Abstract
The presence of mycobacterial antigens in leprosy skin lesions was studied by immunohistological methods using monoclonal antibodies (MAbs) to Mycobacterium leprae-specific phenolic glycolipid I (PGL-I) and to cross-reactive mycobacterial antigens of 36 kd, 65 kd, and lipoarabinomannan (LAM). The staining patterns with MAb to 36 kd and 65 kd were heterogeneous and were also seen in the lesions of other skin diseases. The in situ staining of PGL-I and LAM was seen only in leprosy. Both antigens were abundantly present in infiltrating macrophages in the lesions of untreated multibacillary (MB) patients, whereas only PGL-I was occasionally seen in scattered macrophages in untreated paucibacillary lesions. During treatment, clearance of PGL-I from granulomas in MB lesions occurred before that of LAM, although the former persisted in scattered macrophages in some treated patients. This persistence of PGL-I in the lesions paralleled high serum anti-PGL-I antibody titers but was not indicative for the presence of viable bacilli in the lesions. Interestingly, we also observed a differential expression pattern of PGL-I and LAM in the lesions of MB patients with reactions during the course of the disease as compared with those without reactions. In conclusion, the in situ expression pattern of PGL-I and LAM in MB patients may assist in early diagnosis of reactions versus relapse.
Leprosy represents a spectrum of immunopathological responses to infection with Mycobacterium leprae (Ml.), characterized by histologically different granulomatous skin lesions.1,2 Tissue granulomas vary from predominantly epithelioid cells with the absence or occasionally presence of bacilli at the tuberculoid end of the spectrum (TT) to abundance of bacilli-filled foamy macrophages in lepromatous leprosy (LL). The histopathology of granulomas reflects the patient’s (local) immune response, which may show either strong delayed-type cellular immunity (CMI-DTH) toward the antigens of Ml. at the paucibacillary (PB) tuberculoid pole or Ml.-specific unresponsiveness at the multibacillary (MB) lepromatous pole. In between these polar forms of leprosy, the largest group of patients are the immunologically unstable borderline leprosy patients, classified as borderline tuberculoid (BT), mid-borderline (BB), and borderline lepromatous (BL). A considerable number of the borderline patients (20% to 30%) may undergo acute immunological changes in the course of the disease, such as reversal reaction (RR) and erythema nodosum leprosum (ENL). RR is often accompanied by nerve damage, resulting in disability, and may be due to an augmented CMI-DTH response to antigens of Ml.3-5 ENL is associated with a humoral immune response to antigens of Ml. causing severe tissue damage mediated by local deposition of immune complexes and complement activation.3,6 Some studies showed that augmentation of CMI might be seen in patients undergoing ENL as well.7
The immunopathological spectrum and the associated tissue damage in leprosy is largely considered to be due to the variation in immune responses by the individual host to specific antigens of Ml. as well as to cross-reactive mycobacterial antigens.8,9 Therefore, the identification and characterization of Ml.-specific and cross-reactive mycobacterial antigens that are associated with the different forms of leprosy may provide additional tools for diagnosis and prognosis. To this aim, in the past decade many investigators have investigated the immunogenicity of a large number of protein, carbohydrate, and lipid antigens of Ml. in both humoral and CMI responses in relation to the pathology of leprosy.10
Several immunodominant B-cell antigens have been identified. In general, antibody levels to species-specific epitopes (such as phenolic-glycolipid I (PGL-I) and 36-kd protein) and common mycobacterial antigens (such as lipoarabinomannan (LAM)) are higher in lepromatous patients and diminish toward the tuberculoid pole of the spectrum.11,12 Moreover, elevated levels of anti-PGL-I antibodies (IgM) in untreated borderline leprosy patients are implicated to be associated with manifestation of RR,13 whereas patients with ENL had lower anti-PGL-I serum titers (IgM) than non-ENL patients with comparable bacterial load.14 Among the protein antigenic components recognized by serum antibodies, the cross-reactive components of mycobacteria in the regions of 30 kd and 65 kd are predominantly recognized by lepromatous and tuberculoid leprosy patients, respectively.15-17
On the other hand, antigenic components that are critical in either perpetuation of T-cell activation in tuberculoid lesions or maintenance of T-cell unresponsiveness in lepromatous lesions have yet to be elucidated. T-cell lines and T-cell clones generated from either the lesional skin or peripheral blood from both tuberculoid and lepromatous leprosy patients recognize a large number of different antigenic proteins.10 Moreover, some investigators reported that non-protein antigens, such as LAM and PGL-I, also show T-cell reactivity.18-20 Although several studies have implicated that certain Ml. antigens, in particular the 10-kd and 65-kd heat-shock proteins and the secreted 25-kd and 30-kd (antigen 85 complex) proteins, appear to be immunodominant for T-cell proliferative responses, the recognition of a range of antigens by T cells varied from individual to individual rather than between patient groups.21-26
An alternative approach for studying the association of different Ml. antigens in the immunopathological spectrum of leprosy is to identify the in situ presence of such components in the lesional skin. At present, only few studies on the identifications of in situ mycobacterial antigens related to leprosy skin lesions are known in literature.27-30 These studies demonstrated Ml. antigens in the interstitial space, intracellularly or expressed on the infiltrating cells in both paucibacillary (PB) and multibacillary (MB) lesions, and did not reveal any specific association of particular types of antigens related to the immunopathological spectrum. However, in an earlier study from our laboratory it was reported that differential in situ expression of MAb 3A8 reactive to antigen in the 30-kd region might be associated with the spectral immunopathology of leprosy.31 Presently, we undertook a retrospective study by immunohistochemical methods to identify the in situ presence of other Ml.-specific and cross-reactive antigenic components that might be associated specifically with different forms of leprosy, including the reactional states. We selected a panel of MAbs against antigenic components (36-kd and 65-kd region proteins, PGL-I, and LAM) that were useful in monitoring the humoral/cellular immune responses of leprosy patients. The distribution of these antigens in the lesions of untreated leprosy patients was compared between leprosy patient groups and also with specimens obtained from non-leprosy patients but with other active skin diseases and from normal healthy individuals. We found that the staining pattern of PGL-I and the granular staining pattern of LAM were exclusive for leprosy. The clearance and/or persistence of these two antigenic components were further investigated in lesions during therapy and when the patients were released from treatment. We additionally carried out a comparative study between patients with and without RR or ENL but with comparable bacillary load. Furthermore, the in situ detection of PGL-I by immunohistochemical methods was carried out in parallel with monitoring the anti-PGL-I serum titer and also paralleling the analysis of Ml. rRNA in a number of lesions.
Materials and Methods
Patients and Tissue Specimens
Lesional skin biopsies were obtained from a total of 45 patients attending the leprosy clinic at the Academic Medical Center, Amsterdam (n = 41), the Dijkzigt Hospital, Rotterdam, The Netherlands (n = 2), and the Zimbabwe leprosy control program (n = 2). The skin specimens were snap-frozen in liquid N2 and stored at −70°C until use. All patients were classified according to the clinical and histopathological criteria of Ridley and Jopling.1 Clinical criteria used for the diagnosis of RR were erythematous swelling of the existing lesions, appearance of new lesions, and the onset or worsening of neuritis. Clinical criteria for the diagnosis of ENL were sudden appearance of tender erythematous nodules, in some cases accompanied by fever, leukocytosis, or neuritis. The bacterial index (BI) in the biopsies was evaluated by Fite-Faraco-Wade (FFW) staining.32 In this respect, patients belonging to the spectrum TT/BT and BB/BL/LL are classified as paucibacillary (PB) and multibacillary (MB) with the BI values of ≤1+ and >1+, respectively. The patients were treated with multidrug therapy (MDT) standardized by the World Health Organization and was continued for at least 24 months for MB patients and 6 months for PB patients. Patients experiencing RR or ENL were additionally treated with prednisone or prednisone or thalidomide, respectively. Classification and treatment status of the patients are summarized in Table 1▶ .
Table 1.
