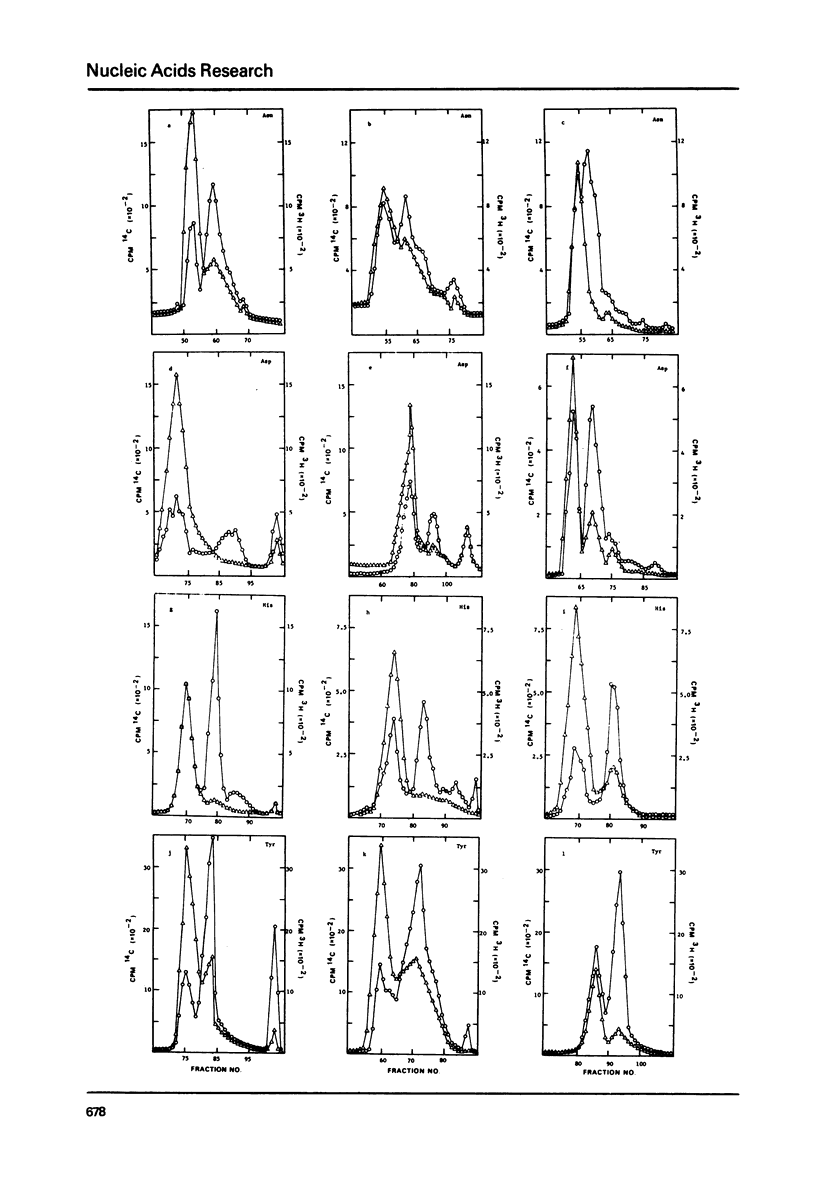
Abstract
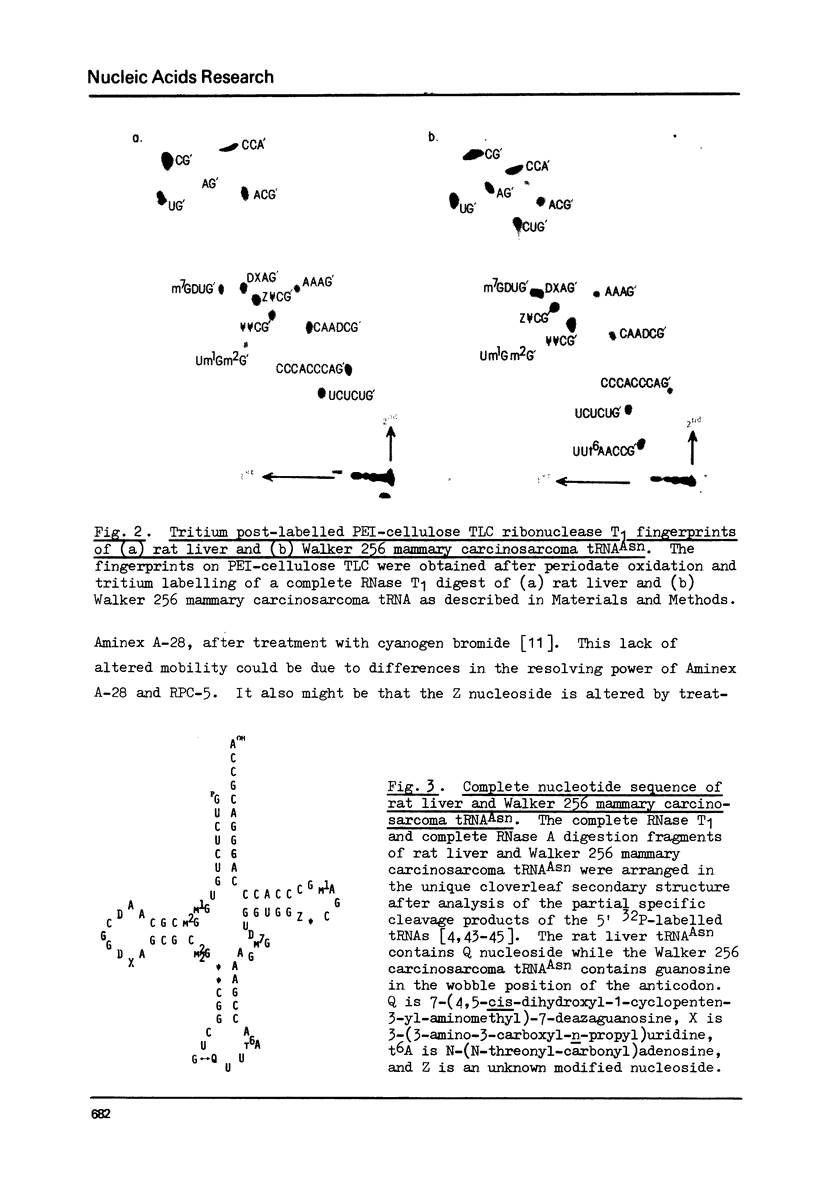
The complete nucleotide sequences of both rat liver and Walker 256 mammary carcinosarcoma tRNAAsn reveal that they are identical except for the nucleotide present in the wobble position of the anticodon loop. The rat liver tRNAAsn contains the Q nucleoside, whereas the tumour tRNAAsn contains an unmodified guanosine. The tRNAs from both tissues also show significant quantitative differences in the chromatographic mobilities for isoaccepting species of tRNAAsp, tRNAAsn, tRNAHis and tRNATyr. In addition, chromatographic shifts upon cyanogen bromide treatment and analyses of the alkaline hydrolysates of these tRNAs demonstrate that those of tumour origin contain significantly less Q and Q nucleoside than their normal rat liver counterparts.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amandaraj M. P., Roe B. A. Purification of human placenta phenylalanine, valine, methionine, glucine, and serine transfer ribonucleic acids. Biochemistry. 1975 Nov 18;14(23):5068–5073. doi: 10.1021/bi00694a006. [DOI] [PubMed] [Google Scholar]
- Baxt W., Hehlmann R., Spiegelman S. Human leukaemic cells contain reverse transcriptase associated with a high molecular weight virus-related RNA. Nat New Biol. 1972 Nov 15;240(98):72–75. doi: 10.1038/newbio240072a0. [DOI] [PubMed] [Google Scholar]
- Bertrand K., Korn L., Lee F., Platt T., Squires C. L., Squires C., Yanofsky C. New features of the regulation of the tryptophan operon. Science. 1975 Jul 4;189(4196):22–26. doi: 10.1126/science.1094538. [DOI] [PubMed] [Google Scholar]
- Blobstein S. H., Gebert R., Grunberger D., Nakanishi K., Weinstein I. B. Structure of the fluorescent nucleoside of yeast phenylalanine transfer ribonucleic acid. Arch Biochem Biophys. 1975 Apr;167(2):668–673. doi: 10.1016/0003-9861(75)90510-x. [DOI] [PubMed] [Google Scholar]
- Borek E., Kerr S. J. Atypical transfer RNA's and their origin in neoplastic cells. Adv Cancer Res. 1972;15:163–190. doi: 10.1016/s0065-230x(08)60374-7. [DOI] [PubMed] [Google Scholar]
- Briscoe W. T., Griffin A. C., McBride C., Bowen J. M. The distribution and properties of aspartyl transfer RNA in human and animal tumors. Cancer Res. 1975 Sep;35(9):2586–2593. [PubMed] [Google Scholar]
- Briscoe W. T., Syrewicz J. J., Marshall M. V., Griffin A. C. Regulation of an aspartyl-tRNA species in BHK cells in culture and in solid tumor form. Biochim Biophys Acta. 1975 Apr 2;383(4):441–445. doi: 10.1016/0005-2787(75)90314-7. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Roe B. A. A two-dimensional thin layer chromatographic procedure for the sequential analysis of oligonucleotides employing tritium post-labeling. Nucleic Acids Res. 1977 Oct;4(10):3563–3572. doi: 10.1093/nar/4.10.