Abstract
Tumor necrosis factor (TNFα) is a proinflammatory cytokine involved in the pathogenesis of inflammatory bowel disease (IBD). Although TNFα has been extensively targeted using systemic drugs, the use of antisense and small interfering RNA (siRNA) to drive down its expression at the site of inflammation should provide important advantages. In this study, native and chemically modified siRNA against TNFα was developed and characterized using a murine model of IBD. siRNA with 2′-O-methyl and propanediol modifications (siTNF-OMe-P) were resistant to nuclease degradation and provided better silencing efficacy in vitro as compared to unmodified siRNA. Every modification reduced nonspecific Toll-like receptor (TLR)-mediated immunomodulation in human peripheral blood mononuclear cells (PBMC) cells. Intrarectal administration of siTNF-OMe-P significantly ameliorated the clinical endpoints and histopathological severity in 5% dextran sulphate sodium (DSS)-treated mice as compared to unmodified and other chemically modified siRNAs. Differential gene expression assessed in siTNF-OMe-P-treated animals correlated with improved colon integrity and reduced TLR activation as compared to all treatment groups. All in all, this study demonstrates that propanediol and 2′-O-methyl modifications have profound functional consequences for siRNA efficacy in vivo. Consequently, this strategy has potential implications for therapeutic intervention in IBD and other diseases.
Introduction
The etiology and pathophysiological mechanisms of inflammatory bowel disease (IBD) are not completely understood. However, the loss of tolerance to intestinal microbiota, the activation of immune cells and the production of proinflammatory cytokines such as TNFα, interleukin-1β, interleukin-6, and interleukin-8 are common features of IBD. It is widely accepted that TNFα plays a prominent role in this process by contributing to the recruitment of immunocompetent cells that amplify the inflammatory response in T cells, macrophages, and mucosal cells.1,2,3,4,5 Indeed, TNFα levels are increased in both serum and mucosa of IBD patients.6,7,8,9 Furthermore, numerous studies have also shown significantly increased TNFα levels in animal models of IBD (2,4,6-trinitrobenzenesulphonic acid and dextran sulphate sodium (DSS)-induced colitis).10,11 TNFα also has deleterious effects on tight junctions, impairing barrier function, and enhancing the immune challenge by luminal antigens.12,13 Mucosal cells may thus be considered putative targets of anti-inflammatory therapy in the early phases of intestinal inflammation.14
Blockade of TNFα has been shown to improve or prevent inflammation in both animal models11,15,16 and humans.17 Anti-TNFα antibodies, such as infliximab, and adalimumab, have proven to be efficacious against IBD in clinical trials. However, anti-TNFα therapies require parenteral administration at relatively high doses to achieve their therapeutic effect at specific diseased sites, thereby increasing the chance of adverse effects such as increased vulnerability of patients to intracellular pathogens,18 lupoid reactions3 or the generation of anti-infliximab antibodies.19 A possible association of infliximab treatment with the development of lymphoma20 has also been suggested. Therefore, strategies providing an organ-selective blockade or inhibition of TNFα effects should improve the safety of these biological agents.
