Abstract
Previous studies have demonstrated that mesenchymal stromal cells (MSCs) enhance cell survival through upregulation and secretion of stanniocalcin-1 (STC1). This study shows that MSC-derived STC1 promotes survival of lung cancer cells by uncoupling oxidative phosphorylation, reducing intracellular reactive oxygen species (ROS), and shifting metabolism towards a more glycolytic metabolic profile. MSC-derived STC1 upregulated uncoupling protein 2 (UCP2) in injured A549 cells in an STC1-dependent manner. Knockdown of UCP2 reduced the ability of MSCs and recombinant STC1 (rSTC1) to reduce cell death in the A549 population. rSTC1-treated A549 cells displayed decreased levels of ROS, mitochondrial membrane potential (MMP), and increased lactate production, all of which were dependent on the upregulation of UCP2. Our data suggest that MSCs can promote cell survival by regulating mitochondrial respiration via STC1.
Introduction
Mesenchymal stem or stromal cells (MSCs) reside in multiple organs, can be isolated and expanded for cell therapy, and have been shown to contribute to tissue repair by several mechanisms.1 MSCs can home and contribute to the tumor stroma but there are conflicting reports as to whether the MSCs support or suppress tumor growth.2,3 We previously observed that MSCs responded to signals from apoptotic cells by upregulation and secretion of stanniocalcin-1 (STC1).4 STC1 is a evolutionarily conserved secreted protein that exerts pleiotropic effects including alteration of mitochondrial function by upregulation of uncoupling protein 2 (UCP2).5,6,7,8,9 Here, we demonstrate that co-culture of MSCs with lung cancer cell lines made apoptotic by H2O2 or chemotherapeutic drugs activated MSCs to secrete STC1. The STC1 reduced apoptosis by upregulating UCP2 in the cancer cells to enhance the increased anaerobic glycolysis that is referred to as the Warburg effect and that promotes the growth of cancers. The results suggest that antibodies or antagonists to STC1 might counteract some of the effects of tumor stroma and provide a useful therapy for some cancers. The results also suggest that therapy using MSCs may itself be a double-edged sword.
Results
To test whether STC1 secreted by MSCs reduced ROS-induced cell death, we used cultures with A549 cells, a line of human alveolar basal epithelial adenocarcinoma cells. The A549 cells were made apoptotic by the addition of 100 µmol/l H2O210,11 and cultured alone or in the presence of MSCs grown on a transwell filter (outlined in Supplementary Figure S1). MSCs promoted the survival of A549 cells as measured by annexin V/propidium iodide (PI) staining and flow cytometry (Figure 1a), and by lactate dehydrogenase release (Figure 1b). STC1 transcripts and protein were upregulated in MSCs stimulated by H2O2 (Figure 1c). Blocking STC1 in the co-culture with anti-STC1 antibodies reduced the ability of the MSCs to promote cell survival (Figure 1d). Addition of recombinant STC1 (rSTC1) (12.5, 25, 50 ng/ml) was sufficient to increase survival of A549 cells exposed to H2O2 (Figure 1e). rSTC1 also promoted survival of A549 cells exposed to H2O2 as measured using a WST8 assay at a 48-hour timepoint (Figure 1f). To test longer term effects, increased the incubation time of the experiments. rSTC1 increased the survival of A549 cells exposed to H2O2 in experiments that were extended for 5 days (Supplementary Figure S2). Similar results were obtained with three additional lung cell lines, two additional lung epithelial adenocarcinoma (H1299 and PC9) and one lung epithelial squamous cell carcinoma (EBC1) lines. However, rSTC1 had no effect on the survival of squamous cancer cell line (LK2).
Figure 1.