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Roe B. A. Sequence studies on human placenta tRNAVal : comparison with the mouse myeloma tRNAVal. Biochem Biophys Res Commun. 1977 Sep 23;78(2):631–640. doi: 10.1016/0006-291x(77)90226-1. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Roe B. A. The application of PEI-cellulose thin-layer chromatography for the resolution of large oligonucleotide fragments of transfer ribonucleic acids. Anal Biochem. 1978 Aug 15;89(1):45–59. doi: 10.1016/0003-2697(78)90725-x. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Roe B. A. The nucleotide sequence of rat liver tRNAAsn. Biochem Biophys Res Commun. 1978 May 15;82(1):235–246. doi: 10.1016/0006-291x(78)90601-0. [DOI] [PubMed] [Google Scholar]
- Cortese R., Landsberg R., Haar R. A., Umbarger H. E., Ames B. N. Pleiotropy of hisT mutants blocked in pseudouridine synthesis in tRNA: leucine and isoleucine-valine operons. Proc Natl Acad Sci U S A. 1974 May;71(5):1857–1861. doi: 10.1073/pnas.71.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM L. J., STEWART H. L. A survey of transplantable and transmissible animal tumors. J Natl Cancer Inst. 1953 Apr;13(5):1299–1377. [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrul E. F., Farkas W. R. Partial purification and properties of the reticulocyte guanylating enzyme. Biochim Biophys Acta. 1976 Sep 6;442(3):379–390. doi: 10.1016/0005-2787(76)90312-9. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., Levinson W. E., Goodman H. M., Bishop J. M. RNA-directed DNA polymerase of Rous sarcoma virus: initiation of synthesis with 70 S viral RNA as template. J Mol Biol. 1973 Sep 5;79(1):163–183. doi: 10.1016/0022-2836(73)90277-5. [DOI] [PubMed] [Google Scholar]
- Farkas W. R., Chernoff D. Identification of the minor guanylated tRNA of rabbit reticulocytes. Nucleic Acids Res. 1976 Oct;3(10):2521–2528. doi: 10.1093/nar/3.10.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S., Li H. J., Nakanishi K., Van Lear G. 3-(3-amino-3-carboxy-n-propyl)uridine. The structure of the nucleoside in Escherichia coli transfer ribonucleic acid that reacts with phenoxyacetoxysuccinimide. Biochemistry. 1974 Jul 2;13(14):2932–2937. doi: 10.1021/bi00711a024. [DOI] [PubMed] [Google Scholar]
- Gallagher R. E., Gallo R. C. Chromatographic analyses of isoaccepting tRNAs from avian myeloblastosis virus. J Virol. 1973 Sep;12(3):449–457. doi: 10.1128/jvi.12.3.449-457.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R. E., Ting R. C., Gallo R. C. A common change of aspartyl-tRNA in polyoma- and SV40 -transformed cells. Biochim Biophys Acta. 1972 Jul 31;272(4):568–582. doi: 10.1016/0005-2787(72)90512-6. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Roe B. A., Anandaraj M. P., RajBhandary U. L. Nucleotide sequence of human placenta cytoplasmic initiator tRNA. Cell. 1975 Nov;6(3):407–413. doi: 10.1016/0092-8674(75)90190-7. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., Olson M. V., Hall B. D. Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5453–5457. doi: 10.1073/pnas.74.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H. J., de Henau S., Crothers D. M. On the physical basis for ambiguity in genetic coding interactions. Proc Natl Acad Sci U S A. 1978 Feb;75(2):610–614. doi: 10.1073/pnas.75.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberger D., Weinstein I. B., Mushinski J. F. Deficiency of the Y base in a hepatoma phenylalanine tRNA. Nature. 1975 Jan 3;253(5486):66–67. doi: 10.1038/253066a0. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Randerath E., Randerath K. A double-labeling procedure for sequence analysis of picomole amounts of nonradioactive RNA fragments. Nucleic Acids Res. 1976 Nov;3(11):2895–2914. doi: 10.1093/nar/3.11.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Nishimura S. Possible anticodon sequences of tRNA His , tRNA Asm , and tRNA Asp from Escherichia coli B. Universal presence of nucleoside Q in the first postion of the anticondons of these transfer ribonucleic acids. Biochemistry. 1972 Jan 18;11(2):301–308. doi: 10.1021/bi00752a024. [DOI] [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Hayashi M., Griffin A. C., Duff R., Rapp F. Chromatographic studies of tyrosyl and phenylalanyl transfer RNA's of liver and tumor cells. Cancer Res. 1973 Apr;33(4):902–905. [PubMed] [Google Scholar]
- Itoh Y. H., Itoh T., Haruna I., Watanabe I. Substitution of guanine for a specific base in tRNA by extracts of Ehrlich ascites tumour cells. Nature. 1977 Jun 2;267(5610):467–467. doi: 10.1038/267467a0. [DOI] [PubMed] [Google Scholar]
- Jacobson E. L., Juarez H., Hedgcoth C., Consigli R. A. An extra species of lysine transfer ribonucleic acid in polyoma virus-transformed cells in tissue culture. Arch Biochem Biophys. 1974 Aug;163(2):666–670. doi: 10.1016/0003-9861(74)90527-x. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B. Role of an isoacceptor transfer ribonucleic acid as an enzyme inhibitor: effect on tryptophan pyrrolase of Drosophila. Nat New Biol. 1971 May 5;231(18):17–19. [PubMed] [Google Scholar]
- Kasai H., Kuchino Y., Nihei K., Nishimura S. Distribution of the modified nucleoside Q and its derivatives in animal and plant transfer RNA's. Nucleic Acids Res. 1975 Oct;2(10):1931–1939. doi: 10.1093/nar/2.10.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Nakanishi K., Macfarlane R. D., Torgerson D. F., Ohashi Z., McCloskey J. A., Gross H. J., Nishimura S. Letter: The structure of Q* nucleoside isolated from rabbit liver transfer ribonucleic acid. J Am Chem Soc. 1976 Aug 4;98(16):5044–5046. doi: 10.1021/ja00432a071. [DOI] [PubMed] [Google Scholar]