Among alternative strategies to block TNFα locally, the use of antisense oligonucleotides and small interfering RNA (siRNA) has received some attention,15,21 whereas antisense strategies addressing other molecular targets of inflammation are already undergoing clinical trials.22,23 Although siRNA is considerably more interesting than antisense as a therapeutic approach, the inherent instability of the RNA moiety has hindered its clinical development.24
The present study describes the efficacy of chemically modified siRNA in modulating IBD symptoms mediated by TNFα after rectal administration of a siRNA–lipoplex complex. Propanediol and 2′-O-methyl-modified siRNA were stable and active in vitro and reduced nonspecific siRNA-mediated Toll-like receptor (TLR) immunomodulation in human peripheral blood mononuclear cells (PBMC) and the colon of diseased mice. These data have immediate implications for therapeutic intervention in IBD alone or in combination with novel oral delivery strategies.25
Results
Negative modulation of TNFα gene expression by siRNA in primary cells
Several siRNA sequences have been extensively used to downregulate TNFα expression. We used one such siRNA26 to verify its efficiency in vitro against endogenous TNFα production by 4T1 mouse breast carcinoma cells. Our data show a substantial inhibition of TNFα mRNA and protein (data not shown) using unmodified siRNA, although this was unable to significantly reduce TNFα when transfected into mouse primary peritoneal macrophages (Figure 1a). Therefore, we studied the capacity of several chemically modified siRNAs (Table 1) to effectively silence TNFα in peritoneal macrophages. Specifically we have examined the biological properties of several modified siRNAs bearing 2′-O-methyl-RNA, locked nucleic acids (LNA), phosphorothioate (PS) linkages and propanediol modification at the 3′-end.27 All these modifications have been introduced only in the sense or passenger strand. Figure 1 shows that the propanediol modification of the 3′-end and a double methylation of the 5′-end of the sense strand of siTNF was significantly more effective at silencing endogenous TNFα than were either unmodified siTNF or any other chemical modifications tested: LNA, PS, OMe alone, or propanediol alone. Differential siRNA efficacy inside the cell is a balance between persistence and its ability to engage RNA-induced silencing complex. In order to study whether our most effective siRNA (siTNF-OMe-P) was resistant to degradation, we subjected all the siRNAs to a degradation assay in the presence of 50% fetal bovine serum (Figure 1b). All modifications improved upon the stability of unmodified siRNA against nuclease degradation as visualized by ethidium bromide staining. However, siTNF-OMe-P was significantly more resistant over time (at 24 hours, 52% of siRNA remained intact by densitometry analysis; data not shown) than all other modifications.
Figure 1.
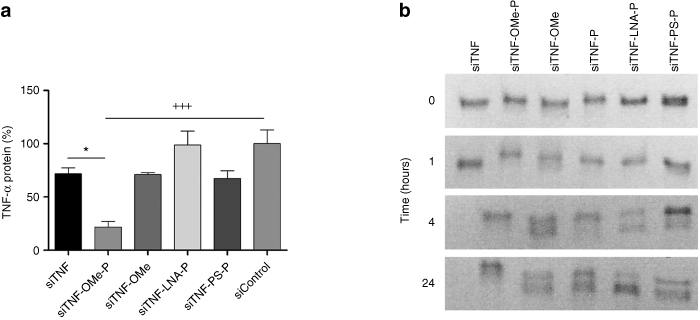
Antitumor necrosis factor (TNFα) silencing efficiency in mouse peritoneal macrophages correlates with small interfering RNA (siRNA) stability. (a) Mouse peritoneal macrophages were transfected with siRNA (100 nmol/l) using DOTAP. After 20 hours of transfection, cells were stimulated with lipopolysaccharide (LPS) (10 ng/ml) for 10 hours and protein levels were determined by enzyme-linked immunoabsorbent assay (ELISA) in cell supernatants. siTNF, unmodified siRNA against TNF-α; siTNF-PS-P, siRNA anti-TNF-α modified with phosphorothioate and propanediol; siTNF-LNA-P, siRNA anti-TNF-α modified with LNA and propanediol; siTNF-OMe, siRNA anti-TNF-α modified with O-methyl; siTNF-OMe-P, siRNA anti-TNFα modified with 2′-O-methyl and propanediol; siControl, negative control siRNA modified with 2′-O-methyl. The data represent the mean ± SEM, n = 3. +++P < 0.001 versus siControl; *P < 0.05 versus siTNF. ANOVA, Bonferroni post-hoc test. (b) siRNAs were incubated with 50% fetal bovine serum for the indicated amount of time (at 37 °C). Thereafter, RNAs were separated using a nondenaturing, 20% polyacrylamide gel.
Table 1. Oligonucleotide sequences of sense and antisense strands and their chemical modifications.
Therefore, we next evaluated the therapeutic efficacy of siTNF-OMe-P in different cell lines expressing endogenous and exogenous mouse TNFα and in vivo in an animal model of colitis induced by 5% DSS.
