Abstract
Glycosylation of natural products, including antibiotics, often plays an important role in determining their physical properties and their biological activity, and thus their potential as drug candidates. The arylomycin class of antibiotics inhibits bacterial type I signal peptidase and is comprised of three related series of natural products with a lipopeptide tail attached to a core macrocycle. Previously, we reported the total synthesis of several A series derivatives, which have unmodified core macrocycles, as well as B series derivatives, which have a nitrated macrocycle. We now report the synthesis and biological evaluation of lipoglycopeptide arylomycin variants whose macrocycles are glycosylated with a deoxy-α-mannose substituent, and also in some cases hydroxylated. The synthesis of the derivatives bearing each possible deoxy-α-mannose enantiomer allowed us to assign the absolute stereochemistry of the sugar in the natural product and also to show that while glycosylation does not alter antibacterial activity, it does appear to improve solubility. Crystallographic structural studies of a lipoglycopeptide arylomycin bound to its signal peptidase target reveal the molecular interactions that underlie inhibition and also that the mannose is directed away from the binding site into solvent which suggests that other modifications may be made at the same position to further increase solubility and thus reduce protein binding and possibly optimize the pharmacokinetics of the scaffold.
Introduction
The clinical and agricultural use of antibiotics imposes a relentless selection pressure on bacteria that has driven the evolution of multidrug resistance in many pathogens, and novel classes of antibiotics are needed.1,2 Bacteria produce a large assortment of antibiotics, possibly to gain advantage over competing microorganisms for limited resources,3–9 and these compounds have proven to be the richest source of antimicrobials for development into therapeutics. While most if not all of these natural products are produced as families of related compounds, the significance of this diversity is debated.10–14 The arylomycins, first isolated in 2002 from a strain of Streptomyces, consist of three related series of compounds, each of which has a conserved C-terminal tripeptide macrocycle attached to an N-terminal lipopeptide (Fig. 1).15–17 The macrocycle of the A series compounds is unmodified, while those of the B series and lipoglycopeptides are nitrated and glycosylated (and in some cases hydroxylated), respectively.15,16
Figure 1.
Structure of the arylomycin derivatives characterized in this study. Arylomycin A-C16, and arylomycin B-C16 correspond to A and B series arylomycins, respectively, while arylomycin C-C16, and BAL4850C correspond to the lipoglycopeptide series of arylomycins (see text for details).
The arylomycins inhibit type I signal peptidase (SPase, EC 3.4.21.89),16,18,19 which is an essential membrane-bound serine endopeptidase with a highly conserved active site that is required to remove the amino-terminal leader (signal) sequence during, or shortly after protein translocation across the cytoplasmic membrane. SPase acts via a unique Ser-Lys catalytic dyad with an unusual nucleophilic attack on the si-face of the substrate, as opposed to the re-face attack characteristic of the more common Ser-His-Asp catalytic triad serine proteases.20 Moreover, its position on the outer surface of the cytoplasmic membrane should make it relatively accessible to inhibitors, although penetration of the outer membrane could limit accessibility in Gram-negative bacteria. While their novel mechanism of action originally generated much enthusiasm, excitement for developing the arylomycins waned when it was concluded that their activity was limited to only a few Gram-positive bacteria.16,17 However, after reporting the first synthesis of an arylomycin, the A series member arylomycin A2 (Fig. 1), as well as several derivatives,21 we demonstrated that they actually have a remarkably broad spectrum of activity,19 including potent activity against both Gram-positive and Gram-negative bacteria, but that activity is limited in some cases by one of at least two resistance mechanisms: target mutation, specifically, the presence of a proline residue in SPase;19 or a second as yet undefined mechanism that confers Streptococcus agalactiae with resistance to the A series derivatives.22 Moreover, following the synthesis of a B series arylomycin, arylomycin B-C16, we demonstrated that the nitro group does not negatively impact activity against bacteria that are sensitive to arylomycin C16, and importantly, that it overcomes the resistance of S. agalactiae and imparts the scaffold with a reasonably potent minimum inhibitory concentration (MIC) of 8 μg/ml.22
Like the lipoglycopeptide arylomycins, many antibiotics are glycosylated, and in some cases the sugar substituents are required for activity.23,24 From a medicinal chemistry perspective, glycosylation can also impact an antibiotic’s potential for development as a therapeutic by affecting its pharmacokinetic properties, including absorption, distribution, metabolism, and excretion, at least in part due to changes in solubility and serum binding.25 The most common sugar substituents are 6-deoxysugars, of which more than a hundred have been identified among different secondary metabolites,26 and indeed the sugar substituent of the lipoglycopeptide arylomycins was identified as deoxy-α-mannose,16 although its absolute stereochemistry was not determined.
The lipoglycopeptide arylomycins have been shown to have moderate activity against several bacteria, inhibiting a strain of Streptococcus pneumoniae with MICs ranging from 8 to >64 μM; a strain of Staphylococcus aureus, with MICs ranging from 32 to >64 μM; and a strain of Haemophilus influenzae, with an MIC of 64 μM.16 While they are not active against intact Escherichia coli, they were shown to have activity against permeabilized mutant strains, leading to the suggestion that their development as therapeutics would require the optimization of outer membrane penetration.16 However, the potential role of the resistance conferring proline has not been examined.
E. coli SPase is 324 amino acids in length, (molecular weight 35,960 Da and pI 6.9)27 and contains two amino-terminal transmembrane segments (residues 4–28 and 59–77), one small cytoplasmic region (residues 29–58), and a large carboxyl-terminal periplasmic catalytic domain (residues 78–324).28,29 Proteinase K digestion,29,30 gene-fusion,31 and disulfide cross-linking studies32,33 are all consistent with both the N- and C- termini of E. coli SPase facing the periplasmic space. The catalytically active periplasmic domain of E. coli SPase (SPase 2–75) has a molecular weight of 27,952 Da34 and pI of 5.6.35 It has been sub-cloned, purified,34 characterized35 and crystallized.36 To date, four crystal structures of E. coli SPase have been reported (all with the Δ2–76 enzyme), including the unbound protein,37 a binary complex with a β-lactam inhibitor,20 a binary complex with an A family arylomycin (arylomycin A2),18 and a ternary complex with arylomycin A2 and β-sultam.38 While these structures have helped elucidate the mechanisms of the molecular recognition underlying the inhibition of SPase by the arylomycins, the effects of macrocycle glycosylation remained unclear.