Clinical and Histopathological Classification of Leprosy Patients (n = 45) at the Commencement of the Study
| Classification | Total (n) | Treatment status (with/without reactional states) | ||||
|---|---|---|---|---|---|---|
| Untreated | Released from treatment (RFT) | |||||
| No reaction (n) | + RR (n) | No reaction (n) | + RR (n) | + ENL (n) | ||
| Paucibacillary (PB) | ||||||
| TT | 6 | 6 | ||||
| BT | 18 | 16 | 1 | 1 | ||
| Multibacillary (PB) | ||||||
| BB/BL | 15 | 9 | 1 | 3 | 1 | 1 |
| LL | 6 | 2 | 3 | 1 |
Patients were classified according to the clinical and histopathological criteria of Ridley and Jopling. TT, tuberculoid leprosy; BT, borderline tuberculoid; BB, mid-borderline; BL, borderline lepromatous; LL, lepromatous leprosy; RR, reversal reaction; ENL, erythema nodosum leprosum; RFT, release from treatment.
A number of the patients presented in Table 1▶ were followed in the course of the disease, and additional biopsies were obtained from the lesional skin of these patients at the end of MDT (release from treatment, RFT) and when patients experienced a RR or ENL. From two MB patients (without reactional states) additional biopsies were obtained on different occasions during treatment. The total number of biopsies investigated in this study, and their BI values, is summarized in Table 2▶ .
Table 2.
Classification, Treatment Status, and Bacilli Index of Lesional Skin Biopsies Generated from 45 Leprosy Patients in the Course of the Disease
| Classification | Treatment status | Number of skin biopsies from patients | |||||
|---|---|---|---|---|---|---|---|
| Without reactions | With RR | With ENL | |||||
| BI | Total (n) | BI | Total (n) | BI | Total (n) | ||
| Paucibacillary (PB) | |||||||
| TT | Untreated | 0 | 6 | ||||
| TT | RFT | 0 | 1 | ||||
| BT | Untreated | 0–1+ | 16 | 4+† | 1 | ||
| BT | RFT | 0 | 6 | 0 | 1 | ||
| Multibacillary (MB) | |||||||
| BB/BL | Untreated | 4–5+ | 9 | 1+ | 1 | ||
| BB/BL | On treatment | 1–4+* | 2* | 0 | 1 | 5+ | 1 |
| BB/BL | On treatment | 4+ | 3 | ||||
| BB/BL | RFT | 0–1+ | 11 | 0 | 1 | 0–1+ | 4 |
| LL | Untreated | 5–6+ | 2 | ||||
| LL | RFT | 0 | 3 | 1+ | 1 |
Patients were classified according to the clinical and histopathological criteria of Ridley and Jopling. The bacterial index (BI) in the biopsies was evaluated by Fite-Faraco-Wade (FFW) staining. The abbreviations are described in the legend of Table 1▶ .
*Follow-up biopsies were taken from two patients on different occasions in the course of treatment. The BI in the follow-up biopsies decreased from 4 to 1+.
†This patient was classified BT based on clinico-histopathological features, irrespective of a BI of 4+. BI: bacterial index.
As controls, skin biopsies from healthy individuals undergoing reconstructive surgery (n = 3) and biopsies from the lesional skin of patients with sarcoidosis (n = 6), psoriasis (n = 3), contact allergy (n = 4), and leishmaniasis (n = 5) were analyzed.
Immunohistochemistry
Monoclonal Antibodies
The details of the MAbs to mycobacterial antigens (working dilutions, isotypes, specificity, and origin) are listed in Table 3▶ . The characteristics of MAbs to mycobacterial 36-kd, 65-kd, PGL-I, and LAM antigens have been described previously.33-36 In addition, data of the MAbs to immunocompetent cell markers are given in Table 3▶ .
Table 3.
Monoclonal Antibodies Directed against Ml. Antigenic Components and Cell Markers
| MAb | Isotype | Specificity | Working dilution | Reference/source |
|---|---|---|---|---|
| Ml. antigenic components | ||||
| F126-5 | IgG1 | 36-kd protein | 1:1000 | 33 |
| F67-2 | IgG1 | 65-kd protein | 1:500 | 34 |
| F30-5 | IgM | LAM | 1:5000 | 35 |
| DZ-1 | IgG1 | PGL-I | 1:1000 | 36 |
| Cell markers | ||||
| Leu4 | IgG1 | CD3, pan T cell | 1:10 | Becton Dickinson, Mountain View, CA |
| Leu4 FITC | IgG1 | CD3, pan T cell | 1:200 | Becton Dickinson |
| EBM-11 | IgG1 | CD68, macrophage | 1:50 | Dakopatts, Glostrup, Denmark |
| EBM-11-Bio | IgG1 | CD68, macrophage | 1:100 | Becton Dickinson* |
| OKT6 | IgG1 | CD1a, Langerhans cell | 1:25 | Ortho Diagnostics, Raritan, NJ |
| OKT6-FITC | IgG1 | CD1a, Langerhans cell | 1:100 | Ortho Diagnostics |
*The MAb was biotinylated in our laboratory.
Immunohistochemical Staining Methods (Single and Double)
Cryostat sections (6 μm) of skin biopsies were air dried overnight, fixed in cold acetone for 10 minutes, and either immediately used or stored at −20°C. Immunoenzyme single staining was performed with a streptavidin-biotin-immunoperoxidase method.37 Briefly, sections were preincubated with 0.1% sodium azide in PBS to inhibit endogenous peroxidase activity followed by further incubation with normal goat serum to block Fc receptors. The sections were then incubated with the pre-evaluated optimal dilution of primary antibodies. The activity of the primary antibody was revealed by incubating the sections with biotinylated rabbit anti-mouse immunoglobulins (Dako, Glostrup, Denmark) and streptavidin/biotinylated horseradish peroxidase complex (SABC; Dako), followed by the visualization of peroxidase activity using H2O2 as substrate and 3-amino-9-ethyl carbazole (AEC; Sigma Chemical Co., St. Louis, MO) as chromogen. Sections were counterstained with hematoxylin. Negative control sections were incubated with the PBS, pH 7.4, instead of the MAb and also with isotype-matched mouse MAbs of irrelevant specificity at a higher concentration of the primary antibody. In this respect, IgG1 and IgM mouse MAbs directed to Asperigellus niger glucose oxidase (clones GO1 and GO8, respectively; Dako) were used. Furthermore, cell-marker-associated isotype-matched MAbs also served as the internal controls for ensuring particularly the specificities of IgG1 MAbs to mycobacterial antigens.
Depending on the combination of the primary antibodies, two different immunoenzyme double-staining methods were used to co-localize antigens and immunocompetent cells.38 In one protocol, the sections were incubated with a mixture of the primary IgM MAb F30-5 to LAM with either the IgG1 MAbs DZ-1 to PGL-I, Leu 4 (CD4), EBM-11 (CD68), or OKT 6 (CD1a) followed by an additional incubation step with a mixture of the isotype-specific secondary antibodies (RAM-IgM-HRP and RAM-IgG1-AP; Southern Biotechnology Associates, Birmingham, AL). In the other protocol, the MAbs DZ-1 was applied in combination with the MAb to immunocompetent cells that were labeled with either fluorescein isothiocyanate (Leu 4 FITC and OKT 6 FITC) or biotin (EBM-11 bio), as previously described.38 For visualization, the alkaline phosphatase activity was developed with Fast Blue BB (Sigma) and followed by revealing peroxidase activity with AEC. Double-stained cells were characterized if both red and blue or a distinct purple color could be discerned within one cell.