siTNF administration and therapeutic efficacy in a DSS colitis model
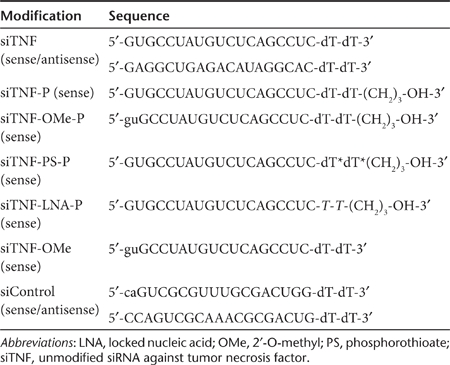
siTNF-OMe-P was found to be very efficacious in both HeLa and 4T1 cells to inhibit the production of mouse TNFα as compared to both unmodified siTNF or siControl (Figure 2a,b). In vivo, experimental rectal administration of siRNA conjugated to lipofectamine 2000 was initiated in 5% DSS-treated mice (56 hours). Target gene silencing was measured in total RNA from colon extracts 16 hours postadministration by quantitative reverse transcriptase -PCR. In the experimental conditions chosen, sham control animals (siControl) showed several-fold increase in TNFα mRNA with respect to healthy mice upon exposure to 5% DSS (Figure 2c). By contrast, TNFα mRNA levels in siTNF-OMe-P-treated animals were not significantly different than healthy mice group.
Figure 2.
In vitro and in vivo potency of chemically modified small interfering RNAs (siRNAs) against tumor necrosis factor (TNFα). (a) Amount of TNFα produced after 24 hours of transfection of 50 nmol/l of siRNAs in HeLa cells expressing mouse TNFα from an exogenous expression cassette. siTNF, unmodified siRNA against TNF-α; siTNF-OMe-P, siRNA anti-TNF-α modified with 2′-O-methyl and propanediol; siControl, negative control siRNA modified with 2′-O-methyl. The data represent the mean ± SEM, n = 3. +++P < 0.001 versus siControl; **P < 0.01 versus siTNF. ANOVA, Bonferroni post-hoc test. (b) Amount of TNFα produced after 24 hours of transfection of 100 nmol/l of siRNAs in 4T1 cells. The data represent the mean ± SEM, n = 3. ++P < 0.01 versus siControl; **P < 0.01 versus siTNF. ANOVA, Bonferroni post-hoc test. (c) TNFα mRNA levels in the colon of an animal model of chronic colitis 56 hours. after rectal infusion of 4 nmol of siTNF-OMe-P (n = 4) or siControl (n = 4) conjugated with lipofectamine 2000. TNFα mRNA was measured by quantitative reverse transcriptase (qRT)-PCR and normalized with Gapdh. The data represent the mean ± SEM with respect to healthy mice (n = 9). Statistical analysis was performed using a non-parametric ANOVA with Kruskal–Wallis post-test. **P < 0.01 versus healthy mice.
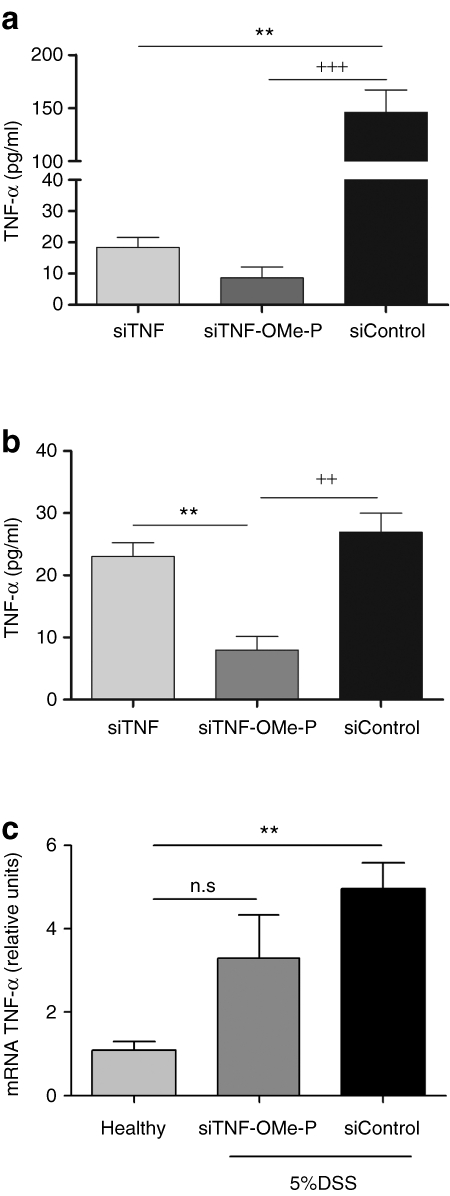
We next sought to ascertain whether, in view of their improved performance in vitro, chemically modified siRNAs had therapeutic potential as compared to unmodified siRNA. To this end, we treated a group of mice on days 2 and 4 using various siRNAs. Clinical endpoints of disease were evaluated 8 days after initiating the addition of 5% DSS. Animals on 5% DSS showed a weight loss over the 8-day period of analysis that was unaffected by the nature of the treatment (data not shown). However, siTNF-OMe-P treatment afforded a significantly ameliorated disease activity index (DAI; Figure 3a) and weight-to-length colon ratio (Figure 3b). Indeed, colons from mice treated with siTNF-OMe-P were less oedematous on necropsia, suggesting reduced inflammation confirmed by lower myeloperoxidase (MPO) activity (Figure 3c), which is a marker of neutrophil infiltration into the colon. Additionally, both the animal survival rate and the degree of hemorrhage in the caecum (Supplementary Figure S3) were clearly improved in animals treated with siTNF-OMe-P, demonstrating