We now report the first synthesis of an arylomycin lipoglycopeptide and its biological characterization, as well as the structural analysis of the binary complex with a related glycosylated and hydroxylated derivative. Total synthesis allowed us to assign the absolute stereochemistry of the deoxy-α-mannose substituent and to determine that the spectrum of activity of the glycosylated derivative is limited by the same mechanisms of resistance as are the A series compounds. The structural analysis revealed that the inhibitor binds in a fashion similar to that previously reported for the A series derivative, and that the sugar is oriented away from the active site and into the aqueous environment. Consistent with these structural studies, in addition to finding that glycosylation does not interfere with SPase binding or activity, we find that it appears to increase aqueous solubility and reduce protein binding based on MIC values in the presence of serum proteins. In all, the data reveal that contrary to previous conclusions,16 glycosylation does not interfere with the antibacterial activity of the arylomycins, including activity against Gram-negative bacteria, and that similar types of modifications might be used to optimize the pharmacokinetics of this promising scaffold.
Results and Discussion
Lipoglycopeptide synthesis
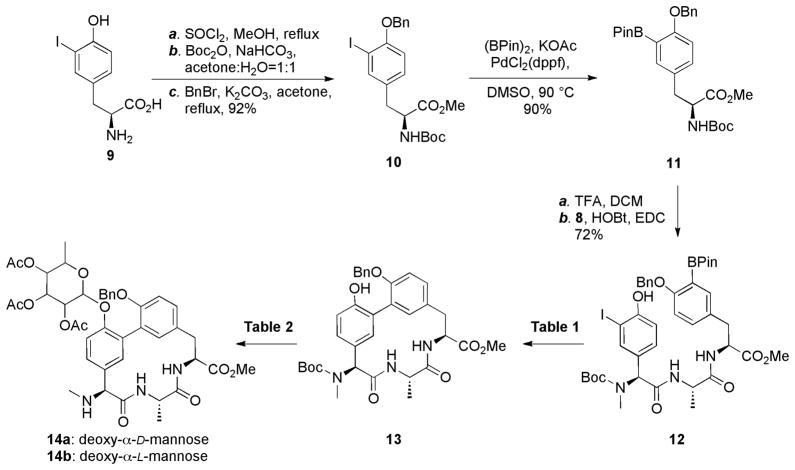
To synthesize the lipoglycopeptide arylomycins, we modified our previously reported syntheses of the arylomycin A21 and B22 series compounds to increase flexibility and material throughput. Because the absolute stereochemistry of the sugar was not known, we targeted the synthesis of both the deoxy-α-L-mannose and the deoxy-α-D-mannose variants. The required lipopeptide 2 was prepared using the procedure of Zhu and co-workers (Scheme 1).39,40 Briefly, the previously reported tripeptide 1 was acylated with isopalmitic acyl chloride, generated in situ from isopalmitic acid and oxalyl chloride in DCM, yielding the fully protected fatty tail, which was hydrolyzed under Nicolaou’s conditions (Me3SnOH/DCE) to afford the lipopeptide 2 in 51% yield.
Scheme 1.
Macrocycle synthesis commenced with the preparation of the hydroxyphenylglycine-alanine iododipeptide 8 from commercially available 4-hydroxyphenylglycine 3 (Scheme 2). Briefly, Boc protection of 3, followed by mono iodization41 afforded 4 in 80% yield, which was subsequently converted into oxazolidinone 5. After reduction by triethylsilane in TFA, followed by reinstallation of the Boc group, the desired N-methyl amino acid 6 was obtained without racemization. Acid 6 was then coupled to L-Ala-OMe to afford dipeptide ester 7. Finally, hydrolysis of the methyl ester using Me3SnOH provided iododipeptide 8 in quantitative yield. This sequence produced 8 in seven steps with 40% overall yield, which required only one column purification for product 7.
Scheme 2.
The protected tyrosine pinacol boronic ester 11 was prepared from iodotyrosine in four steps (Scheme 3). Briefly, triply protected iodotyrosine 10 was prepared from commercially available iodotyrosine 9 in greater than 90% yield with only one column purification. The boronic ester of compound 11 was installed via a Miyama reaction,42 and then deprotection, followed by HOBt/EDC-mediated coupling to dipeptide 8 provided tripeptide 12. To optimize macrocyclization of 12, we first employed conditions that we found to be optimal for the cyclization of the bis-methyl phenol protected A or B series macrocycle cores (PdCl2(dppf)/NaHCO3, DMF). However, in this case the desired product was obtained in less than 25% yield, suggesting that while the free phenol may minimize the epimerization of 12,39 it also appears to adversely affect the reaction. Thus we re-optimized the cyclization conditions (Table 1) and found that Pd(tBu3P)2 and K2CO3 provided 13 in a satisfactory 48% yield.
Scheme 3.
Table 1.
Cyclization conditions
| entrya | catalyst | base | yield |
|---|---|---|---|
| 1 | PdCl2(dppf) | K2CO3 | 25%c |
| 2 | Pd(0)(Pt-Bu3)2 | K2CO3 | 48%c |
| 3 | PEPPSI™-IPrb | K2CO3 | <10%d |
| 4 | PdCl2(PPh3)2 | K2CO3 | <10%d |
| 5 | Pd(OAc)2(SPhos)2 | K2CO3 | <10%d |
| 6 | Pd2(dba)3+SPhos | K2CO3 | <10%d |
| 7 | Pd(PPh3)4 | K2CO3 | <10%d |
| 8 | Pd(0)(Pt-Bu3)2 | Cs2CO3 | <10%d |
| 9 | Pd(0)(Pt-Bu3)2 | CsF | <10%d |
| 10 | Pd(0)(Pt-Bu3)2 | NaOH | <10%d |
| 11 | Pd(0)(Pt-Bu3)2 | K3PO4 | <10%d |
All the reactions were carried out in DMSO at 90 °C for 24 h.