Nucleic Acid Sequence Based Amplification (NASBA)
To detect the presence of live bacilli, in parallel with immunohistochemical detection of antigens, identification of M. leprae 16 S rRNA was carried out in some of the skin biopsies. This method was performed as described previously.39
ELISA
The titers of antibodies to PGL-I (IgM) in patient serum was analyzed by ELISA using a standard synthetic neoglycoprotein (DBSA, Gigg Dissach) supplied by the World Health Organization. The details of this ELISA test were described previously.40
Results
Single Immunoenzyme Staining Pattern: Exclusive Reactivities of MAbs DZ-1 (PGL-I) and F30-5 (LAM) in Leprosy Skin Lesions
The in situ reactivities of MAbs to 36 kd, 65 kd, LAM, and PGL-I in the lesional skin of various forms of untreated leprosy and in control skin biopsies are summarized in Table 4▶ . The staining patterns were granular, diffuse, or membrane bound. Diffuse and membrane-bound staining patterns with MAbs to 36 kd and 65 kd were found in both leprosy and control skin biopsies, whereas the granular staining patterns with MAbs to LAM, 36 kd, and 65 kd and the staining pattern with MAb to PGL-I were seen only in leprosy biopsies.
Table 4.
In Situ Reactivity of MAbs to Different Mycobacterial Epitopes (LAM, PGL-I, 36 kd, and 65 kd) as Analyzed by Immunohistochemistry: Semiquantitative Analysis in the Lesional Skin of Untreated Leprosy Patients and Control Skin Biopsies
| Patient classification | BI | n | Positive staining by MAb (antigen)/number of biopsies tested and intensity of staining and characteristics | |||
|---|---|---|---|---|---|---|
| F30-5 (LAM) | DZ-1 (PGL-I) | F126-5 (36 kd) | F67-2 (65 kd) | |||
| Leprosy patients | ||||||
| Paucibacillary (PB) | ||||||
| TT | 0 | 6 | 0 /6 | 0 /6 | 3 /6 | 3 /6 |
| +† | +‡ | |||||
| BT | 0–1+ | 16 | 0 /16 | 3 /16 | 13 /16 | 5 /16 |
| +¶ | +*† | +‡ | ||||
| Multibacillary (MB) | ||||||
| BB/BL | 4–5+ | 9 | 9/9, 3/9 | 9/9, 9/9 | 9/9, 3/9 | 9/9, 3/9, 1/9 |
| +++,§ +¶ | +++,§ ++¶ | ++,*†§ +¶ | ++,§+,¶ RT-PCR‡ | |||
| LL | 5–6+ | 2 | 2 /2 | 2 /2 | 2 /2 | 2 /2 |
| +++§ | +++§∥ | +++†§ | +++§ | |||
| Other skin tissue | ||||||
| Sarcoidosis | 6 | 0 /6 | 0 /6 | 6 /6 | 1 /6 | |
| +++*† | +† | |||||
| Psoriasis | 3 | 0 /3 | 0 /3 | 2 /3 | 1 /3 | |
| +** | +‡ | |||||
| Contact allergy | 4 | 0 /4 | 0 /4 | 0 /4 | 4 /4 | |
| +‡ | ||||||
| Leishmaniasis | 5 | 3 /5 | 0 | 5 /5 | 2 /5 | |
| +†† | +++*† | +†† | ||||
| Normal skin | 3 | 0 /3 | 0 /3 | 0 /3 | 0 /3 |
The in situ presence of Ml.-specific or cross-reacting mycobacterial antigens was determined by immunohistochemical analysis using MAbs. The staining patterns were evaluated on the basis of intensity (grading: + to +++) and also on the basis of the specific staining characteristic and distribution in the tissue.
*Staining is associated with the cell membrane of immunocompetent cells at the site of juxtapositionally opposed T cells and macrophages.
†Diffusely stained interstitial space of the cellular infiltrate, and occasional intracytoplasmic diffused staining.
‡Membrane-bound staining of cells as a necklace around and associated with the basal layer of the epidermis.
§Granular staining of discrete, whole, or fragmented bacilli.
¶Intracellular staining within scattered cells.
∥Staining as dots within the infiltrate.
**Membrane-bound staining of scattered cells.
††Few sporadic individual cells stained intracytoplasmatic and membrane bound within the infiltrate.
Figure 1, A–D and E–H▶ , illustrate the representative staining patterns with the MAbs to PGL-I, LAM, 65 kd, and 36 kd in untreated MB and PB leprosy lesions, respectively. It can be seen that the staining patterns differed between the specimens from MB and PB patients. In all untreated MB lesions (n = 11) a granular staining pattern with MAbs to LAM, 65 kd, and 36 kd was seen in the granuloma resembling the presence of fragmented/granular bacilli (Figure 1, B–D)▶ . In 3/11 MB lesions, these MAbs also stained scattered macrophages surrounding the granuloma. The MAb to PGL-I stained as clumps in the granuloma as well as in macrophages surrounding the granuloma in all MB lesions (Figure 1A)▶ . In contrast, the infiltrate of untreated PB lesions (n = 22) did not show a granular staining patterns with the MAbs to LAM, 65 kd, and 36 kd (Figure 1, E–H)▶ , but in 3/22 lesions, cells surrounding the granuloma were positively stained with the MAb to PGL-I (see inset in Figure 1E▶ ).
Figure 1.
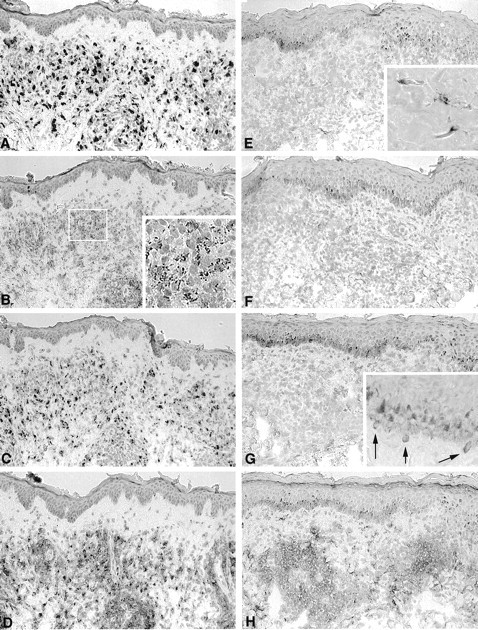
Representative staining patterns with MAbs to PGL-I, LAM, 65 kd, and 36 kd in the lesional skin of untreated MB and PB patients. Immunoperoxidase staining of sequential sections of a MB lesion (BI 4+; A to D) and of a PB lesion (BI 0; E to H) with MAbs against PGL-I (A and E), LAM (B and F), 65 kd (C to G), and 36 kd (D to H). All sections were counterstained with hematoxylin. Immunoperoxidase single staining; magnification, ×125. Note the dark staining in the basal layer is due to melanin-containing melanocytes and not due to immunostaining.
In addition to the granular staining pattern, the MAb to 36-kd protein stained the cell surface as well as diffuse cytoplasma of infiltrate cells and the interstitial space of the granuloma in all MB and in 16/22 PB leprosy lesions (Figure 1, D and H▶ , respectively). The same staining pattern was seen in the lesional skin of patients with sarcoidosis and leishmaniasis, whereas in 2/3 psoriasis lesions the membrane of these macrophage-like cells not associated with the granuloma were also stained. The MAb to 65-kd protein stained the surface of cells associated with the papillary dermis and attached to the basal layer of the epidermis in 8/21 PB lesions and in 1/11 MB lesions (Figure 1G▶ , inset). This staining was also seen in one of three psoriasis patients and in all patients with contact allergy. In one sarcoidosis lesion a diffuse staining with the MAb to 65 kd was seen within the interstitial space of the cellular infiltrate, whereas few sporadic individual cells were stained within cytoplasma and/or as membrane bound in 2/5 leishmaniasis lesions, which was also seen with the MAb against LAM in 3/5 leishmaniasis lesions. Normal skin was negative for all MAbs.
From this study it appears that the staining with MAb to PGL-I and the granular staining with MAb to LAM are seen only in leprosy lesions and not in any of the other non-leprosy skin biopsies studied. The in situ presence of LAM and PGL-I was therefore subsequently investigated in further detail.