that the beneficial effects observed locally on colon inflammation correlated with significant amelioration of the physiological state of the animal. Therefore, siTNF-OMe-P treatment produces significant improvements in qualitative and quantitative clinical outcomes of colitis in treated animals, as compared to siTNF (unmodified siRNA against TNF-α) and siControl-treated animals. Clinical improvement was mirrored at the histological level (Figure 3d,e). Both inflammatory infiltrates and crypt damage scores were ameliorated with siTNF-OMe-P treatment. Mice exposed to DSS in their drinking water exhibited crypt pathology, including crypt shortening, erosion of the epithelial layer, hyperplasia, and an inflammatory cell infiltrate in the lamina propria. These signs of disease only decreased in the colon of mice treated with siTNF-OMe-P.
Figure 3.
Clinical parameters of colitis in the animals treated with small interfering RNA (siRNA) antitumor necrosis factor (TNFα). Fifty C57BL/6 mice were administered 5% dextran sulphate sodium (DSS) in drinking water and subsequently treated on days 2 and 4 with 4 nmol of various siRNAs. Some animals died before the end of the study and were not included in the analysis. Animals that survived up to 8 days (healthy controls, n = 6; siControl, n = 7; siTNF, n = 3; siTNF-OMe, n = 5; and siTNF-OMe-P = 8), were killed to sample the distal colon and perform a histopathological evaluation. (a) disease activity index (DAI) was evaluated from unbiased determination of rectal bleeding, feces consistency, and overall appearance. (b). Weight-over-length ratio of the colon. (c) Myeloperoxidase activity in colon extracts. (d) H&E stained specimens (×200). (e) Histological score was assessed by the Dieleman method. Error bars represent the SEM of the mean. Statistical analysis was performed by ANOVA with Bonferroni post-hoc test. ***P < 0.001, **P < 0.01 or *P < 0.05 versus healthy mice, and +++P < 0.001, +P < 0.05 versus siControl.
Gene array analysis in IBD mice treated with various siRNAs anti-TNFα
To gain insight into the molecular mechanisms underlying the therapeutic benefit provided by siTNF-OMe-P in this murine model of colitis, we analyzed the differential gene expression in colitic mice treated with various modified or unmodified TNFα specific siRNAs and siControl (eight mice/treatment were analyzed without pooling) and compared them to healthy animals, using a Mouse Gene ST 1.0 microarray (Affymetrix, Santa Clara, CA) consisting of 25,000 genes. This study identified circa 4,000 genes that were modulated significantly by the 5% DSS treatment. These genes were tagged as “pathology-related” genes. “siRNA-specific” genes were identified for each treatment group as those that were differentially expressed in each treatment group.
A heatmap was built using all “pathology-related” genes that allowed for a statistical similarity test per group (Figure 4a). This study showed that siTNF (unmodified siRNA against TNFα) and siControl are related in terms of their gene modulation pattern, whereas siTNF-OMe-P is closer to siTNF-OMe. Pattern-similarity assignment correlated well with the therapeutic potential observed through objective clinical parameters (Figure 3).
Figure 4.