PEPPSI™-IPr: (1,3-Diisopropylimidazol-2-ylidene)(3-chloro-pyridyl) palladium(II) dichloride.58
Isolated yield.
Based on LC-MS.
The only remaining challenge was to glycosylate the free phenol of the macrocycle without O- to C-glycoside rearrangement under the required Lewis acidic reaction conditions (Table 2).43 Due to the low nucleophilicity of the phenol and the steric hindrance of the neighboring O-benzyl group, we first attempted to glycosylate 13 using glycosyl bromides 15 in the presence of AgOTf and 4 Å molecular sieves in DCM, but no glycosylated product was detected. We then investigated trichloroacetimidate 16a as a glycosyl donor. TMSOTf-promoted glycosylation, either in catalytic or super-stoichiometric quantities, yielded the desired macrocycle in less than 40% yield. However, 10 equivalents of BF3-Et2O44 resulted in simultaneous glycosylation and Boc deprotection, and yielded the desired glycosylated macrocycle 14a in 76% yield. Similarly, 14b was obtained in 70% yield using the same conditions.
Table 2.
Glycosylation conditions.
| entrya | glycosyl donor | lewis acid | productb | yieldc |
|---|---|---|---|---|
| 1 |
 (15) |
AgOTf (4 eq) | NRd | 0 |
| 2 |
 (16a) |
TMSOTf (0.5 eq) | Mixture | NDe |
| 3 | 16a | TMSOTf (2.2 eq) | Mixture | 40% |
| 4 | 16a | BF3-Et2O (10 eq) | 14a | 76% |
| 5 |
 (16b) |
BF3-Et2O (10 eq) | 14b | 70% |
All reactions were carried out in anhydrous DCM with 4 Å molecular sieves; details are provided in Supporting Information.
Product ratios determined by LC-MS.
Yields were isolated yields.
No product detected.
Not determined.
With lipopeptide tail 2 and both glycosylated macrocycles 14a and 14b in hand, it only remained to couple and deprotect the two halves of the molecule. Compound 2 was coupled to 14a or 14b to provide the fully protected natural products 17a and 17b. Global deprotection was carried out in three mild reactions to avoid the possible elimination of the D-Ser hydroxyl group. Pd/C catalyzed hydrogenation, NaOMe induced deacetylation, and hydrolysis with Me3SnOH provided the candidate natural product candidates 18a and 18b in greater than 50% yield. Comparison of the 1H NMR spectra of the two candidate natural products with that of the authentic natural product (kindly provided by Dr. Sheng-Bing Peng, Eli Lilly) revealed that while almost all of the proton resonances of the 18a spectrum are nearly identical to the natural product, those of the sugar are significantly different (Fig. S1). In contrast, the 1H spectra of 18b and the natural product are virtually identical, as are the 13C spectra. Thus, we conclude that the lipopeptide arylomycins are glycosylated with deoxy-α-L-mannose. Furthermore, similarities in the NMR spectra in the region corresponding to the sugar of the different members of the lipoglycopeptides,16 suggest that they are all glycosylated with deoxy-α-L-mannose.
While the arylomycins, including the lipoglycopeptides, are naturally lipidated with different fatty acids ranging in length from 12 to 16 carbons, our analysis of the A and B series compounds were performed with a straight chain C16 tail.21 Thus, for systematic comparison of biological activity, we used the above protocol but with the straight chain C16 lipid, to synthesize the corresponding lipoglycopeptide derivative. As expected, this synthesis proceeded with indistinguishable yields. For simplicity, we refer to these derivatives as arylomycin A-C16, arylomycin B-C16, and arylomycin C-C16, corresponding to the A, B, and lipoglycopeptide compounds, respectively (Fig. 1).
Structural analysis of a lipoglycopeptide arylomycin bound to SPase
Structural studies focused on analysis of the glycosylated and hydroxylated lipoglycopeptide antibiotic BAL4850C and SPase Δ2–76. BAL4850C contains the same sugar that is characteristic of the lipoglycopeptide class of arylomycins, but is differentiated from arylomycin C-C16 by macrocyclic hydroxylation and lipidation by an unsaturated C16 fatty acid (Fig. 1). All attempts to soak BAL4850C into preformed crystals of SPase Δ2–76 were unsuccessful, so we instead developed a co-crystallization method which yielded well ordered crystals that diffracted to beyond 2.4 Å resolution (Table 3). The initial Fo - Fc difference map revealed a well-defined rod-shape density corresponding to the N-terminal tripeptide, as well as ring-shape electron density corresponding to the three residue macrocycle core of the inhibitor with clear electron density for the mannose substituent (Fig. 2A). The modeled L-stereochemistry for the mannose substituent is consistent with the electron density, but the resolution is not high enough to independently confirm the assigned stereochemistry. The electron density of the C16 fatty acid tail of the lipoglycopeptide is weak at 1.0 σ, suggesting the fatty acid tail is disordered. Similarly, no density was observed for the fatty acid tail in the reported complex with arylomycin A2.18
Table 3.