Cellular Localization of Mycobacterial Cell-Wall-Associated Antigens PGL-I and LAM
Double staining with the MAb to either PGL-I or LAM together with the markers for macrophages, T cells, or Langerhans cells was carried out in MB leprosy lesions. The staining pattern with MAbs to LAM and PGL-I was found intracellularly in CD68+ macrophages. These CD68+ macrophages containing mycobacterial antigens appear to be frequently located adjacent to T cells and as scattered cells surrounding the granuloma. Representative illustrations of such double staining are shown in Figure 2▶ . The in situ detection of LAM and T cells, and LAM and macrophages, are presented in Figure 2, A and B▶ , respectively. No double staining was found with the MAbs to LAM and PGL-I and the marker for CD1a-positive Langerhans cells (data not shown).
Figure 2.
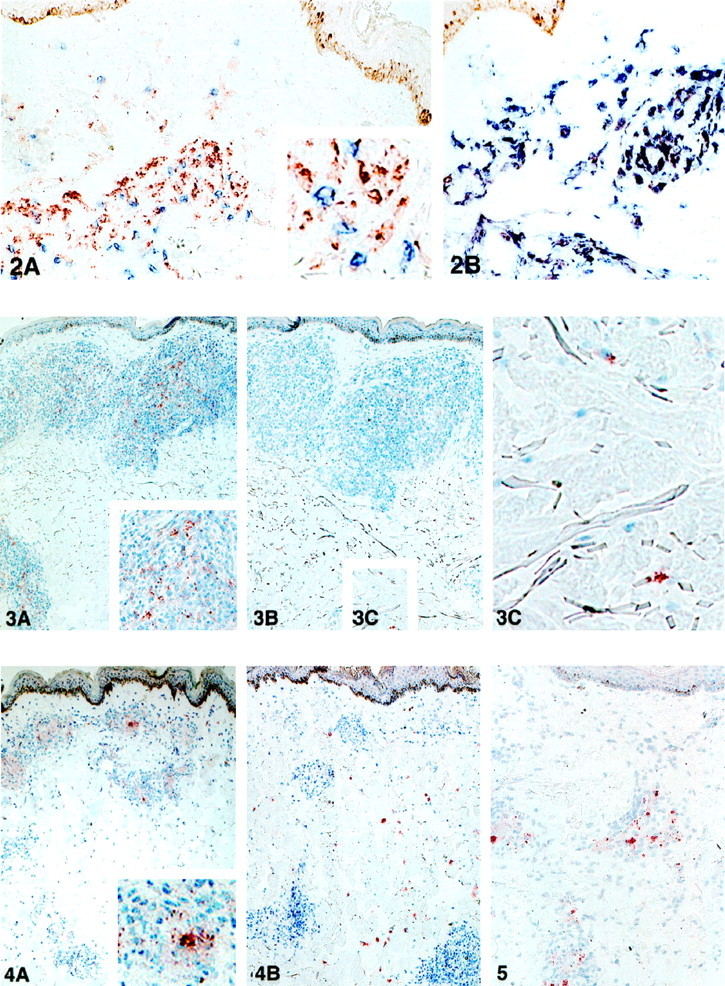
Figure 2. A and B: In situ co-localization of Ml. antigens and immunocompetent cells. Sequential sections of the lesion of an untreated MB patient (BI 4+) were double stained with the MAb to LAM (red) with either the MAb to T cell marker (anti-CD3, blue; A) or the MAb to macrophage marker (anti-CD68, blue; B). Note the intracellular presence of LAM within macrophages. Immunohistochemical double staining; magnification, ×160.
Figure 3. A and B: In situ detection of LAM and PGL-I in the course of the disease. Sequential sections of the lesion of a MB patient (1 year after the onset of the treatment, BI 2+) were stained with the MAb to LAM (A) and the MAb to PGL-I (B). C: Inset from B, showing scattered cells stained with the MAb to PGL-I. Immunoperoxidase single staining, hematoxylin counterstaining; magnification, ×40.
Figure 4.In situ detection of LAM and PGL-I in the course of the disease with RR. Sequential sections of the lesion of a MB patient with RR (BI 4+) in the course of treatment were stained with the MAb to LAM (A) and the MAb to PGL-I (B). Immunoperoxidase single staining, hematoxylin counterstaining; magnification, ×40.
Figure 5.In situ detection of LAM in the lesional skin of a MB patient with ENL after treatment. A section of the lesion of a MB patient experiencing an ENL 2 years after being released from treatment (BI 0–1+) stained with the MAb to LAM. Immunoperoxidase single staining, hematoxylin counterstaining; magnification, ×80.
In Situ Expression of PGL-I and LAM in Leprosy Patients during and after Treatment: Clearance and Persistence of Antigens
We extended our studies with the MAbs to LAM and PGL-I in a longitudinal follow-up of MB patients during treatment and of MB and PB patients after release from treatment.
We investigated the clearance of these antigens in the lesions of two MB patients during treatment. Biopsies were obtained on different occasions during the therapy. In the lesions of both patients we observed that the expression of both LAM and PGL-I had decreased from the granuloma during the treatment, paralleling a decrease in BI, but the staining with the MAb to PGL-I became negative within the granulomas before that of LAM (illustrated in Figure 3, A and B▶ ). However, the expression of PGL-I persisted in scattered macrophages, mainly in the deep dermis, similar to that found in untreated PB lesions (see inset in Figure 3C▶ ).
Figure 3.
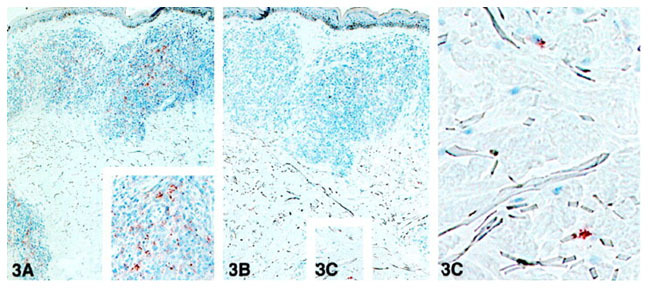
A and B: In situ detection of LAM and PGL-I in the course of the disease. Sequential sections of the lesion of a MB patient (1 year after the onset of the treatment, BI 2+) were stained with the MAb to LAM (A) and the MAb to PGL-I (B). C: Inset from B, showing scattered cells stained with the MAb to PGL-I. Immunoperoxidase single staining, hematoxylin counterstaining; magnification, ×40.
The presence of LAM and PGL-I in the lesional skin of PB and MB patients after the release from treatment is summarized in Table 5▶ . After treatment, the staining with MAbs to LAM and PGL-I was negative in the granuloma in the lesions of 13/14 MB patients and paralleled the decreased BI (BI 0–1+). However, in scattered macrophages, the staining with the MAb to PGL-I persisted in 6/14 lesions. In 2/14 skin lesions a granular staining with MAbs to both LAM and PGL-I was seen perivascularly in the lesion or in nerve fibers (data not shown). In the lesion of 1/7 PB patients, persistence of PGL-I was seen in scattered macrophages.
Table 5.
In Situ Detection of LAM and PGL-I in the Lesional Skin of MB and PB Patients Released from Treatment
| Patients RFT | Localization | Positive staining pattern | |
|---|---|---|---|
| LAM* | PGL-I† | ||
| MB (n = 14; BI 0–1+) | Granuloma | 1 /14 | 1 /14 |
| Scattered cells | 0 /14 | 6 /14 | |
| Other locations‡ | 2 /14 | 2 /14 | |
| PB (n = 7; BI 0) | Granuloma | 0 /7 | 0 /7 |
| Scattered cells | 0 /7 | 1 /7 | |
| Other locations | 0 /7 | 0 /7 |
In situ presence of LAM and PGL-I was determined by immunohistochemical analysis using MAbs F 30-5 and DZ-1, respectively. MB, multibacillary; PB, paucibacillary; RFT, release from treatment.
*Granular staining pattern.
†Staining pattern was as clumps (see inset in Figure 3C▶ ).
‡Around the vessel or nerve fibers.