- Kasai H., Oashi Z., Harada F., Nishimura S., Oppenheimer N. J., Crain P. F., Liehr J. G., von Minden D. L., McCloskey J. A. Structure of the modified nucleoside Q isolated from Escherichia coli transfer ribonucleic acid. 7-(4,5-cis-Dihydroxy-1-cyclopenten-3-ylaminomethyl)-7-deazaguanosine. Biochemistry. 1975 Sep 23;14(19):4198–4208. doi: 10.1021/bi00690a008. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Alterations in SVT2 cell transfer RNAs in response to cell density and serum type. Biochim Biophys Acta. 1975 Mar 10;383(2):131–139. doi: 10.1016/0005-2787(75)90254-3. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Borek E. Changes in transfer RNA's in human malignant trophoblastic cells (BeWo line). Cancer Res. 1976 Aug;36(8):2932–2936. [PubMed] [Google Scholar]
- Kuchino Y., Borek E. Tumour-specific phenylalanine tRNA contains two supernumerary methylated bases. Nature. 1978 Jan 12;271(5641):126–129. doi: 10.1038/271126a0. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan T. F., Gilham P. T., Söll D. An improved method for the purification of tRNA by chromatography on dihydroxyboryl substituted cellulose. Nucleic Acids Res. 1975 Jun;2(6):853–864. doi: 10.1093/nar/2.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan S., Körner A., Low K. B., Söll D. Regulation of biosynthesis of aminoacyl-tRNA synthetases and of tRNA in Escherichia coli. I. Isolation and characterization of a mutant with elevated levels of tRNAGln 1. J Mol Biol. 1977 Dec 25;117(4):1013–1031. doi: 10.1016/s0022-2836(77)80010-7. [DOI] [PubMed] [Google Scholar]
- Okada N., Harada F., Nishimura S. Specific replacement of Q base in the anticodon of tRNA by guanine catalyzed by a cell-free extract of rabbit reticulocytes. Nucleic Acids Res. 1976 Oct;3(10):2593–2603. doi: 10.1093/nar/3.10.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Shindo-Okada N., Nishimura S. Isolation of mammalian tRNAAsp and tRNATyr by lectin-Sepharose affinity column chromatography. Nucleic Acids Res. 1977 Feb;4(2):415–423. doi: 10.1093/nar/4.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Shindo-Okada N., Sato S., Itoh Y. H., Oda K., Nishimura S. Detection of unique tRNA species in tumor tissues by Escherichia coli guanine insertion enzyme. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4247–4251. doi: 10.1073/pnas.75.9.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Yasuda T., Nishimura S. Detection of nucleoside Q precursor in methyl-deficient E.coli tRNA. Nucleic Acids Res. 1977 Dec;4(12):4063–4075. doi: 10.1093/nar/4.12.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen C. E., Penhoet E. E. Chromatographic and functional comparison of human placenta and HeLa cell tyrosine transfer ribonucleic acids. Biochemistry. 