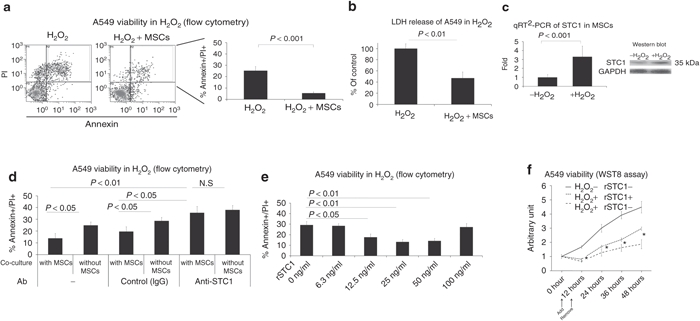
MSCs reduced ROS-induced cell death and cytotoxicity in A549s in a STC1-dependent manner. (a) A549 cells were incubated in a 6-well plate in culture medium containing H2O2 (100 µmol/l) with or without MSCs on a transwell filter (Supplementary Figure S1). Seven hours later, cell survival was assayed by flow cytometry for annexin-V staining and PI incorporation. (b) A549 cells were grown as in a and assayed for viability by LDH release. (c) STC1 expression in MSCs cultured in conditioned media with or without H2O2 (100 µmol/l) as evaluated using RT-PCR and western blot. (d) A549 cells were exposed to H2O2 (100 µmol/l) in the presence or absence of MSCs. Each culture was then incubated with control IgG or anti-STC1 antibodies and assayed for cell viability by flow cytometry. (e) Recombinant human STC1 was added to A549 cells exposed to H2O2 (100 µmol/l) and assayed by flow cytometry. (f) A549 cells were exposed to H2O2 (100 µmol/l) with or without rSTC1 and assayed every 12 hours up to 48 hours for viability by WST8 assay. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MSC, mesenchymal stromal cell; LDH, lactate dehydrogenase; PI, propidium iodide, ROS, reactive oxygen species; rSTC1, recombinant stanniocalcin-1; RT-PCR, reverse transcription-PCR.
We next sought to determine whether the STC1-mediated increased survival of A549 cells could be attributed to decreased ROS production. Knockdown of STC1 in MSCs with short interfering RNA (siRNA) (Supplementary Figure S3) inhibited cytoprotection of H2O2-injured A549 cells (Figure 2a). A549 cells grown alone or in the presence of MSCs were exposed to H2O2 for 4 hours and assayed by flow cytometry for ROS production. The co-cultures displayed a 30% reduction in ROS compared to A549 cells cultured alone (Figure 2b). Knockdown of STC1 in MSCs by siRNA resulted in increased ROS production in the A549 cells exposed to H2O2 compared to ROS production by A549 cells co-cultured with MSCs transduced with a nonspecific siRNA (Figure 2c). In addition, as expected, addition of rSTC1 decreased ROS in A549 cells cultured with H2O2 to control levels (Figure 2d). Addition of rSTC1 also reduced ROS in three other cell lines cultured in the presence of H2O2 (HL1299, PC9, ad EBC1 in Supplementary Figure S4). A fourth cell line (LK2) was unresponsive to rSTC1. Unexpectedly, STC1 reduced ROS in one cell line (PC9) incubated in the absence of H2O2 (Supplementary Figure S4). To expand our observations to a more clinically relevant model of ROS induction, we damaged A549 cells with the chemotherapeutic drug, paclitaxel.12 Treatment of A549 cells with paclitaxel increased ROS production to the same extent as H2O2 (Figure 2e). Treatment of cells with the ROS scavenger N-acetyl cysteine, as well as STC1, reduced ROS production. To test whether H2O2 had any effect on the biochemical action of STC1, we exposed 500 µg/ml rSTC1 to 10 mmol/l H2O2 for 1 hour. H2O2-exposed STC1 was as effective at reducing ROS production as nonexposed rSTC1 (Figure 2e, last column).
Figure 2.