Characteristic Immunostaining Patterns of PGL-I and LAM in Skin Lesions of Patients in Reactional States (RR and ENL)
We investigated the in situ staining pattern of LAM and PGL-I in the lesions of patients undergoing RR or ENL and compared these with those found in patients with similar bacterial load. The staining characteristics are summarized in Table 6▶ .
Table 6.
In Situ Detection of LAM and PGL-I in the Lesional Skin of MB and PB Patients Undergoing RR and ENL in the Course of the Disease
| Patients with reactions | Localization | Positive staining pattern | |
|---|---|---|---|
| LAM* | PGL-I† | ||
| RR (n = 4; BI 4+) | Granuloma | 4 /4 | 0 /4 |
| Scattered cells | 0 /4 | 4 /4 | |
| RR (n = 4; BI 0–1+) | Granuloma | 0 /4 | 0 /4 |
| Scattered cells | 0 /4 | 4 /4 | |
| ENL (n = 1; BI 5+) | Granuloma | 1 /1 | 1 /1 |
| Scattered cells | 0 /1 | 1 /1 | |
| ENL (n = 5; BI 0–2+) | Granuloma | 4 /5 | 3‡ /5 |
| Scattered cells | 1 /5 | 4 /5 |
In situ presence of LAM and PGL-I was determined by immunohistochemical analysis using MAbs F 30-5 and DZ-1, respectively. RR, reversal reaction; ENL, erythema nodosum leprosum.
*Granular staining pattern.
†Staining pattern was as clumps.
‡In two lesions the staining was weak.
Four MB patients (BI 4+) underwent a RR (three were on treatment and one was untreated). In all lesions, the staining with the MAb to PGL-I was absent in the infiltrates, but many scattered macrophages surrounding the granuloma were stained (Figure 4B)▶ . The staining pattern differed in this respect from the staining pattern with the MAb to PGL-I in lesions of MB patients without RR (see Figure 1A▶ and Table 4▶ ). Similar to that of MB lesions without RR, the infiltrates were still positively stained with the MAb to LAM, which was not present in scattered cells (see Figures 4A and 1B▶ ▶ ). The staining patterns with MAb to LAM and PGL-I in the lesions of four patients with a RR at the time their BI was 0–1+ (one BT/RFT, one BL/RFT, one BL/on treatment, and one BB/untreated), showed the absence of both LAM and PGL-I in the granuloma, but showed scattered cells positive for PGL-I, and did not differ in this respect from other lesions with BI 0–1+.
Figure 4.

In situ detection of LAM and PGL-I in the course of the disease with RR. Sequential sections of the lesion of a MB patient with RR (BI 4+) in the course of treatment were stained with the MAb to LAM (A) and the MAb to PGL-I (B). Immunoperoxidase single staining, hematoxylin counterstaining; magnification, ×40.
On the other hand, five MB patients (RFT, BI 0–2+) were diagnosed with an ENL. In 4/5 patients, the lesions showed extensive staining in the infiltrates with the MAb to LAM (illustrated in Figure 5▶ ). This staining pattern differed in this respect from that seen in the lesions of MB patients released from treatment without ENL (BI 0–1+) that were characterized by the absence of LAM in the granuloma (Table 5)▶ . The staining with the MAb to PGL-I showed varied staining patterns indicating also varied persistence of this antigen. The significance of persistence of LAM in ENL has been further confirmed by a follow-up study of one of these patients. Initially the patient (BI 5+) developed an ENL during treatment, and the infiltrates were found positive for LAM and PGL-I. The patient was subsequently treated for 2 years. After treatment, the patient was followed for 2 more years. During this period the lesions showed persistence of LAM and PGL-I in the infiltrates. The patient developed a second episode of ENL (BI 0), and the granular staining with MAbs to LAM was still extensively positive in the infiltrate whereas the staining with the MAb to PGL-I at the same site was negative.
Figure 5.
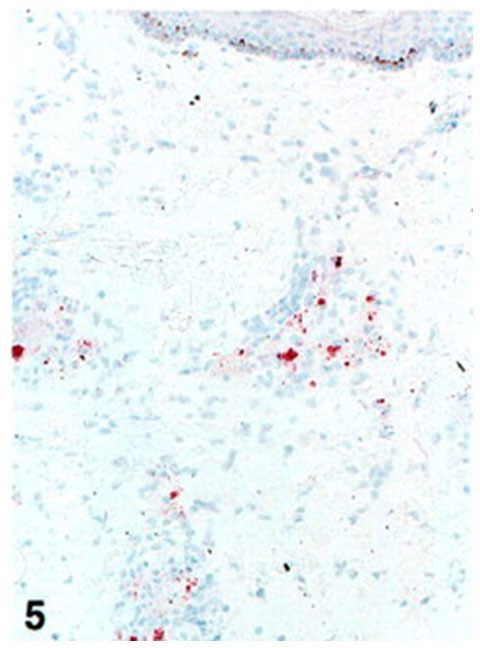
In situ detection of LAM in the lesional skin of a MB patient with ENL after treatment. A section of the lesion of a MB patient experiencing an ENL 2 years after being released from treatment (BI 0?1+) stained with the MAb to LAM. Immunoperoxidase single staining, hematoxylin counterstaining; magnification, ×80.
In Situ Detection of Tissue PGL-I Combined with Serological Data of Anti-PGL-I Antibody Titers and the in Situ Presence of Viable Bacilli
In the present study we observed persistence of PGL-I in scattered macrophages in a number of lesional skin biopsies with BI 0–1+. We investigated whether the retention of such PGL-I within these lesions might be associated with high anti-PGL-I (αPGL-I) antibody levels in the serum of these patients and/or with the presence of live bacteria within these lesions.
In a panel of 41 patients we combined the detection of PGL-I in the lesions (BI 0–1+), with the αPGL-I antibody levels in serum. This panel included both untreated and treated PB patients, treated MB patients, and patients undergoing reactional states (see Table 2▶ ). The results are presented in Table 7▶ . It can be seen that in 30/41 patients the in situ presence or absence of PGL-I in the lesion paralled the positive or negative αPGL-I serum titer. However, this apparent agreement was not statistically significant, which might be because eight patients showed persistence of PGL-I in the lesions without elevated αPGL-I antibody titers. This latter group consisted of three PB and five MB patients. Remarkably, three untreated MB patients (BI 4+) additionally did not show high αPGL-I serum titers, whereas they showed high density of PGL-I in the lesions (see patient R 92-33 and R 89-81 in Table 8▶ ). The lesions of three other patients showed no detectable in situ PGL-I antigens, but high αPGL-I titers were found in the serum.
Table 7.
Absence or Presence of PGL-I in the Lesional Skin of Leprosy Patients (n = 41; BI: 0–1+) Combined with the Presence or Absence of High Anti-PGL-I Antibody Titer (OD >0.500) in the Corresponding Serum
| Number of patients | In situ PGL-I* | High anti-PGL-I antibody titer in serum† |
|---|---|---|
| 12 | + | + |
| 18 | − | − |
| 8 | + | − |
| 3 | − | + |
*In situ presence of PGL-I was determined by immunohistochemical analysis using MAb DZ-1.
†Anti-PGL-I antibodies in serum were determined by ELISA. Serum values were considered high when OD was >0.500.
Table 8.