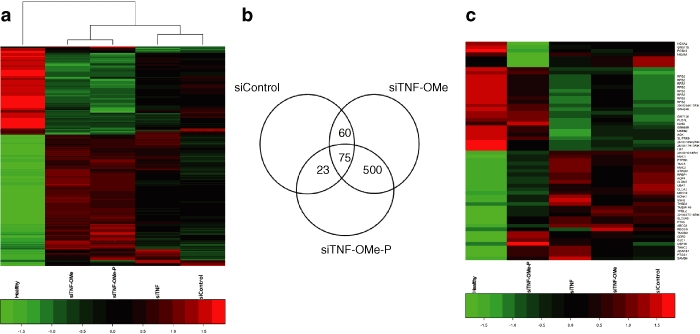
Differential gene expression in a 5% dextran sulphate sodium (DSS) murine colitis model after specific small interfering RNA (siRNA) treatments. (a) Heat map of differentially expressed genes after treatment with various siRNAs. Treatments are grouped by similarity based on their effects on gene expression. Horizontal strips represent genes and columns show treatment protocols. The absolute-fold changes of gene ratio are color coded as shown in the bar below. (b) Venn diagram of significantly upregulated or downregulated genes upon treatment. Intersections indicate the number of differentially expressed genes in response to specific treatments. (c) Clustering analysis of genes significantly altered by treatment with siTNF-OMe-P (total 60 genes). This study was conducted at the Institute of Biomedical Research of Barcelona, located in the Barcelona Science Park (PCB).
Differentially expressed genes in a given treatment subgroup were analyzed according to the Venn diagram shown in Figure 4b. Note that most of the genes that were significantly altered (500) exhibited similar regulation in siTNF-OMe and siTNF-OMe-P and were differently regulated in siControl and siTNF. Only 60 genes were exclusively affected by siTNF-OMe-P treatment (Supplementary Table S1). A heatmap of these genes showed strong pattern similarity with healthy controls for most genes (Figure 4c). Among them, several genes implicated in tissue repair, such as cldn-7 (claudin 7) and ssh-2, were upregulated in the siTNF-OMe-P group as compared to controls, whereas other genes present in a healthy colon epithelium (mucin1 and 2 and aqp4) were found to closely match the levels found in healthy controls. When siTNF-OMe-P treatment group was stringently compared pair wise against siControl or siTNF (Supplementary Table S1), several keratin genes, including keratin 4, were found to be upregulated (more than twofold induction and probability of differentially expressed higher than 0.95). Crabp2, upregulated by agents that induce keratinocyte differentiation such as retinoic acid was similarly upregulated, together with Ga733 (Tacstd2; murine epithelial glycoprotein), a protein involved in cell-adhesion, all in all suggesting that siTNF-OMe-P treatment helps re-establish a healthy colon epithelium.
Pathways differentially enriched in colons treated with siTNF-OMe-P
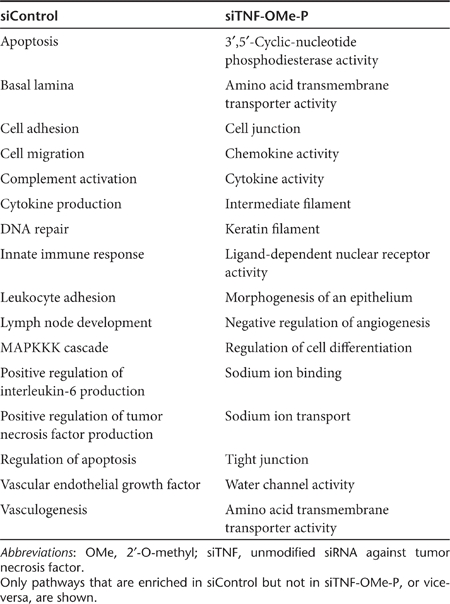
In order to gain insight into the molecular mechanism mediating the therapeutic effects evidenced for siTNF-OMe-P, we performed a gene ontology enrichment analysis comparing siTNF-OMe-P and siControl (Table 2). Several pathology-related pathways (apoptosis, innate immune response, cytokine production, leukocyte adhesion, lymph node development, etc.) were significantly enriched in siControl treatment but not in siTNF-OMe-P. By contrast, several pathways related to tissue repair and regeneration (cell junction, tight junction, morphogenesis of epithelium, regulation of cell differentiation, etc.), and to the normal physiology of the colon epithelium (keratin filaments, water channel activity, sodium transport, amino acid transport, etc.), were significantly enriched in siTNF-OMe-P but not in siControl.
Table 2. Gene ontology pathways enriched upon treatment with siControl or siTNF-OMe-PGO pathways enriched.