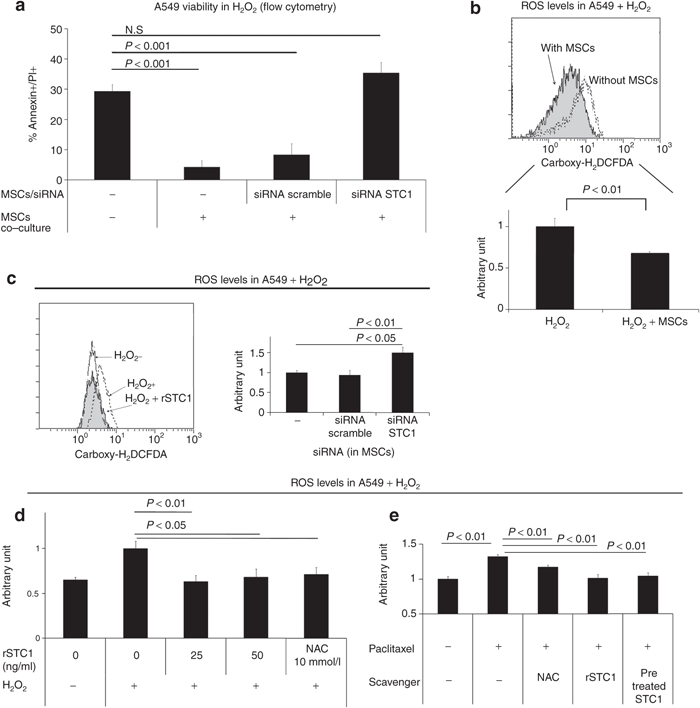
MSCs decreased ROS in H2O2-exposed A549 cells in a STC1-dependent manner. (a) A549 cells were incubated in culture medium containing H2O2 (100 µmol/l) with or without MSCs on a transwell filter. MSCs were transfected with a control or STC1 directed siRNA. Cells were assayed for viability using annexin V/PI staining. (b) A549 cells were incubated in a 6-well plate in culture medium containing H2O2 (100 µmol/l) with or without MSCs on a transwell filter. Four hours later, each culture was assayed for ROS using the dye, carboxy-H2DCFDA. Upper panel, representative flow cytometry data. Lower panel, quantification of ROS from three experiments. (c) MSCs transfected with scrambled or STC1-specific siRNA were co-cultured with A549 in medium containing H2O2 (100 µmol/l). Left panel: A549 cells were assayed for ROS using carboxy-H2DCFDA. Right panel: siRNA knockdown of STC1 in MSCs measured by real-time PCR. (d) rSTC1 was added to the A549 cell culture medium containing H2O2 (100 µmol/l). After 4 hours, A549 cells were assayed for ROS using carboxy-H2DCFDA. (e) NAC (N-acetylcysteine, 10 mmol/l), rSTC1 (50 ng/ml), and H2O2-pretreated rSTC1 (50 ng/ml) were added to A549 cells exposed to paclitaxel (5 µmol/l). Cells were assayed for ROS using carboxy-H2DCFDA. MSC, mesenchymal stromal cell; PI, propidium iodide; ROS, reactive oxygen species; siRNA, short interfering RNA; rSTC1, recombinant stanniocalcin-1.
Previously, STC1 was shown to induce UCP2 expression in macrophages and thereby decrease ROS production.7 To confirm whether STC1 increased UCP2 in A549 cells cultured with H2O2, rSTC1 was added to the culture medium and UCP2 expression was assayed in the A549 cells by real-time PCR and western blot. The rSTC1 increased expression of UCP2 (Figure 3a). We next tested whether STC1 produced by A549 cells contributed to the UCP2 production in the same cells incubated with H2O2. As expected, knockdown of STC1 in the A549 cells with a specific siRNA decreased the levels of messenger RNA for UCP2 (Figure 3b). The expression of UCP2 was also upregulated in A549s when co-cultured with MSCs and addition of anti-STC1 antibodies reduced this effect (Figure 3c).
Figure 3.
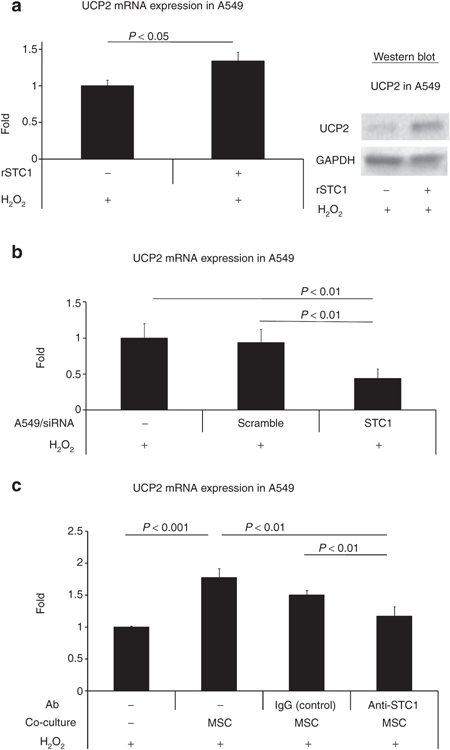
Autocrine and paracrine upregulation of UCP2 by STC1. (a) A549 cells were exposed to rSTC1 (50 ng/ml) for 4 hours and assayed for UCP2 expression by real-time PCR (left panel) and western blot (right panel). (b) A549 cells cultured in medium containing H2O2 (100 µmol/l) were assayed for UCP2 expression by real-time PCR following knockdown of STC1 by siRNA. (c) A549 cells were cultured in medium containing H2O2 (100 µmol/l) with/without MSCs. Antibody vehicle only, control IgG, or anti-STC1 antibodies (500 ng/ml) were added to the co-cultured A549 cells. MSC, mesenchymal stromal cell; siRNA, short interfering RNA; STC1, stanniocalcin-1; UCP2, uncoupling protein 2.