High Serum Anti-PGL-I Antibody Titer and in Situ Detection of PGL-I and Viable Ml. Bacilli in Skin Biopsy Specimen of Untreated Leprosy Patients and in Those in the Course of the Disease
| Untreated patients | Follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Classification | Patient number | BI | αPGL-I* | PGL-I† | Ml. RNA‡ | Treatment status | BI | αPGL-I* | PGL-I† | Ml. RNA‡ |
| TT | CPB05 | 0 | − | − | + | RFT | 0 | − | − | − |
| TT | R90-36 | 0 | ND | − | + | ND | ||||
| BT | R91-18 | 0 | − | − | + | RFT | 0 | − | − | − |
| BT | R92-24 | 0 | − | − | − | RFT | 0 | − | − | − |
| BT | R92-11 | 0 | − | − | − | ND | ||||
| BT | R87-60 | 0 | + | + | ++ | ND | ||||
| BT | R88-14 | 0 | − | + | + | ND | ||||
| BL | R91-19 | 4+ | + | + | +++ | ND | ||||
| BB | R92-33 | 4+ | − | + | +++ | On treatment (1 year) | 1+ | − | + | + |
| BL | R90-18 | ND | ND | ND | ND | RFT | 0 | + | + | − |
| BL | R88-4 | 4+ | + | + | +++ | RFT | 0 | − | − | − |
| BL | R89-81 | 4+ | − | + | +++ | RFT | 1+ | − | + | − |
| BL | R90-32 | ND | ND | ND | ND | RFT | 0 | + | + | +§ |
| BL | R89-121 | 5+ | + | + | − | RFT | 0 | + | + | − |
| BL | R90-21 | 4+ | + | + | +++ | RFT | 0 | + | + | − |
| BL | R88-1 | ND | ND | ND | ND | RFT | 1+ | + | + | ++¶ |
| RFT | 0 | + | + | − |
RFT, released from treatment; other abbreviations (TT, BT and BL) are as in Table 1▶ .
*Anti-PGL-I antibody titer in serum was measured by ELISA; serum values were considered high with OD >0.500.
†PGL-I is detected by immunohistochemical analysis using MAb DZ-1.
‡Ml. RNA was detected by NASBA.
§Patient with poor compliance of therapy.
¶Patient was investigated for possible relapse.
We investigated whether the persistence of PGL-I in those lesions with BI 0–1+ is associated with the presence of viable bacilli. The NASBA of Ml. 16 S rRNA in a panel of biopsies has previously been employed.39 We combined the data from the previous study with those obtained in the present study (summarized in Table 8▶ ). Lesions of untreated MB patients (BI ≥ 4+) were positive for the NASBA signal and the presence of in situ PGL-I, with the exception of one patient (R 89-121). In the particular case of this patient, the negative NASBA signal in the lesion may indicate that despite positive BI all bacilli were dead. When MB patients were released from treatment with BI 0–1+ in the lesions, only 2/8 lesions showed a positive NASBA signal whereas in 7/8 lesions PGL-I was still detectable. In the lesion of one MB patient on treatment (R92-33, BI 0–1+) still viable bacilli were present. On the other hand, in the lesions of 5/7 untreated PB patients (BI 0) the NASBA signal was positive whereas in only two of those lesions were in situ PGL-I antigens detectable. Lesions of treated PB patients were negative for both the NASBA signal and in situ presence of PGL-I. Taken together, the in situ presence of PGL-I in lesional skin (BI 0–1+) is not necessarily associated with the presence of viable bacilli in the same lesion.
Discussion
Identification of Ml. antigenic determinant and their cellular localization in the lesional skin of leprosy patients may improve our understanding of leprosy pathology. The present study was designed to assess in situ expression of different mycobacterial antigenic determinants in various forms of leprosy lesions using a panel of MAbs. These antigens have previously been reported to be involved in T-cell-dependent or humoral immune responses.
We observed that the differing types of staining pattern with the MAbs against the 36-kd and 65-kd cross-reacting proteins and the cell wall products LAM and PGL-I are related to different types of leprosy. The granular expression of 36-kd and 65-kd proteins and LAM was restricted to leprosy and paralleled the BI of the lesions. However, diffuse or membrane-bound staining patterns with the MAbs to the 36-kd and 65-kd protein were found in both leprosy and various other skin diseases. The staining of LAM and PGL-I appeared to be specific for leprosy and predominated in MB lesions. The dynamics of the expression of these two antigens was therefore studied in the course of the disease. We found that the dynamics in the expression of these antigens in the lesions of MB patients appears to be associated with immunopathological phenomena in leprosy such as RR and ENL. Moreover, we observed retention of PGL-I in a number of lesions with low numbers or absence of bacilli (BI 0–1+) that paralleled apparently with high serum antibody titers against this antigen, but not necessarily with the presence of viable bacilli in the lesions.
The in situ expression of mycobacterial antigenic determinants either in the interstitial space or expressed on the infiltrating cells in both PB and MB lesions have been implicated in local immune responses.27-29,31,41 In concordance with these studies, in the present study the MAbs against the 36-kd protein stained the cell membrane of immunocompetent cells in untreated PB and MB lesions at the site of interacting T lymphocytes and macrophages and also stained diffusely the interstitial space of the cellular infiltrate. The MAbs to 65 kd stained the membrane of scattered cells that were associated with the basal layer of the epidermis of, predominantly, PB lesions and MB lesions with RR (data not shown). Interestingly, the diffuse and membrane-bound staining patterns by MAbs to both 36 kd and 65 kd were not restricted to leprosy lesions. In the context of this finding, it is tempting to speculate that, on one hand, the in situ presence of mycobacterial antigenic determinants may also be involved in the pathogenesis of other skin diseases. In this respect it is noteworthy that a previous study from our laboratory showed that psoriatic patients had significant IgG antibody levels in serum against mycobacterial hsp65 and other dominant mycobacterial antigens, implicating a role for mycobacterial antigens in this disease.42 On the other hand, host cellular antigens, expressed during inflammatory skin diseases, may be cross-reactive to mycobacterial epitopes. Antigenic similarities between mycobacteria and normal human skin components were shown previously.43,44 We additionally observed that few sporadic individual cells were stained within cytoplasma and as membrane bound with MAb to 65 kd and LAM in leishmaniasis lesions. As these patients came from endemic areas, possibility for a co-infection with mycobacteria cannot be ruled out in these lesions.
On the other hand, the granular expression of the MAb to 36-kd and 65-kd proteins and LAM and the staining with MAb to PGL-I was restricted exclusively to the lesions of MB patients. The granular staining pattern by any of these MAbs was negative in the lesions of untreated PB patients, with the exception of the occasional presence of PGL-I in scattered macrophages. The dynamics of the in situ presence of PGL-I and LAM was further studied in relation to treatment of MB lesions. The clearance of PGL-I from the infiltrates was found to occur before that of LAM, whereas PGL-I persisted in macrophages in the deep dermis, even after treatment. So far, retention of mycobacterial antigens, including PGL-I and LAM, despite prolonged chemotherapy, has also been described previously for nerves45,46 and lymph nodes47 of leprosy patients. To our knowledge, this is the first report that describes the exclusive retention of these two antigens in the infiltrate that can be correlated with the dynamics of leprosy pathology.
In this respect, the importance of the present study is that in situ dynamics in the expression of LAM and PGL-I appears to be associated with the occurrence of reactional states. The lesions of MB patients with RR, as compared with those of untreated MB patients without RR, can be recognized by the absence of PGL-I in the granulomas but abundant presence of PGL-I in scattered macrophages. In this respect, the staining with this MAb may assist in distinguishing the lesions from multibacillary patients with RR from those with a relapse. However, the implications of the in situ PGL-I in scattered macrophages in these lesions with the occurrence of RR cannot be drawn from these results. Another interesting finding of the present study is that the lesions of 4/5 MB patients with the occurrence of an ENL after treatment showed persistence of LAM was seen in the infiltrates. Such a staining pattern was absent in the lesions of MB patients released from treatment without ENL. It is, therefore, suggestive that the expression of LAM in the lesions of MB patients that are released from treatment may be indicative that the patient is at risk for developing an ENL, as was the case for one patient in the present study. Other investigators have suggested that LAM mediates the pathogenesis of ENL via the induction of high levels of tumor necrosis factor-α.48 In this context, retention of LAM in the skin may account for the elevated levels of tumor necrosis factor-α in serum of patients with ENL49 and subsequently mediate immunopathological manifestations, such as fever. Moreover, LAM can play a role in T-cell activation as this antigen has been shown to induce T-cell proliferation in a CD1b- and CD1c-restricted manner.18,50
Another aspect of the present study was that persistence of PGL-I in scattered cells in a number of lesions with low numbers or absence of bacilli (BI 0–1+) appeared to be associated with high antibody titers against this antigen in the serum, but not necessarily with the presence of viable bacilli in the lesions. In this respect, the persistence of PGL-I in the lesional skin may account for high anti-PGL-I serum titers in patients even years after treatment. In this context, it is noteworthy from the literature that in the lesions of leprosy patients M. leprae-specific antibodies are produced that therefore might account for the parallel occurrence of in situ retention of PGL-I antigen and elevated systemic antibody titres.51 As skin is considered to be an immunocompetent tissue,52 it is not surprising that the presence of antigens in skin may reflect systemic humoral immune responses.