Chemical modifications result in reduced innate immune system activation
SiRNA and other RNA and DNA oligonucleotides stimulate innate immune response through the activation of TLR7/8 and 3.28 Gene ontology data indicated that innate immune response pathway was enriched in siControl but not siTNF-OMe-P. Therefore, we aimed to ascertain TLR activation capacity for all siRNAs, using a TLR7/8 response assay in PBMC. It is important to note that murine anti-TNFα siRNAs do not inhibit the expression of h-TNFα, a marker of TLR activation in this assay. As shown in Figure 5a, all chemically modified siRNAs exhibited reduced capacity to induce h-TNFα release by PBMC compared to unmodified siRNA (siTNF). We then examined TLR gene activation in vivo by comparing the differential expression of several TLR genes present in the gene array shown previously. TLR genes 2, 3, 4, 7, and 9 were significantly downregulated (two-way ANOVA) after siTNF-OMe-P treatment (Figure 5b) when compared to siTNF-treated mice, whereas siTNF-OMe was ineffective (data not shown).
Figure 5.
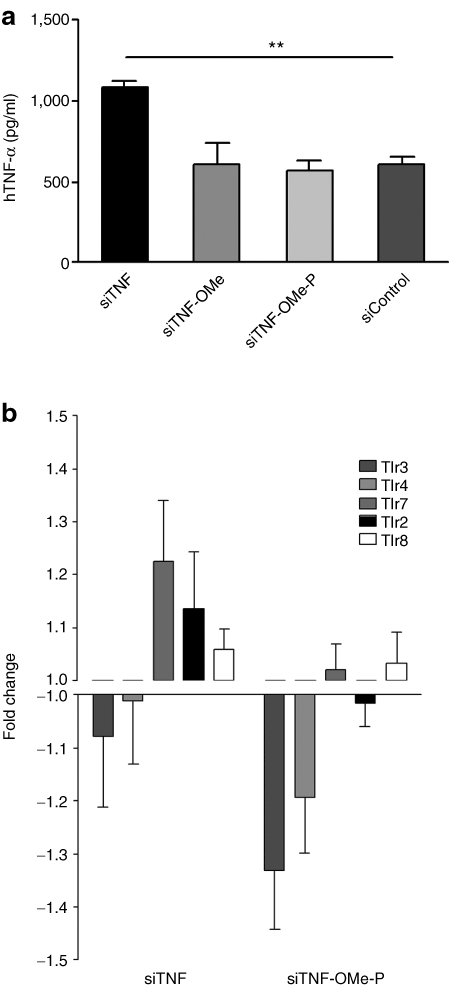
Modulation of Toll-like receptor (TLR)-response mediated by small interfering RNAs (siRNAs). Differential absolute gene expression for several TLR genes was identified using an Affymetrix Mouse Gene ST 1.0 microarray. (a) Peripheral blood mononuclear cells (PBMC) were treated with various siRNAs /DOTAP (10 nmol/l) for 18 hours. Stimulation of innate immune response by siRNAs was monitored by measuring the levels of h-tumor necrosis factor (TNFα) produced by PBMC. The data represent the mean ± SEM, n = 3; *P < 0.05 and **P < 0.01 versus siTNF. Statistical analysis was performed by ANOVA with Bonferroni post-hoc test. (b) Changes in gene expression induced by treatment with siTNF and siTNF-OMe-P. Statistical analysis was performed by two-way ANOVA. The data represent the mean ± SEM, n = 8.
Discussion
TNFα plays a major role in inflammatory responses and has therefore been identified as a major therapeutic target in different chronic inflammatory diseases, including IBD and arthritis. The importance of TNFα has also been demonstrated in a variety of animal models11,29 using wild-type and TNFα knockout mice, as well as in DSS-induced colitis.15 Consequently, neutralization of TNFα by antibodies, soluble receptors or antisense oligonucleotides has shown significant success in attenuating experimental colitis.3,15,18,19,20,21 Zhang et al.30 reported efficient silencing of TNFα mRNA after rectal delivery of unmodified siRNA in 5% DSS-treated mice, although no clinical improvement was observed.