Data Collection and Refinement Statistics
| Crystal Parameters | |
| Space group | P43212 |
| a,b,c (Å) | 72.0, 72.0, 262.6 |
| Data Collection Statistics | |
| Wavelength (Å) | 1.5418 |
| Resolution (Å) | 32.2 – 2.4 (2.5 – 2.4)a |
| Total Reflections | 247952 (28360) |
| Unique reflections | 26680 (2605) |
| Rmergeb | 0.105 (0.44) |
| Mean (I)/σ (I) | 10.4 (5.1) |
| Completeness (%) | 99.6 (100.0) |
| Redundancy | 9.3 (10.9) |
| Refinement Statistics | |
| Protein molecules (chains) in A.U. | 2 |
| Residues | 432 |
| Inhibitors | 2 |
| Water molecules | 57 |
| Total number of atoms | 3617 |
| Rcrystc/Rfreed (%) | 24.5/26.5 |
| Average B-factor (Å2) (all atoms) | 56.5 |
| Rmsd on angles (°) | 1.098 |
| Rmsd on bonds (Å) | 0.007 |
The data collection statistics in brackets are the values for the highest resolution shell.
Rmerge = ΣhklΣi|Ii (hkl)-〈I(hkl)〉|/ΣhklΣiIi(hkl) where Ii (hkl) is the intensity of an individual reflection and 〈I(hkl)〉 is the mean intensity of that reflection.
Rcryst = Σhkl||Fobs| − |Fcalc||/Σhkl|Fobs| where Fobs and Fcalc are the observed and calculated structure-factor amplitudes, respectively.
Rfree is calculated using 5% of the reflections randomly excluded from refinement.
Figure 2.
View of lipoglycopeptide BAL4850C within the active site and substrate-binding groove of SPase. Due to weak electron density, the fatty acid tail is not included. A. Electron density for lipoglycopeptide BAL4850C bound within the active site and substrate-binding groove of SPase. A cross-validated 2Fo-Fc electron density map contoured at 1s (blue) is shown with the lipoglycopeptide shown in stick representation and colored by element (carbon: yellow, oxygen: red, nitrogen: blue). The SPase protein is shown in cartoon representation and colored in grey. The catalytic residues (Ser91 and Lys146) are labeled and colored by element (carbon: grey, oxygen: red, nitrogen: blue). B. The molecular surface of SPase with basic residues in blue, acidic residues in red, and all others in grey. The bound lipoglycopeptide is shown and colored as in panel A. The S1 and S3 binding sites of SPase are labeled. Chain B within the asymmetric unit was used to make the figure.
The refined structure revealed that the arylomycin is positioned within the SPase binding site with its C-terminal macrocycle oriented towards the catalytic residues and both the peptide backbone and side chains tightly packed within the substrate binding groove (Fig. 2 and 3). Similar to the complex of SPase Δ2–76 and arylomycin A2,18 one C-terminal carboxylate oxygen (O45) interacts with three catalytically essential residues of the enzyme, including the nucleophile Ser91 Oγ, the general base Lys146 Nζ, and a component of the oxyanion hole, Ser89 Oγ. (Note that the numbering system used with previous SPase structures18,20,37,38 is different by one residue due an error in the originally reported sequence of the E. coli protein.30 The sequencing error occurred in the cytoplasmic region, between the two N-terminal transmembrane segments, which is not present in SPaseΔ2–76, and therefore does not affect the register of the residues within the crystal structures, only the residue numbering system. The numbering system used in the currently reported structure matches that in the Swiss-Prot sequence data base, accession number: P00803.) The other C-terminal carboxylate oxygen (O44) is hydrogen-bonded to Ile145 N and the general base Lys146 Nζ. The peptide backbone of the arylomycin forms eleven direct hydrogen-bonds with SPase, forming parallel β-strand interactions with the β-sheets that make up the substrate binding groove (SPase residues 143–146 and 82–86), seven of which are mediated by the macrocycle tripeptide and four by the N-terminal tripeptide. Macrocycle residues N33 and N28 form hydrogen-bonds with Asp143 O and Gln86 O, respectively, and there is a conserved water molecule (water 14 in Chain A and water 15 in Chain B) within hydrogen-bonding distance of O45. Finally, the C30 methyl group of Ala6 is directed toward the S3 substrate-specificity pocket and is in van der Waals contact with the side chain of SPase residue Ile145. Clearly, the macrocycle provides the majority of the interactions involved in SPase recognition. Interestingly, although the deoxy-α-L-mannose is in van der Waals contact with SPase residue Pro88, it is predominantly solvent exposed (Fig. 2 and 3).
Figure 3.
Interactions between lipoglycopeptide BAL4850C and SPase. Due to weak electron density, the fatty acid tail is not included. A. SPase is shown in line representation and colored by element (carbon: green, oxygen: red, nitrogen: blue, residues labeled in black). The lipoglycopeptide is shown in stick and colored by element (carbon: yellow, nitrogen: blue, oxygen: red, atoms labeled in red). Chain B within the asymmetric unit was used to make the figure. B. Schematic representation of lipoglycopeptide BAL4850C interactions with the active site and substrate binding groove of SPase. Dashed lines indicate hydrogen bonds and distances are in Å (average value for the two molecules in asymmetric unit). The residues involved in van der Waals interactions are symbolized by arches.
A superposition of the structures of the three SPase-arylomycin complexes solved to date reveals that very little adjustment within the protein is needed to accommodate the sugar (Fig. 4). Moreover, the presence of the sugar does not appear to significantly alter the structure of the macrocycle, which the superposition reveals is similar, and engages the protein in a similar way, in each complex. However, the superposition also reveals that the binding mode for the three N-terminal residues (D-MeSer2–D-Ala3–Gly4) of the inhibitor is more variable, particularly at D-Ala3. These observations are consistent with the macrocycle mediating the majority of interactions between the inhibitor and the enzyme and suggest that the N-terminal peptidic tail may be more flexible.
Figure 4.
Superposition of the active sites of SPase-arylomycin complexes. The SPase active site residues are shown as thin lines and labeled. The arylomycin molecules are shown in ball and stick representation. The lipoglycopeptide BAL4850C complex (PDB: 3S04) is black, the ternary complex with arylomycin A2 and a β-sultam (PDB ID: 3IIQ)38 is red, and the arylomycin A2 complex (PDB ID: 1T7D)18 is green. Due to weak electron density, the fatty acid tail is not included in the BAL4850C and arylomycin A2 complexes.