To confirm that upregulation of UCP2 was the primary mechanism for the reduction of ROS, UCP2 in the A549 cells was knocked down with a specific siRNA. Downregulation of UCP2 in A549 cells was confirmed by real-time PCR and western blot (Figure 4a). Knockdown of UCP2 in A549 cells abolished the ability of rSTC1 to increase viability of A549 cells injured with H2O2 (Figure 4b). Also, knockdown of UCP2 by siRNA impaired the ability of rSTC1 to decrease ROS compared to the controls (Figure 4c). In order to corroborate the role of UCP2, we next assayed whether rSTC1 affected traditional pathways for ROS resistance in A549 cells. rSTC1had no effect on catalase, glutathione peroxidase or thioredoxin reductase (Supplementary Figure S5). These results suggested ROS reduction induced by rSTC1 was not dependent on these enzyme reactions but rather the upregulation of UCP2 in A549 cells.
Figure 4.
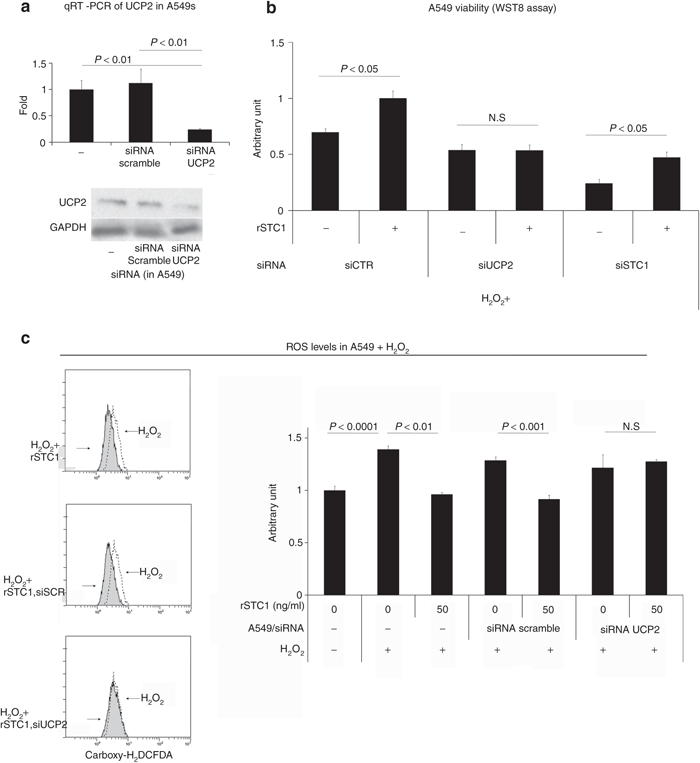
STC1-mediated reduction of ROS is dependent on UCP2. (a) A549 cells were transfected with siRNA-targeting UCP2 and assayed for UCP2 by real-time PCR (upper panel) and western blot assays (lower panel). (b) A549 cells were cultured in H2O2 (100 µmol/l) and transfected with control, UCP2 or STC1 directed siRNA. For each condition, cells were tested for the ability of rSTC1 to promote viability. (c) A549 cells were transfected with siRNA-targeting UCP2 or a scrambled siRNA control (siSCR) and cultured in medium containing H2O2 (100 µmol/l) and rSTC1 (50 ng/ml). After 4 hours, cells were assayed for ROS using carboxy-H2DCFDA. Left panel: representative flow cytometry images. Right panel: quantification of flow cytometry data from multiple experiments. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; qRT-PCR, quantitative reverse transcription-PCR; ROS, reactive oxygen species; siRNA, short interfering RNA; STC1, stanniocalcin-1; UCP2, uncoupling protein 2.