In the present study we used the same TNFα-specific siRNA sequence as that described by Zhang et al.,30 but added several chemical modifications to the molecule. Specifically, chemical modifications (LNA, 2′-O-methyl (OMe), and PS) known to improve the stability and potency of siRNA were introduced into the same nucleotide sequence and were compared with propanediol modification at the 3′-end of the siRNA, which, according to previous results, exhibited an improved chemical stability in the context of antisense delivery.31 Several studies have shown improved serum stability of single-stranded and double-stranded nucleic acids by blocking 3′-exonuclease activity, the most important nuclease activity in serum, with modifications at the 3′-end.32 In this study, the silencing capacity of unmodified or chemically modified siTNFs was first tested in mouse peritoneal macrophages. This study showed that among the chemical modifications introduced into the siTNFs studied, propanediol modification at the 3′-end showed the greatest silencing capacity (Figure 1a). Similarly, siTNF-OMe-P was strikingly effective in the context of difficult to transfect cells such as 4T1 mammary carcinoma cells, or HeLa cells cotransfected with an expression plasmid for mTNFα (Figure 2a,b). The difference in efficacy could be partially accounted for by increased serum stability of siTNF-OMe-P (Figure 1b). This assay preferentially shows double-stranded RNA since staining is performed with ethidium bromide. All siRNAs, except for siTNF-OMe-P, showed the late appearance of a second, faster mobility band that could represent degradation products. Propanediol could stabilize siTNF-OMe-P molecule by presumably blocking 3′-exonuclease activity. However, it is not clear whether other biological variables including off-target effects, stimulation of cellular protective mechanisms, or RNA-induced silencing complex activation may be also justify successful siRNA-mediated silencing when utilizing OMe-P modification.28
We consistently found that when 5% DSS colitic mice were treated with the siRNAs described in this study, only siTNF-OMe-P was able to reduce TNFα mRNA and improve upon clinical endpoints such as the DAI, colon weight/length ratio, MPO activity, and histopathology, as compared to unmodified siTNF, siTNF-OMe, or siControl. Interestingly, unmodified siTNF worsened animal survival and caecal inflammation under visual inspection (Supplementary Figure S3b) as compared to siControl. These observations are not consistent with results published by Zhang et al.30 using the same protocol with unmodified siRNA against TNFα. Authors showed an important reduction of murine TNFα mRNA in the distal colon of treated mice, whereas in our study TNFα mRNA was not reduced as much. In several studies involving acute DSS colitis,33,34 complete neutralization by mAb against TNFα or its absence35,36 failed to block or even exacerbated disease parameters. This has prompted some authors.15 to suggest that disease protection in the acute form of DSS colitis may be dependent on the degree of TNFα inhibition attained. A small amount of TNFα may actually be protective in the early stages of disease development. Antisense siRNA (or low dose mAb treatment) may not totally block all TNFα production and, hence, might improve treatment efficacy in the acute model.15
The proinflammatory action of TNFα is critical for disease initiation, whereas its anti-inflammatory activity helps to resolve the disease. This dual role for TNFα has also been demonstrated in the pathogenesis of experimental autoimmune encephalomyelitis,37 thereby indicating that it may be beneficial to maintain certain levels of TNFα.
Given the lack of information regarding the mechanism of action by which siRNAs against TNFα might contribute to improving colitis, we decided to perform a whole-genome differential expression analysis with colitic mice treated with the siRNAs featured in our study. Our major interest was to gain insight into the genes involved in the therapeutic effects observed upon local siTNF-OMe-P delivery. Only 60 genes were exclusively modulated by siTNF-OMe-P treatment. Most of them were unchanged as compared to healthy controls, and hence were found in normal colon epithelium. More interesting information was obtained by analyzing the enrichment of gene ontology pathways in either the siTNF-OMe-P or siControl group versus healthy controls. Upregulated pathways present in siControl are also described in other studies of differential gene expression induced by DSS,1,38 and include cytokine production, innate immune response, lymph node development, and angiogenesis. None of those pathways were enriched by siTNF-OMe-P treatment. Instead, several pathways related to tissue repair, normal colon function and chemokine modulation were observed, suggesting that colon inflammation is inhibited and tissue repair is activated. Similar results have been found using antisense oligonucleotides targeting CD40 in a 2,4,6-trinitrobenzenesulphonic acid inflammatory model.39 Interestingly, antisense complexes were also introduced using a lipoplex enema, suggesting that directly access to the colon epithelia is an interesting strategy for the treatment of colitis.