Biological activity
The antibacterial activity of the lipoglycopeptide arylomycin C-C16 was characterized by determining the MIC required to inhibit the growth of several Gram-positive bacteria (Table 4). Against S. epidermidis, S. pyogenes, S. pneumoniae, C. glutamicum, and R. opacus arylomycin C-C16 has activity that is indistinguishable from the analogous A series compound. Moreover, like arylomycin A-C16, but unlike arylomycin B-C16, arylomycin C-C16 has no activity against S. agalactiae strain COH1, demonstrating that the ability of the nitro substituent to impart the B series scaffold with activity against this pathogen is unique.
Table 4.
MICs of Arylomycin A- and C-C16 (μg/ml)a
| Strain | Arylomycin A-C16 | Arylomycin C-C16 |
|---|---|---|
| S. epidermidis RP62A | 0.25 | 0.25 |
| S. epidermidis PAS9001b | 8 | 8 |
| S. agalactiae COH-1 | >128 | >128 |
| S. pyogenes MGAS-1 | 8 | 16 |
| S. pneumoniae R800 | 8 | 8 |
| C. glutamicum DSM 44475 | 4 | 4 |
| R. opacus DSM 1069 | 2 | 1 |
We next determined the activity of arylomycin C-C16 against S. aureus, E. coli, and P. aeruginosa. As with both the A and B series compounds, arylomycin C-C16 has no activity against these pathogens. Also as with the A and B series compounds, arylomycin C-C16 does have significant activity against the mutant pathogens where the arylomycin resistance-conferring proline residue is mutated to a residue that does not confer resistance (P29S in the S. aureus protein, and P84L in the E. coli and P. aeruginosa proteins)19 (Table 5). Moreover, the addition of polymyxin B nonapeptide, which permeabilizes the outer membrane of Gram-negative bacteria, did not significantly affect the MICs. Thus, while the spectrum of arylomycin C-C16 is limited by the same resistance mechanisms as is the A series compound, in contrast to previous conclusions,16 the presence of the sugar does not impair the inhibitor’s ability to penetrate the outer membrane of Gram-negative bacteria.
Table 5.
MICs of Arylomycin C-C16 (μg/ml) against mutants known to be sensitive to arylomycin A- and B-C16
Although not unprecedented among therapeutics,45–48 from a drug development perspective, the fatty acid tails of the arylomycins might prove to be a liability, for example due to decreased solubility and increased serum binding. Thus, to begin to examine the effects of glycosylation on aqueous solubility and serum binding we redetermined the MICs of arylomycin A-C16 and arylomycin C-C16 against wild type S. epidermidis and sensitized E. coli in the presence of pooled human serum (25 – 100%) or bovine serum albumin (4 – 10%) in MHIIB. While the value of MICs varied under the different conditions tested, the MIC observed for arylomycin C-C16 was consistently 2- to 4-fold lower than that of arylomycin A-C16 under these conditions. These small but reproducible effects are consistent with glycosylation increasing the concentration of the free inhibitor available for SPase binding.
Conclusions
Our previous demonstration that the arylomycin class of antibiotics has a broader spectrum of antibacterial activity than previously appreciated, and that where resistance does exist, it results from the presence of a specific proline mutation in the target SPase protein,19 makes the arylomycin scaffold promising for development as a therapeutic. Toward the further exploration of this class of natural product antibiotics, we found that the lipoglycopeptide variants can be by synthesized in reasonable yield, with the key steps being a Pd(tBu3P)2/K2CO3-mediated macrocycle cyclization and a BF3-Et2O-mediated trichloroacetimidate glycosylation. Characterization of the synthetic product allowed us to unambiguously determine that the natural products are glycosylated with deoxy-α-L-mannose, and contrary to previous reports,16 we found that glycosylation does not appear to interfere significantly with activity. The structural analysis revealed that the lipoglycopeptides bind SPase in a manner analogous to the arylomycin A series compounds with the sugar moiety oriented away from the enzyme active site and largely solvent exposed. Nonetheless, the structural analysis also revealed that the hydrophobic portion of the sugar interacts with active site residues, and that glycosylation does affect the interactions between the peptidic position of the inhibitors tail and SPase. Thus, it remains possible that glycosylation affects activity against bacteria that were not examined in the current study. While the selection pressure, if any, that favors glycosylation of the arylomycin scaffold in nature remains unclear, it does appear that glycosylation increases the solubility of the scaffold, an important pharmacokinetic attribute for any candidate therapeutic. Thus, derivatization at the same position with other substituents, e.g. other sugars, phosphates, sulfates etc., might further improve the pharmacokinetic properties of the arylomycin scaffold and aid in its potential development as a therapeutic. Experiments to test this hypothesis are currently in progress.
Experimental Section
General experimental procedures
Dry solvents were purchased from Acros. Commercially available amino acids were purchased from Bachem (Torrence, CA), Chem-Impex (Wood Dale, IL), or Novabiochem (EMD Chemicals, Gibbstown, NJ). Celite 545 filter aid (not acid washed) was purchased from Fisher. Anhydrous 1-hydroxybenzotriazole (HOBt) was purchased from Chem-Impex. All other chemicals were purchased from Fisher/Acros or Aldrich. Reactions were magnetically stirred and monitored by thin layer chromatography (TLC) with 0.25 mm Whatman pre-coated silica gel (with fluorescence indicator) plates. Flash chromatography was performed with silica gel (particle size 40–63 μm, EMD chemicals). 1H and 13C NMR spectra were recorded on Bruker DRX 500, or Bruker DRX 600 spectrometers. Chemical shifts are reported relative to either chloroform (δ 7.26) or methanol (δ 3.31) for 1H NMR, and either chloroform (δ 77.16) or methanol (δ 49.00) for 13C NMR. High resolution time-of-flight mass spectra were measured at the Scripps Center for Mass Spectrometry. ESI mass spectra were measured on either an HP Series 1100 MSD or a PESCIEX API/Plus. For all compounds exhibiting atropisomerism or isolated as semi-pure mixtures, NMR spectra are provided in Supporting Information. Yields refer to chromatographically and spectroscopically pure compounds unless otherwise stated.