1976 Oct 19;15(21):4649–4654. doi: 10.1021/bi00666a016. [DOI] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal F. H., Simonds J. S., Twardzik D., Oskarsson M. Effects of simian virus 40-induced transformation on isoaccepting species of transfer RNA from mouse fibroblasts. J Virol. 1973 Dec;12(6):1616–1619. doi: 10.1128/jvi.12.6.1616-1619.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath E., Yu C. T., Randerath K. Base analysis of ribopolynucleotides by chemical tritium labeling: a methodological study with model nucleosides and purified tRNA species. Anal Biochem. 1972 Jul;48(1):172–198. doi: 10.1016/0003-2697(72)90181-9. [DOI] [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E. Analysis of nucleic acid derivatives at the subnanomole level. 3. A tritium labeling procedure for quantitative analysis of ribose derivatives. Anal Biochem. 1969 Apr 4;28(1):110–118. doi: 10.1016/0003-2697(69)90162-6. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E., Chia L. S., Nowak B. J. Base analysis of ribopolynucleotides by chemical tritium labeling: an improved mapping procedure for nucleoside trialcohols. Anal Biochem. 1974 May;59(1):263–271. doi: 10.1016/0003-2697(74)90032-3. [DOI] [PubMed] [Google Scholar]
- Rizzino A., Mastanduno M., Freundlich M. Partial derepression of the isoleucine-valine enzymes during methionine starvation is Salmonella typhimurium. Biochim Biophys Acta. 1977 Mar 18;475(2):267–275. doi: 10.1016/0005-2787(77)90017-x. [DOI] [PubMed] [Google Scholar]
- Robert M. S., Smith R. G., Gallo R. C., Sarin P. S., Abrell J. W. Viral and cellular DNA polymerase: comparison of activities with synthetic and natural RNA templates. Science. 1972 May 19;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Anandaraj M. P., Chia L. S., Randerath E., Gupta R. C., Randerath K. Sequence studies on tRNAPhe from placenta: comparison with known sequences of tRNAPhe from other normal mammalian tissues. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1097–1105. doi: 10.1016/0006-291x(75)90470-2. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Chen E. Y., Tsen H. Y. Studies on the ribothymidine content of specific rat and human tRNAs: a postulated role for 5-methyl cytosine in the regulation of ribothymidine biosynthesis. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1339–1347. doi: 10.1016/0006-291x(76)90343-0. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Stankiewicz A. F., Chen C. Y. Chromatographic behavior of several mammalian tRNAs on acylated dihydroxyl-borate cellulose and Aminex A-28. Nucleic Acids Res. 1977 Jul;4(7):2191–2204. doi: 10.1093/nar/4.