USA. 2004;101:14443–14448. doi: 10.1073/pnas.0401346101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solozobova V, Rolletschek A, Blattner C. Nuclear accumulation and activation of p53 in embryonic stem cells after DNA damage. BMC Cell Biol. 2009;10:46. doi: 10.1186/1471-2121-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabapathy K, Klemm M, Jaenisch R, Wagner EF. Regulation of ES cell differentiation by functional and conformational modulation of p53. EMBO J. 1997;16:6217–6229. doi: 10.1093/emboj/16.20.6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu D, et al. Ets1 is required for p53 transcriptional activity in UV-induced apoptosis in embryonic stem cells. EMBO J. 2002;21:4081–4093. doi: 10.1093/emboj/cdf413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmid P, Lorenz A, Hameister H, Montenarh M. Expression of p53 during mouse embryogenesis. Development. 1991;113:857–865. doi: 10.1242/dev.113.3.857. [DOI] [PubMed] [Google Scholar]
- 47.Rinon A, et al. p53 coordinates cranial neural crest cell growth and epithelial-mesenchymal transition/delamination processes. Development. 2011;138:1827–1838. doi: 10.1242/dev.053645. [DOI] [PubMed] [Google Scholar]
- 48.Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127:1323–1334. doi: 10.1016/j.cell.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Ventura A, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 50.Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Issaeva N, et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10:1321–1328. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 52.Shangary S, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci USA. 2008;105:3933–3938. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- 54.Feldser DM, et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature. 2010;468:572–575. doi: 10.1038/nature09535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Junttila MR, et al. Selective activation of p53-mediated tumour suppression in high-grade tumours. Nature. 2010;468:567–571. doi: 10.1038/nature09526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jonkers J, et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 57.Lewandoski M, Martin GR. Cre-mediated chromosome loss in mice. Nat Genet. 1997;17:223–225. doi: 10.1038/ng1097-223. [DOI] [PubMed] [Google Scholar]
- 58.Wong ES, et al. A simple procedure for the efficient derivation of mouse ES cells. Methods Enzymol. 2010;476:265–283. doi: 10.1016/S0076-6879(10)76015-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.