All preparative reverse phase chromatography was performed using Dynamax SD-200 pumps connected to a Dynamax UV-D II detector (monitoring at 220 nm) on a Phenomenex Jupiter C18 column (10 μm, 2.12 × 25 cm, 300 Å pore size). All solvents contained 0.1% TFA; Solvent A, H2O; Solvent B, 90% acetonitrile/10% H2O. All samples were loaded onto the column at 0% B, and the column was allowed to equilibrate ~10 min before a linear gradient was started. Retention times are reported according to the linear gradient used and the % B at the time the sample eluted.
Production and co-crystallization of SPase Δ2–76 and lipoglycopeptide BAL4850C and data collection
SPase Δ2–76 was expressed and purified as described previously.36 Prior to co-crystallization, SPase Δ2–76 (18.0 mg/ml in 20 mM Tris-HCl, pH 7.4, and 0.5% Triton-100) was combined with the lipoglycopeptide BAL4850C (10 mM in DMSO) in a 1:1 molar ratio and incubated on ice for one hour.
Co-crystallization trials for SPase Δ2–76 in complex with BAL4850C (provided by Basilea Pharmaceutica International Ltd., Grenzacherstrasse 487, CH-4058, Basel, Switzerland) were carried out by the sitting-drop vapor diffusion method. The final optimized reservoir condition that produced high quality crystals for data collection was 22% (w/v) PEG 4000 and 0.2 M KCl. The drop consisted of 2 μl of protein/inhibitor complex described above, 2 μl of reservoir solution, and 2 μl of 0.025 M n-dodecyl-β-D-maltoside (DDM). This 6 μl drop was equilibrated over 1 ml of reservoir solution at 20 °C. The crystals formed from a light precipitate after approximately two weeks and had an average size of ~0.2 × 0.1× 0.5 mm.
Before data collection, the crystal was transferred by a pipette from the growth drop to a cryoprotectant composed of 24% w/v PEG 4000, 0.2 M KCl, 0.008 M DDM, 20% glycerol) for 30 s. The crystal was mounted on a Hampton Research loop and flash-cryo-cooled by directly placing it into a gaseous nitrogen stream at 100K. The X-rays (wavelength 1.5418 Å) were generated from Cu Ka radiation via a Rigaku MicroMax-007 Microfocus X-ray rotating-anode generator running at 40 Kv and 20 mA and equipped with Osmic Confocal VariMax High Flux optics. The crystal-to-detector distance was set to 200 mm. All frames (280) were recorded on a R-AXIS IV++ imaging-plate detector with a 0.5° oscillation angle and an exposure time of 240 s per frame. The data revealed diffraction to beyond a resolution of 2.4 Å. Data were collected, indexed, and scaled using the program CrystalClear.49 The crystals belong to the tetragonal space group P43212. The unit cell dimensions were determined to be a = 72.0 Å, b = 72.0 Å, and c = 262.6 Å. The Matthews coefficient (Vm) is 3.03 Å3/Da for two molecules in the asymmetric unit. The fraction of the crystal volume occupied by solvent was 59.3%, calculated by the program Matthews in the CCP4i suite of programs.50,51 For crystal and data collection statistics see Table 3.
Phasing, model building, and refinement
A molecular replacement solution was found using the program Phaser in the CCP4i suite of programs.51 The atomic coordinates used for the search model was taken from a 2.5 Å crystal structure of SPase Δ2–76 (PDB code, 1T7D; molecule A).18 The topology and parameter files for the inhibitor were generated using the program PRODRG.52 Coordinates for the inhibitor were manually docked into clear electron difference density (Fo - Fc) near the active site. In addition, the main chain trace and the side chain assignments for the dynamic regions corresponding to residues Phe197-Asn201 and Asp305-Leu315 in chain B were built in manually. Water molecules were added to well-defined peaks (2.0 σ and greater into the Fo – Fc maps). Model building and analysis was performed with the program Coot.53,54 Refinement of the structure was carried out using the program Refmac 5 in the CCP4i suite as well as CNS.51,55 The cycles of refinement were carried out for both protein model and inhibitor model using rigid body and restrained NCS refinement in the program Refmac 5, and simulated annealing, energy minimization, and B-factor refinement was performed in CNS. In addition, a cycle of TLS refinement was carried out using the TLS Motion Determination Sever and restrained TLS refinement protocol of Refmac 5 within the CCP4i suite.51 In all cycles of refinement, 5% of the reflections were set aside for cross-validation. Final refinement and analysis statistics of the complex are provided in Table 3. The stereochemistry of the structure model was analyzed with the program PROCHECK.56 No stereochemical outliers were observed in the Ramachandran plot, with 96.3% of the residues in the preferred regions. An all atom superposition of the arylomycin complexes was performed with the program Pymol57 using molecule B of the lipoglycopeptide arylomycin complex (PDB: 3S04), molecule A of the ternary complex with arylomycin A2 and a β-sultam (PDB: 3IIQ),38 and molecule A of the arylomycin A2 complex structure (PDB: 1T7D).18 Figures were prepared using the programs ISIS Draw version 2.5 (MDL Information Systems, Inc.), and PyMol.57 The atomic coordinates (accession code: 3S04) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org).