We next tested the effects of STC1 on mitochondrial membrane potential (MMP) as assayed by flow cytometry using JC1 dye. rSTC1 decreased MMP of A549 cells incubated under control conditions (Figure 5a), incubated with H2O2 (Figure 5a), or incubated under conditions of hypoxia and acidosis (Supplementary Figure S6). In contrast, rSTC1 increased MMP in A549 cells in which UCP2 was knocked down with a siRNA and the cells were exposed to H2O2 (Figure 5b). As expected, rSTC1 had no effect on MMP in the presence of a blocking antibody to STC1 (Figure 5c). Two additional lung epithelial adenocarcinoma (H1299 and PC9) and one lung epithelial squamous cell carcinoma (EBC1) lines behaved similarly when assayed for MMP with JC1 dye following incubation with H2O2 and rSTC1 (Supplementary Figure S7). One squamous cancer cell line (LK2) did not respond to rSTC1. The conditioned media of A549 cells was then assayed for the presence of lactate that is produced by anaerobic glycolysis. rSTC1 induced a 33% increase in lactate production in the presence or absence of H2O2 (Figure 5d). The increase in anaerobic glycolysis (about 0.4 pmol of lactate per cell equivalent to 0.2 pmol glucose) was not sufficient to be detectable by an increase in glucose utilization under the same conditions (Figure 5e).
Figure 5.
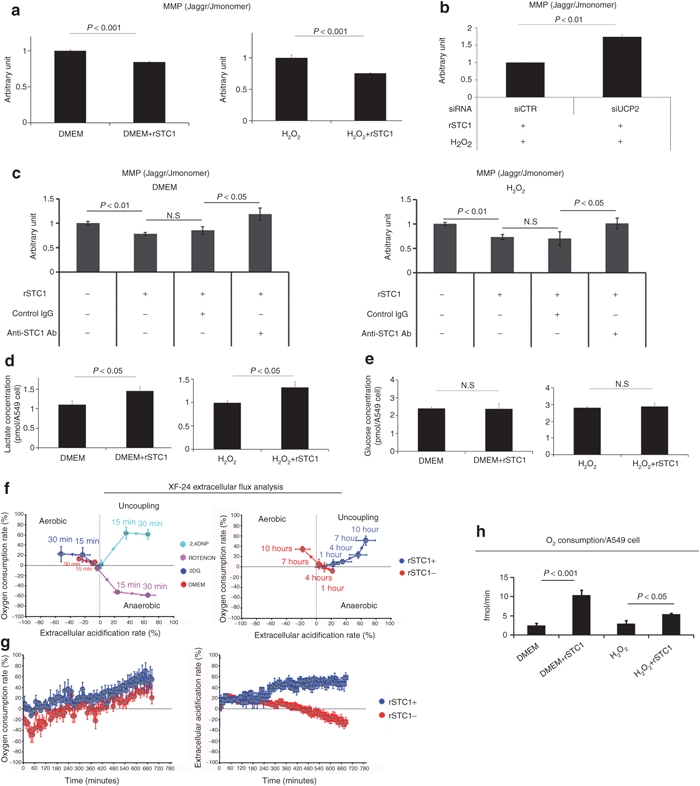
STC1 promotes anaerobic glycolysis in A549 cells. (a) A549 cells were cultured with or without H2O2 (100 µmol/l) and rSTC1 (50 ng/ml). Four hours later, cells were assayed for MMP by JC1 dye incorporation. Left panels, representative flow cytometry data. Right panels, quantification of MMP from multiple experiments. (b) A549 cells were cultured in H2O2 (100 µmol/l) and transfected with control (siCTR) or siRNA directed towards UCP2. Cells were assayed for MMP by JC1 dye incorporation. (c) A549 cells were cultured in the absence (left panel) or presence (right panel) of H2O2. Cells were treated with nonspecific IgG or anti-STC1 antibodies and assayed for MMP by JC1 dye incorporation. (d) A549 cells were cultured with or without H2O2 (100 µmol/l) and rSTC1 (50 ng/ml). Four hours later, cells were assayed for lactate production using a colorimetric assay. (e) A549 cells were cultured with or without H2O2 (100 µmol/l) and rSTC1 (50 ng/ml). Four hours later, conditioned medium was assayed for glucose levels using a colorimetric assay. As indicated in text, the increase in anaerobic glycolysis was detected by assays of lactate production but too small to detect by assay of glucose. (f) A549 cells were cultured on XF24-well plates to allow injection of compounds and subsequent detection of OCR and ECAR by the Seahorse XF Extracellular Flux Analyzer. Left panel: validation of the system. Cells cultured in DMEM were transferred to assay medium, exposed to test compounds, and then analyzed for OCR and ECAR for 30 minutes with 2-deoxyglucose (2-DG) to stimulate aerobic metabolism (aerobic), rotenone (ROTENON) to inhibit mitochondrial metabolism (anaerobic), and 2,4-dinitrophenol (2,4-DNP) to uncouple oxidative phosphorylation (uncoupling). Right panel: cells cultured in similar conditions were exposed to DMEM with or without rSTC1 (final concentration; 50 ng/ml) for 10 hours. (g) A549 cells were cultured as in a, and analyzed by the Seahorse system for 12 hours following injection of culture medium with or without rSTC1(50 ng/ml). (h) A549 cells were cultured with or without H2O2 (100 µmol/l) and rSTC1 (50 ng/ml), and oxygen consumption rate was measured over time using a Clarke electrode. DMEM, Dulbecco's modified Eagle medium, ECAR, extracellular acidification rate; MMP, mitochondrial membrane potential; OCR, oxygen consumption rate; rSTC1, recombinant stanniocalcin-1; UCP2, uncoupling protein 2.