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B. A. Studies on human tRNA. I. The rapid, large scale isolation and partial fractionation of placenta and liver tRNA. Nucleic Acids Res. 1975 Jan;2(1):21–42. doi: 10.1093/nar/2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B. A., Tsen H. Y. Role of ribothymidine in mammalian tRNAPhe. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3696–3700. doi: 10.1073/pnas.74.9.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R., Giveon D., Kimhi Y., Littauer U. Z. Abundance of tRNAPhe lacking the peroxy Y-base in mouse neuroblastoma. Biochemistry. 1976 Nov 30;15(24):5258–5262. doi: 10.1021/bi00669a010. [DOI] [PubMed] [Google Scholar]
- Saneyoshi M., Nishimura S. Selective modification of 4-thiouridylate residue in Escherichia coli transfer RNA with cyanogen bromide. Biochim Biophys Acta. 1970 Apr 15;204(2):389–399. doi: 10.1016/0005-2787(70)90158-9. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Singhal R. P., Griffin G. D., Novelli G. D. Separation of transfer ribonucleic acids on polystyrene anion exchangers. Biochemistry. 1976 Nov 16;15(23):5083–5087. doi: 10.1021/bi00668a021. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Grüter F., Gauss D. H. Collection of published tRNA sequences. Nucleic Acids Res. 1978 May;5(5):r15–r27. [PMC free article] [PubMed] [Google Scholar]
- Taylor M. W., Wang S., Kothari R. M., Hung P. P. Chromatographic analyses of isoaccepting tRNAs from avian tumor viruses. J Virol. 1974 Nov;14(5):1092–1098. doi: 10.1128/jvi.14.5.1092-1098.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Venegas A., Weinberg F., Bishop R., Rutter W. J. Structure of yeast phenylalanine-tRNA genes: an intervening DNA segment within the region coding for the tRNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):190–194. doi: 10.1073/pnas.75.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C. Transfer RNA into RNA tumor viruses. Prog Nucleic Acid Res Mol Biol. 1977;20:131–160. doi: 10.1016/s0079-6603(08)60471-7. [DOI] [PubMed] [Google Scholar]
- White B. N. Chromatographic changes in specific tRNAs after reaction with cyanogen bromide and sodium periodate. Biochim Biophys Acta. 1974 Jul 11;353(3):283–291. doi: 10.1016/0005-2787(74)90021-5. [DOI] [PubMed] [Google Scholar]
- White B. N., Tener G. M. Activity of a transfer RNA modifying enzyme during the development of Drosophila and its relationship to the su(s) locus. J Mol Biol. 1973 Mar 15;74(4):635–651. doi: 10.1016/0022-2836(73)90054-5. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Mutations affecting tRNATrp and its charging and their effect on regulation of transcription termination at the attenuator of the tryptophan operon. J Mol Biol. 1977 Jul 15;113(4):663–677. doi: 10.1016/0022-2836(77)90229-7. [DOI] [PubMed] [Google Scholar]