Determination of antimicrobial activity
Antimicrobial activity was examined using thirteen bacterial strains, Staphylococcus epidermidis RP62A, Staphylococcus aureus NCTC 8325, Escherichia coli MG1655, Pseudomonas aeruginosa PAO1, Staphylococcus epidermidis RP62A SpsIB(S29P) (PAS9001), Staphylococcus aureus NCTC 8325 SpsB(P29S) (PAS8001),19 Escherichia coli MG1655 LepB(P84L) (PAS0260),19 Pseudomonas aeruginosa PAO1 LepB(P84L) (PAS2008),19 Rhodococcus opacus DSM 1069, Streptococcus agalactiae COH-1, Streptococcus pyogenes M1-5448, Streptococcus pneumoniae R800, and Corynebacterium glutamicum ATCC 44475. Minimum Inhibitory Concentrations (MICs) were determined from at least three independent experiments using the CLSI broth microdilution method. Briefly, inocula were prepared by suspending bacteria growing on solid media into the same type of broth used in the MIC experiment and diluting to a final concentration of 1 × 107 colony forming units/ml. 5 μl of this suspension were added to the wells of a 96-well plate containing 100 μl of media with the appropriate concentrations of compound. The MICs of E. coli, P. aeruginosa, S. aureus, S. epidermidis, R. equi, R. opacus, and C. glutamicum were determined in Cation-adjusted Mueller Hinton II broth. MICs of S. pyogenes and S. pneumoniae were determined in Todd Hewitt broth. The MICs of S. agalactiae were determined in Cation-adjusted Mueller Hinton II broth and in Todd Hewitt broth (MIC values differed by at most two fold between these two media). In all cases MICs were defined as the lowest concentration of compound to inhibit visible growth.
Supplementary Material
Scheme 4.
Acknowledgments
This work was supported by the Office of Naval Research (Awards N000140310126 and N000140810478 to F.E.R) and the National Institutes of Health (AI081126 to F.E.R). We also acknowledge support from the Canadian Institute of Health research (to M.P.), the National Science and Engineering Research Council of Canada (to M.P), the Michael Smith Foundation for Health Research (to M.P.), and the Canadian Foundation of Innovation (to M.P.).
Footnotes
Supporting Information Available. Full characterization of synthetic intermediates and compound 18a; supporting data and NMR spectra; complete Ref. 16. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Nat Rev Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 2.Hancock REW. Nat Rev Drug Discov. 2007;6:28. [Google Scholar]
- 3.Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. Nat Rev Microbiol. 2010;8:251–259. doi: 10.1038/nrmicro2312. [DOI] [PubMed] [Google Scholar]
- 4.Baltz RH. J Ind Microbiol Biotechnol. 2006;33:507–513. doi: 10.1007/s10295-005-0077-9. [DOI] [PubMed] [Google Scholar]
- 5.Czaran TL, Hoekstra RF, Pagie L. Proc Natl Acad Sci USA. 2002;99:786–790. doi: 10.1073/pnas.012399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Costa VM, Griffiths E, Wright GD. Curr Opin Microbiol. 2007;10:481–489. doi: 10.1016/j.mib.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Laskaris P, Tolba S, Calvo-Bado L, Wellington L. Environ Microbiol. 2010;12:783–796. doi: 10.1111/j.1462-2920.2009.02125.x. [DOI] [PubMed] [Google Scholar]
- 8.Lynch M. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8597–8604. doi: 10.1073/pnas.0702207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez JL. Proc Biol Sci. 2009;276:2521–2530. doi: 10.1098/rspb.2009.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischbach MA, Clardy J. Nat Chem Biol. 2007;3:353–355. doi: 10.1038/nchembio0707-353. [DOI] [PubMed] [Google Scholar]
- 11.Firn RD, Jones CG. Mol Microbiol. 2000;37:989–994. doi: 10.1046/j.1365-2958.2000.02098.x. [DOI] [PubMed] [Google Scholar]
- 12.Firn RD, Jones CG. Nat Prod Rep. 2003;20:382–391. doi: 10.1039/b208815k. [DOI] [PubMed] [Google Scholar]
- 13.Stone MJ, Williams DH. Mol Microbiol. 1992;6:29–34. doi: 10.1111/j.1365-2958.1992.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 14.Williams DH, Stone MJ, Hauck PR, Rahman SK. J Nat Prod. 1989;52:1189–1208. doi: 10.1021/np50066a001. [DOI] [PubMed] [Google Scholar]
- 15.Holtzel A, Schmid DG, Nicholson GJ, Stevanovic S, Schimana J, Gebhardt K, Fiedler HP, Jung G. J Antibiot. 2002;55:571–577. doi: 10.7164/antibiotics.55.571. [DOI] [PubMed] [Google Scholar]
- 16.Kulanthaivel P, et al. J Biol Chem. 2004;279:36250–36258. doi: 10.1074/jbc.M405884200. [DOI] [PubMed] [Google Scholar]
- 17.Schimana J, Gebhardt K, Holtzel A, Schmid DG, Sussmuth R, Muller J, Pukall R, Fiedler HP. J Antibiot. 2002;55:565–570. doi: 10.7164/antibiotics.55.565. [DOI] [PubMed] [Google Scholar]
- 18.Paetzel M, Goodall JJ, Kania M, Dalbey RE, Page MGP. J Biol Chem. 2004;279:30781–30790. doi: 10.1074/jbc.M401686200. [DOI] [PubMed] [Google Scholar]