To determine whether uncoupling of oxidative phosphorylation by STC1 increased nonphosphorylating respiration in A549 cells, we measured metabolic flux using an automated instrument (Seahorse Extracellular Flux Analyzer; Seahorse Bioscience, North Billerica, MA), and using a Clarke electrode to measure oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) over time following injection of test compounds. To validate the adequacy of the measurements of OCR and ECAR, control experiments were performed with 2-deoxyglucose (2-DG) to stimulate aerobic metabolism (increased OCR, decreased ECAR), rotenone to promote anaerobic metabolism (decreased OCR, increased ECAR), and 2,4-dinitrophenol (2,4-DNP) to uncouple oxidative phosphorylation (increased OCR, increased ECAR). The expected responses were observed (Figure 5f, left panel). Treatment of the A549 cells with rSTC1 produced an increase in OCR and ECAR characteristic of uncoupling of oxidative phosphorylation, whereas control cells respired via aerobic metabolism (Figure 5f, right panel). Raw data of OCR and ECAR over 12 hours are displayed in Figure 5g. To confirm these data and investigate the effect of rSTC1 in H2O2-exposed cells, A549 cells were treated with rSTC1 in the absence or presence of H2O2 and oxygen consumption was measured using a Clarke electrode system. As expected, treatment of A549 cells with rSTC1 in the absence or presence of H2O2 led to an increase in OCR (Figure 5).
Discussion
The microenvironment provided by the stromas of tumors is a key determinant for the propagation and metastases of cancer cells.13,14,15 The stromas can arise from a variety of cells, including bone marrow MSCs, tissue resident MSCs or even endothelial cells that can transform into MSC-like cells.16,17,18 Previous reports demonstrated that co-culture of MSCs with leukemia cells increased the cancer-characteristic Warburg effect of excessive anaerobic glycolysis by upregulating the expression of UCP2 in the leukemia cells.19,20 By uncoupling oxidative phosphorylation, UCP2 increases the efficiency of mitochondria in generating electrons to reduce ROS.7,21 UCP2 can thereby contribute to the aberrant redox system of many cancer cells that allows them to proliferate in the presence of carcinogenic environments that generate ROS.22 The stromas of tumors provide a large number of factors that enhance propagation and metastases of tumors and that probably vary with the stromas of different cancers.23 The results here suggest that STC1 secreted by MSC-like cells in tumor stroma plays a critical role in enhancing the Warburg effect and making tumors resistant to ROS. Therefore blocking antibodies or antagonists to STC1 may be attractive candidates as an adjunct therapy for γ-radiation and other therapies that increase ROS. The results also highlight additional considerations that need to be addressed in order to fully understand the use of MSCs as a cell therapy. By understanding the processes that make MSCs a “double-edged sword”22 in the context of some disease states, we may be able to modify the cells or culture conditions such that the therapeutic outcomes are more predictable.
Materials and Methods
For details, see Supplementary Materials and Methods.
Cell culture and reagents. Frozen vials of passage 1 human bone marrow MSCs from three different donors were obtained from Tulane University (http://www.som.tulane.edu/gene_therapy/distribute.shtml; currently http://medicine.tamhsc.edu/irm/msc-distribution.html). For STC1 blocking and western blot assays, goat anti-STC1 antibodies were used as reported in our previous studies4,5 (R&D Systems, Minneapolis, MN).