In conclusion, in the present study we demonstrated that LAM and PGL-I were differently expressed in the lesional skin of leprosy patients in the course of the disease. The lesions of MB patients with RR (BI 4+) and of MB patients released from treatment with ENL (BI 0–2+) showed specific characteristics in this respect, as compared with those patients with comparable bacterial load but without reactions, and may therefore assist as diagnostic markers. The implications of these in situ characteristics in the immunological etiology of the reactional states will be the subject of future investigations.
Acknowledgments
Dr. C. van der Loos and Mr. A. J. Tigges are kindly acknowledged for their help in immunohistochemical staining techniques. We were very grateful for patient material that was collected in collaboration with Mr. B. Matamera. MAbs F30-5, F67-2, and F126-5 were kindly provided by Dr. A. H. J. Kolk from the Department of Biomedical Research (Royal Tropical Institute (Amsterdam, The Netherlands). DZ-1 was kindly provided by Prof. J. T. Douglas, University of Hawaii, Honolulu, HI.
Footnotes
Address reprint requests to Dr. Pranab K. Das, Research Laboratory Pathology, Academic Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands. E-mail: p.k.das@amc.uva.nl.
Supported by grants from the Netherlands Leprosy Relief Association (The Netherlands) and the Q.M. Gastmann Wichers Foundation (The Netherlands) and carried out under the research programs of ODP/DE1 and ODP/PA2 of the Van Loghem Immunology Institute of the Faculty of Medicine, Academic Medical Center, University of Amsterdam. C.E. Verhagen and A. Buffing are the recipients of Netherlands Leprosy Relief Association maintenance grants.
References
- 1.Ridley DS, Jopling WH: Classification of leprosy according to immunity: a five-group system. Int J Lepr 1966, 34:255-273 [PubMed] [Google Scholar]
- 2.Das PK, Grange JM: Mycobacteria in relation to tissue immune response and pathogenesis. Rev Med Microbiol 1993, 4:15-23 [Google Scholar]
- 3.Ridley DS: Reactions in leprosy. Lepr Rev 1969, 40:77-81 [DOI] [PubMed] [Google Scholar]
- 4.Barnetson RSC, Bjune G, Pearson JMH, Kronvall G: Cell mediated and humoral immunity in ‘reversal reactions’. Int J Lepr 1976, 44:267-274 [PubMed] [Google Scholar]
- 5.Lienhardt C, Fine PEM: Type 1 reaction, neuritis and disability in leprosy. What is the current epidemiological situation? Lepr Rev 1994, 65:9-33 [DOI] [PubMed] [Google Scholar]
- 6.Wemambu SNC, Turk JL, Waters MFR, Rees RJW: Erythema nodosum leprosum: A clinical manifestation of the Arthus phenomenon. Lancet 1969, ii:933-935 [DOI] [PubMed] [Google Scholar]
- 7.Modlin RL, Gebhard JF, Taylor CR, Rea TH: In situ characterization of T lymphocyte subsets in the reactional states of leprosy. Clin Exp Immunol 1983, 53:17-24 [PMC free article] [PubMed] [Google Scholar]
- 8.Shields MJ: The importance of immunologically effective contact with environmental mycobacteria. Ratledge C Stanford J eds. The Biology of the Mycobacteria. 1983, :pp 343-415 Academic Press, London [Google Scholar]
- 9.Grange JM: Environmental mycobacteria and human disease. Lepr Rev 1991, 62:353-361 [DOI] [PubMed] [Google Scholar]
- 10.Thole JER, Wieles B, Clark-Curtiss JE, Ottenhoff THM, Rinke de Witt TF: Immunological and functional characterization of Mycobacterium leprae protein antigens: an overview. Mol Microbiol 1995, 18:791-800 [DOI] [PubMed] [Google Scholar]
- 11.Sengupta U: Mycobacterium leprae antigens and their utility in immunodiagnostics of leprosy. Trop Med Parasitol 1990, 41:361-362 [PubMed] [Google Scholar]
- 12.Roche PW, Britton WJ, Failbus SS, Neupane KD, Theuvenet WJ: Serological monitoring of the response to chemotherapy in leprosy patients. Int J Lepr 1993, 61:35-43 [PubMed] [Google Scholar]
- 13.Roche PW, Theuvenet WJ, Britton WJ: Risk factors for type-1 reactions in borderline leprosy patients. Lancet 1991, 338:654-657 [DOI] [PubMed] [Google Scholar]
- 14.Levis WR, Meeker HC, Schuller-Levis G, Sersen E, Schwerer B: IgM and IgG antibodies to phenolic glycolipid I from Mycobacterium leprae in leprosy: insight into patient monitoring, erythema nodosum leprosum, and bacillary persistence. J Invest Dermatol 1986, 86:529-534 [DOI] [PubMed] [Google Scholar]
- 15.Rumschlag HS, Shinnick TM, Cohen ML: Serological responses of patients with lepromatous and tuberculoid leprosy to 30-, 31- and 32-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol 1988, 26:2200-2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das PK, Rambukkana A, Baas JG, Groothuis DG, Halperin M: Enzyme-linked immunosorbent assay for distinguishing serological responses of lepromatous and tuberculoid leprosies to the 29/33-kilodalton doublet and 64-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol 1990, 28:379-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Launois P, N’Diaye Niang M, Drowart A, van Vooren JP, Sarthou JL, Lalu T, Millan J, Huygen K: IgG response to purified 65- and 70-kDa mycobacterial heat shock proteins and to antigen 85 in leprosy. Int J Lepr 1994, 62:48-54 [PubMed] [Google Scholar]
- 18.Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro RJ, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ, Modlin RL: CD1-restricted T cell recognition of microbial lipoglycan antigens. Science 1995, 269:227-230 [DOI] [PubMed] [Google Scholar]
- 19.Mehra V, Brennan PJ, Rada E, Convit J, Bloom BR: Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature 1984, 308:194-196 [DOI] [PubMed] [Google Scholar]
- 20.Koster FT, Scollard DM, Umland ET, Fishbein DB, Hanly WC, Brennan PJ, Nelson KE: Cellular and humoral immune response to phenolic glycolipid antigen (PhenGL-I) in patients with leprosy. J Clin Microbiol 1987, 25:551-556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SP, Stoker NG, Grant KA, Handzel ZT, Hussain R, McAdam KPWJ, Dockrell HM: Cellular immune responses of leprosy contacts to fractionated Mycobacterium leprae antigens. Infect Immun 1989, 57:2475-2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulle H, Schoel B, Chiplunkar S, Gangal S, Deo MG, Kaufmann SHE: T-cell responses of leprosy patients and healthy contacts toward separated protein antigens of Mycobacterium leprae. Int J Lepr 1992, 60:44-53 [PubMed] [Google Scholar]
- 23.Launois P, N’Diaye Niang MB, Sarthou JL, Rivier F, Drowart A, Van Vooren JP, Millan J, Huygen K: T-cell stimulation with purified mycobacterial antigens in patients and healthy subjects infected with Mycobacterium leprae: secreted antigen 85 is another immunodominant antigen. Scand J Immunol 1993, 38:167-176 [DOI] [PubMed] [Google Scholar]
- 24.Janson AAM, Klatser PR, van der Zee R, Cornelisse YE, de Vries RRP, Thole JER, Ottenhoff THM: A systematic molecular analysis of the T cell-stimulating antigens from Mycobacterium leprae with T cell clones of leprosy patients. J Immunol 1991, 147:3530-3537 [PubMed] [Google Scholar]