- 19.Smith PA, Roberts TC, Romesberg FE. Chem Biol. 2010;17:1223–1231. doi: 10.1016/j.chembiol.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paetzel M, Dalbey RE, Strynadka NC. Nature. 1998;396:186–190. doi: 10.1038/24196. [DOI] [PubMed] [Google Scholar]
- 21.Roberts TC, Smith PA, Cirz RT, Romesberg FE. J Am Chem Soc. 2007;129:15830–15838. doi: 10.1021/ja073340u. [DOI] [PubMed] [Google Scholar]
- 22.Roberts TC, Smith PA, Romesberg FE. J Nat Prod. 2011;74:956–961. doi: 10.1021/np200163g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weymouth-Wilson AC. Nat Prod Rep. 1997;14:99–110. doi: 10.1039/np9971400099. [DOI] [PubMed] [Google Scholar]
- 24.Baltz RH. Chem Biol. 2002;9:1268–1270. doi: 10.1016/s1074-5521(02)00289-2. [DOI] [PubMed] [Google Scholar]
- 25.Cao H, Hwang J, Chen X. In: Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry. Tiwari VK, Mishra BB, editors. Research Signpost; Kerala, India: 2011. pp. 411–431. [Google Scholar]
- 26.Piepersberg W, Distler J. In: Biotechnology: Products of Secondary Metabolism. Rehm H-J, Reed G, editors. Vol. 7. Wiley-VCH Verlag GmbH; 1997. pp. 397–488. [Google Scholar]
- 27.Barrett AJ, Rawlings ND. Arch Biochem Biophys. 1995;318:247–250. doi: 10.1006/abbi.1995.1227. [DOI] [PubMed] [Google Scholar]
- 28.Bilgin N, Lee JI, Zhu HY, Dalbey R, von Heijne G. EMBO J. 1990;9:2717–2722. doi: 10.1002/j.1460-2075.1990.tb07458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore KE, Miura S. J Biol Chem. 1987;262:8806–8813. [PubMed] [Google Scholar]
- 30.Wolfe PB, Wickner W, Goodman JM. J Biol Chem. 1983;258:12073–12080. [PubMed] [Google Scholar]
- 31.San Millan JL, Boyd D, Dalbey R, Wickner W, Beckwith J. J Bacteriol. 1989;171:5536–5541. doi: 10.1128/jb.171.10.5536-5541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalbey RE, Wickner W. Science. 1987;235:783–787. doi: 10.1126/science.3544218. [DOI] [PubMed] [Google Scholar]
- 33.Whitley P, Nilsson L, von Heijne G. Biochemistry. 1993;32:8534–8539. doi: 10.1021/bi00084a020. [DOI] [PubMed] [Google Scholar]
- 34.Kuo DW, Chan HK, Wilson CJ, Griffin PR, Williams H, Knight WB. Arch Biochem Biophys. 1993;303:274–280. doi: 10.1006/abbi.1993.1283. [DOI] [PubMed] [Google Scholar]
- 35.Tschantz WR, Paetzel M, Cao G, Suciu D, Inouye M, Dalbey RE. Biochemistry. 1995;34:3935–3941. doi: 10.1021/bi00012a010. [DOI] [PubMed] [Google Scholar]
- 36.Paetzel M, Chernaia M, Strynadka N, Tschantz W, Cao G, Dalbey RE, James MN. Proteins. 1995;23:122–125. doi: 10.1002/prot.340230115. [DOI] [PubMed] [Google Scholar]
- 37.Paetzel M, Dalbey RE, Strynadka NC. J Biol Chem. 2002;277:9512–9519. doi: 10.1074/jbc.M110983200. [DOI] [PubMed] [Google Scholar]
- 38.Luo C, Roussel P, Dreier J, Page MG, Paetzel M. Biochemistry. 2009;48:8976–8984. doi: 10.1021/bi9009538. [DOI] [PubMed] [Google Scholar]
- 39.Dufour J, Neuville L, Zhu J. Chem Eur J. 2010;16:10523–10534. doi: 10.1002/chem.201000924. [DOI] [PubMed] [Google Scholar]
- 40.Dufour J, Neuville L, Zhu JP. Synlett. 2008;15:2355–2359. [Google Scholar]
- 41.Edgar KJ, Falling SN. J Org Chem. 1990;55:5287–5291. [Google Scholar]
- 42.Ishiyama T, Murata M, Miyaura N. J Org Chem. 1995;60:7508–7510. [Google Scholar]
- 43.Li Y, Wei G, Yu B. Carbohydr Res. 2006;341:2717–2722. doi: 10.1016/j.carres.2006.08.022. and references therein. [DOI] [PubMed] [Google Scholar]
- 44.Nicolaou KC, Mitchell HJ, Jain NF, Winssinger W, Hughes R, Bando T. Angew Chem Int Edit. 1999;38:240–244. [Google Scholar]
- 45.Beauregard DA, Williams DH, Gwynn MN, Knowles DJ. Antimicrob Agents Chemother. 1995;39:781–785. doi: 10.1128/AAC.39.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breukink E, de Kruijff B. Nat Rev Drug Discov. 2006;5:321–332. doi: 10.1038/nrd2004. [DOI] [PubMed] [Google Scholar]
- 47.Kim SJ, Schaefer J. Biochemistry. 2008;47:10155–10161. doi: 10.1021/bi800838c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagarajan R. J Antibiot (Tokyo) 1993;46:1181–1195. doi: 10.7164/antibiotics.46.1181. [DOI] [PubMed] [Google Scholar]
- 49.Pflugrath JW. Acta Crystallogr D Biol Crystallogr. 1999;55:1718–1725. doi: 10.1107/s090744499900935x. [DOI] [PubMed] [Google Scholar]
- 50.Matthews BW. J Mol Biol. 1968;33:491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- 51.Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 52.van Aalten DM, Bywater R, Findlay JB, Hendlich M, Hooft RW, Vriend G. J Comput Aided Mol Des. 1996;10:255–262. doi: 10.1007/BF00355047. [DOI] [PubMed] [Google Scholar]
- 53.Emsley P, Cowtan K. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 54.Kleywegt GJ. Acta Crystallogr D Biol Crystallogr. 2007;63:94–100. doi: 10.1107/S0907444906022657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 56.Laskowski RA, McArthur MW, Moss DS, Thornton JM. J Appl Cryst. 1993;26:283–291. [Google Scholar]
- 57.DeLano WL. The PyMol Molecular Graphics System. DeLano Scientific; San Carlos, CA: 2002. [Google Scholar]
- 58.Sigma-Aldrich ChemFiles. 2006;3(6) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.