Viability assay. Viability was measured by two independent methods, annexin V/PI staining and lactate dehydrogenase release assay. Cell proliferation was measured using 4-[3-(2-methoxy-4-nitrophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate sodium salt (WST-8 assay).
Measurement of ROS. ROS were measured using acetoxymethyl ester dye, 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, according to the manufacturer's instructions (C2938; Molecular Probes, Eugene, OR).
Measurement of MMP. MMP was measured using JC1 dye, according to the manufacturer's instructions (Molecular Probes).
Lactate, glucose, catalase, glutathione peroxidase, and thioredoxin reductase activity measurements. Lactate and glucose concentrations as well as catalase, glutathione peroxidase, and thioredoxin reductase activity in the culture medium were measured using commercial colorimetric assays (BioVision, Mountain View, CA) and a microplate reader.
Oxygen consumption measurements. OCRs were measured using an S1 Clarke electrode disc (Hansatech, Norfolk, UK).
XF-24 OCR and ECAR measurements. OCR and ECAR were measured using the XF24 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA) according to manufacturer's instructions and as reviewed previously.24
Statistical analyses. All experiments were performed a minimum of three times in triplicate. Analysis of variance followed by a Tukey's post-hoc test was performed for experiments with more than two groups; otherwise, a two-tailed, unpaired Student's t-test was performed. Statistical analyses were performed using Smith's Statistical Package (http://www.economics.pomona.edu/StatSite/SSP.html).
SUPPLEMENTARY MATERIAL Figure S1. Setup of the co-culture experiments. Figure S2. rSTC1 promotes survival in other lung epithelial cell lines after 5 days. Figure S3. Knockdown of STC1 in MSCs by siRNA. Figure S4. Other lung epithelial cells also reduce ROS in response to rSTC1. Figure S5. rSTC1 does not affect the activity of other canonical antioxidant enzymes. Figure S6. rSTC1 reduces MMP in A549 cells grown in hypoxia or acidosis. Figure S7. rSTC1 reduced MMP in other lung epithelial cell lines. Materials and Methods.
Acknowledgments
This work was supported by grants from the NIH (HL073755, HL073252, and P01 HL075161), the Louisiana Gene Therapy Research Consortium, and the Ministry of Education, Culture, Sports, Science, and Technology in Japan (21590980 and 20390229). Thanks to Hosoon Choi (Institute for Regenerative Medicine, Texas A&M HSC COM), Dong-Ki Kim (Institute for Regenerative Medicine, Texas A&M HSC COM), Charles Claypool (Institute for Regenerative Medicine, Texas A&M HSC COM), Tatsuro Fukuhara and Yuji Kubo for aid in reviewing the manuscript. Thanks as well to Haruhiko Yamaguchi, Takefumi Shimoyama, Toru Nakayama, Shinichi Sasaka for their assistance with oxygen measurements and with the Seahorse XF24. D.J.P. is the member of the advisory board of Temple Therapeutics LLC. The other authors declared no conflict of interest.
Supplementary Material
Setup of the co-culture experiments.
rSTC1 promotes survival in other lung epithelial cell lines after 5 days.
Knockdown of STC1 in MSCs by siRNA.
Other lung epithelial cells also reduce ROS in response to rSTC1.
rSTC1 does not affect the activity of other canonical antioxidant enzymes.
rSTC1 reduces MMP in A549 cells grown in hypoxia or acidosis.
rSTC1 reduced MMP in other lung epithelial cell lines.