- 25.Thole JER, Janson AAM, Kifle A, Howe RC, McLean K, Nurilygn A, Filley E, Shannon EJ, Bulla GJ, Hermans J, De Vries RRP, Frommel D, Rinke De Wit TF: Analysis of T-cell and B-cell responses to recombinant M. leprae antigens in leprosy patients and in healthy contacts: significant T cell responses to antigens in M. leprae nonresponders. Int J Lepr 1995, 63:369-380 [PubMed] [Google Scholar]
- 26.Klatser PR, Janson AAM, Thole JER, Buhrer S, Bos C, Soebono H, De Vries RRP: Humoral and cellular immune reactivity to recombinant M. leprae antigens in HLA-type leprosy patients and healthy controls. Int J Lepr 1997, 65:178-189 [PubMed] [Google Scholar]
- 27.Khanolkar SR, Mackenzie CD, Lucas SB, Hussen A, Girdhar BK, Katoch K, McAdam KPWJ: Identification of Mycobacterium leprae antigens in tissues of leprosy patients using monoclonal antibodies. Int J Lepr 1989, 57:652-658 [PubMed] [Google Scholar]
- 28.Narayanan RB, Ramu G, Sinha S, Sengupta U, Malaviya GN, Desikan KV: Demonstration of Mycobacterium leprae specific antigens in leprosy lesions using monoclonal antibodies. Int J Lepr 1985, 57:258-264 [PubMed] [Google Scholar]
- 29.Narayanan RB, Girdhar BK, Malaviya GN, Sengupta U: In situ demonstration of Mycobacterium leprae antigens in leprosy lesions using monoclonal antibodies. Immunology Lett 1990, 24:179-184 [DOI] [PubMed] [Google Scholar]
- 30.Wang T, Izumi S, Butt KI, Kawatsu K, Maeda Y: Demonstration of PGL-I and LAM-B antigens in paraffin sections of leprosy skin lesions. Jpn J Leprosy 1992, 61:165-174 [DOI] [PubMed] [Google Scholar]
- 31.Rambukkana A, Das PK, Burggraaf JD, Young S, Faber WR, Thole JER, Harboe M: Heterogeneity of monoclonal antibody-reactive epitopes on mycobacterial 30-kilodalton-region proteins and the secreted antigen 85 complex and demonstration of antigen 85B on the Mycobacterium leprae cell wall surface. Infect Immun 1992, 60:5172-5181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridley DS: Skin Biopsy in Leprosy, ed 2. Basel, Ciba-Giegy, 1985
- 33.Klatser PR, de Wit MYL, Kolk AHJ, Hartskeerl RA: Characterization of murine B-cell epitopes on the Mycobacterium leprae proline-rich antigen by use of synthetic peptides. Infect Immun 1991, 59:433-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchanan TM, Nomaguchi H, Anderson DC, Young RA, Gillis TP, Britton WJ, Ivanyi J, Kolk AHJ, Closs O, Bloom BR, Mehra V: Characterisation of antibody-reactive epitopes on 65-kilodalton protein of Mycobacterium leprae. Infect Immun 1987, 55:1000-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolk AHJ, Ly Ho M, Klatser PR, Eggelte TA, Kuijper S, de Jonge S, van Leeuwen J: Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol 1984, 58:511-521 [PMC free article] [PubMed] [Google Scholar]
- 36.Fujiwara T, Minagawa F, Sakamoto Y, Douglas JT: Epitope mapping of twelve monoclonal antibodies against the phenolic glycolipid-I of M. leprae. Int J Lepr 1997, 65:477-86 [PubMed] [Google Scholar]
- 37.Hsu SM, Raine L, Fanger H: Use of avidin biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981, 29:577-580 [DOI] [PubMed] [Google Scholar]
- 38.Van der Loos CM, Becker AE, van den Oord JJ: Practical suggestions for successful immunoenzyme double-staining experiments. Histochem J 1993, 25:1-13 [DOI] [PubMed] [Google Scholar]
- 39.Van der Vliet GME, Cho S-N, Kampirapap K, Van Leeuwen J, Schukkink AF, Van Gemen B, Das PK, Faber WR, Walsh GP, Klatser PR: Use of NASBA RNA amplification for detection of Mycobacterium leprae in skin biopsies from untreated and treated leprosy patients. Int J Lepr 1996, 64:396-403 [PubMed] [Google Scholar]
- 40.Brett SJ, Payne SN, Gigg J, Burgess P, Gigg R: Use of synthetic glycoconjugates containing the Mycobacterium leprae specific immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin Exp Immunol 1986, 64:476-83 [PMC free article] [PubMed] [Google Scholar]
- 41.Mshana RN, Belehu A, Stoner GL, Harboe M, Haregewoin A: Demonstration of mycobacterial antigens in leprosy tissues. Int J Lepr 1982, 50:1-10 [PubMed] [Google Scholar]
- 42.Rambukkana A, Das PK, Witkamp L, Young S, Meinardi MMHM, Bos JD: Antibodies to mycobacterial 65-kDa heat shock protein and other immunodominant antigens in patients with psoriasis. J Invest Dermatol 1993, 100:87-92 [DOI] [PubMed] [Google Scholar]
- 43.Van den Akker TW, Naafs B, Kolk AHJ, De Glopper-van der Veer E, Chin RA, Lien A, Van Joost T: Similarity between mycobacterial and human epidermal antigens. Br J Dermatol 1992, 127:352-358 [DOI] [PubMed] [Google Scholar]
- 44.Naafs B, Kolk AHJ, Chin A, Lien RAM, Faber WR, Van Dijk G, Kuijper S, Stolz E, Van Joost T: Anti-Mycobacterium leprae monoclonal antibodies crossreact with human skin: an alternative explanation for the immune responses in leprosy. J Invest Dermatol 1990, 94:685-688 [DOI] [PubMed] [Google Scholar]
- 45.Mshana RN, Humber DP, Harboe M, Belehu A: Demonstration of mycobacterial antigens in nerve biopsies from leprosy patients using peroxidase-antiperoxidase immunoenzyme technique. Clin Immunol Immunopathol 1983, 29:359-368 [DOI] [PubMed] [Google Scholar]
- 46.Shetty VP, Uplekar MW, Antia NH: Immunohistological localization of mycobacterial antigens within the peripheral nerves of treated leprosy patients and their significance to nerve damage in leprosy. Acta Neuropathol 1994, 88:300-306 [DOI] [PubMed] [Google Scholar]
- 47.Barros U, Ladiwala U, Birdi TJ, Antia NH: Localization and retention of mycobacterial antigen in lymph nodes of leprosy patients. Br J Exp Pathol 1987, 68:733-741 [PMC free article] [PubMed] [Google Scholar]
- 48.Barnes PF, Chatterjee D, Brennan PJ, Rea TH, Modlin RL: Tumor necrosis factor production in patients with leprosy. Infect Immun 1992, 60:1441-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarno EN, Grau GE, Vieira LMM, Nery JA: Serum levels of tumor necrosis factor alpha and interleukin 1β during leprosy reactional states. Clin Exp Immunol 1991, 84:103-108 [PMC free article] [PubMed] [Google Scholar]
- 50.Beckman EM, Melian A, Behar SM, Sieling PA, Chatterjee D, Furlong ST, Matsumoto R, Rosat JP, Modlin RL, Porcelli SA: CD1c restricts responses of mycobacteria-specific T cells: evidence for antigen presentation by a second member of the human CD1 family. J Immunol 1996, 157:2975-2803 [PubMed] [Google Scholar]
- 51.Lai A, Fat RFM, Chan Pin Lin M, Van Fewth R, Harboe M: In vitro synthesis of antimycobacterial antibodies in biopsies from skin lesions of leprosy patients. Infect Immun 1980, 27:227-230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bos JD: Skin immune system (SIS). Cutaneous Immunology and Clinical Immunodermatology. Edited by Bos JD. New York, CRC Press, 1997