REFERENCES
- da Silva Meirelles L, Chagastelles PC., and, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119 Pt 11:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Bergfeld SA., and, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev. 2010;29:249–261. doi: 10.1007/s10555-010-9222-7. [DOI] [PubMed] [Google Scholar]
- Klopp AH, Gupta A, Spaeth E, Andreeff M., and, Marini F 3rd. Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth. Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R.et al. (2009Multipotent stromal cells (MSCs) are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1 (STC-1) Stem Cells 27670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GJ, DiMattia GD., and, Prockop DJ. Stanniocalcin-1 regulates extracellular ATP-induced calcium waves in human epithelial cancer cells by stimulating ATP release from bystander cells. PLoS ONE. 2010;5:e10237. doi: 10.1371/journal.pone.0010237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard JP, McCudden CR, Tanega C, James KA, Ratkovic S, Staples JF.et al. (2007The respiratory effects of stanniocalcin-1 (STC-1) on intact mitochondria and cells: STC-1 uncouples oxidative phosphorylation and its actions are modulated by nucleotide triphosphates Mol Cell Endocrinol 26490–101. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang L, Abdelrahim M, Cai Q, Truong A, Bick R.et al. (2009Stanniocalcin-1 suppresses superoxide generation in macrophages through induction of mitochondrial UCP2 J Leukoc Biol 86981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberg JA, Serlachius M, Lankila P, Penkowa M, Hidalgo J., and, Andersson LC. Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke. 2007;38:1025–1030. doi: 10.1161/01.STR.0000258113.67252.fa. [DOI] [PubMed] [Google Scholar]
- Zhang K, Lindsberg PJ, Tatlisumak T, Kaste M, Olsen HS., and, Andersson LC. Stanniocalcin: A molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci USA. 2000;97:3637–3642. doi: 10.1073/pnas.070045897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann C, Zeiher AM., and, Dimmeler S. Shear stress inhibits H2O2-induced apoptosis of human endothelial cells by modulation of the glutathione redox cycle and nitric oxide synthase. Arterioscler Thromb Vasc Biol. 1997;17:3588–3592. doi: 10.1161/01.atv.17.12.3588. [DOI] [PubMed] [Google Scholar]
- Hirai H, Kubo H, Yamaya M, Nakayama K, Numasaki M, Kobayashi S.et al. (2003Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines Blood 1021619–1621. [DOI] [PubMed] [Google Scholar]
- Selimovic D, Hassan M, Haikel Y., and, Hengge UR. Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell Signal. 2008;20:311–322. doi: 10.1016/j.cellsig.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Erkan M, Reiser-Erkan C, Michalski CW., and, Kleeff J. Tumor microenvironment and progression of pancreatic cancer. Exp Oncol. 2010;32:128–131. [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW.et al. (2007Mesenchymal stem cells within tumour stroma promote breast cancer metastasis Nature 449557–563. [DOI] [PubMed] [Google Scholar]
- Langley RR., and, Fidler IJ. The seed and soil hypothesis revisited-The role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley RG, Weber W, Rouleau C, Yao M, Honma N, Kataoka S.et al. (2009Human mesenchymal stem cells from bone marrow express tumor endothelial and stromal markers Int J Oncol 34619–627. [DOI] [PubMed] [Google Scholar]
- Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R., and, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2011;17:514. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B.et al. (2009Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression PLoS ONE 4e4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudio I, Fiegl M., and, Andreeff M. Mitochondrial uncoupling and the Warburg effect: molecular basis for the reprogramming of cancer cell metabolism. Cancer Res. 2009;69:2163–2166. doi: 10.1158/0008-5472.CAN-08-3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudio I, Fiegl M, McQueen T, Clise-Dwyer K., and, Andreeff M. The warburg effect in leukemia-stroma cocultures is mediated by mitochondrial uncoupling associated with uncoupling protein 2 activation. Cancer Res. 2008;68:5198–5205. doi: 10.1158/0008-5472.CAN-08-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh-Hamad D. Mammalian stanniocalcin-1 activates mitochondrial antioxidant pathways: new paradigms for regulation of macrophages and endothelium. Am J Physiol Renal Physiol. 2010;298:F248–F254. doi: 10.1152/ajprenal.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya A, Das I, Chandhok D., and, Saha T. Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid Med Cell Longev. 2010;3:23–34. doi: 10.4161/oxim.3.1.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augsten M, Hägglöf C, Peña C., and, Ostman A. A digest on the role of the tumor microenvironment in gastrointestinal cancers. Cancer Microenviron. 2010;3:167–176. doi: 10.1007/s12307-010-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrick DA, Neilson A., and, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Setup of the co-culture experiments.
rSTC1 promotes survival in other lung epithelial cell lines after 5 days.
Knockdown of STC1 in MSCs by siRNA.
Other lung epithelial cells also reduce ROS in response to rSTC1.
rSTC1 does not affect the activity of other canonical antioxidant enzymes.
rSTC1 reduces MMP in A549 cells grown in hypoxia or acidosis.
rSTC1 reduced MMP in other lung epithelial cell lines.