The importance of TLR activation in assessing the performance of siRNA drugs has only been recognized in the last couple of years.28 TLR3 has been shown to mediate the protective effects of systemic poly (I:C) in DSS-induced acute colitis.40 Interestingly, unmodified siTNF direct stimulation of TLR3 in the colon was not protective in our system, probably because of the concomitant stimulation of several proinflammatory cytokines and TLRs by the unmodified siRNA. siTNF also stimulated the expression of TLR7/8, 2, 4, and 13, whereas siRNA chemically modified with OMe-P were effective in partially suppressing these genes. These data are in agreement with results obtained by transfecting PBMC, a good model to test TLR7/8 activation,41 with all siRNAs. Moreover, gene array data indicated that the double modification 2′OMe and propanediol conferred improved TLR activation with respect to 2′OMe alone. It has been shown that incorporation of 2′OMe modifications can dramatically increase nuclease resistance in serum-rich environments as compared to unmodified double-stranded RNA.42 Additionally, and depending on the position, as few as two 2′OMe modifications can prevent immune stimulation mediated through TLR-7 pathways.43 The mechanism by which 2′OMe modification reduces immune stimulation is not well established. It has been suggested that human TLRs might be preferentially activated by pathogen-associated RNA that contains fewer modified nucleosides than does host RNA.44 Addition of 2′OMe modifications to positions 1 and 2 of the passenger strand, as in the present study, inhibited the incorporation of this strand into the Ago2:RNA-induced silencing complex, thereby reducing off-target effects by blocking the interaction with Ago2.45 The synergy between 2′OMe and propanediol modifications with respect to TLR inhibition has not been previously reported. Regardless of the mechanism of action for propanediol, our results in vivo were consistent with data obtained in vitro, clearly showing that siTNF-OMe-P was the only treatment resulting in improved objective clinical endpoints in 5% DSS colitic mice. In addition, the propanediol and 2′-O-methyl modifications are easy and unexpensive to be incorporated in RNA, yet a large benefit can be obtained with the use of these modifications. For these reasons, we believe the findings described in this study are of potential interest in the development of therapeutic applications of siRNAs.
In conclusion, this study has demonstrated that siTNF-OMe-P treatment in murine DSS colitis resulted in the reduction of the DAI, the colon weight/length ratio, the extent of neutrophile infiltration and the microscopic evidence of inflammation, findings which are in agreement with its silencing ability demonstrated both in vitro and in vivo. Furthermore, we describe the successful application of chemically modified siRNA against TNFα in DSS-induced colitis and the differential gene expression associated with this treatment. These results will help to develop siRNA therapeutics for the treatment of colon inflammation.
Materials and Methods
Oligoribonucleotide synthesis. Oligoribonucleotides were prepared using solid phase methodology. The syntheses were carried out with an Applied Biosystems (Foster City, CA) (Model 3400) DNA synthesizer using a 1-µmol scale. The sense anti-TNFα sequences shown in Table 1 siTNF-P (sense): 5′-GUG CCU AUG UCU CAG CCU C-dT-dT-(CH2)3-OH-3′; siTNF-OMe-P (sense): 5′-guG CCU AUG UCU CAG CCU C-dT-dT-(CH2)3-OH-3′; siTNF-PS-P (sense): 5′-GUG CCU AUG UCU CAG CCU C-dT*dT*(CH2)3-OH-3′; siTNF-LNA-P (sense): 5′-GUG CCU AUG UCU CAG CCU C-T-T-(CH2)3-OH-3′) were assembled on a controlled pore glass support functionalized with dimethoxytrityl-propanediol46 in order to obtain oligoribonucleotides carrying propanediol at the 3′-end. dT stands for thymidine. Lower case bold letters (g, u) refer to 2′-O-methyl-RNA units. An asterisk (*) indicates the presence of PS linkages in the polymer backbone, while the T represents a thymidine where the sugar is modified with a LNA nucleoside. Detailed synthesis and quality control of oligonucleotides are provided as Supplementary Materials and Methods and Supplementary Figure S1. The sequences obtained from commercial sources were: sense nontargeting negative control (sense sequence 5′-caG UCG CGU UUG CGA CUG G-dT-dT-3′), antisense nontargeting negative control (antisense sequence 5′-CCA GUC GCA AAC GCG ACU G-dT-dT-3′), antisense or guide anti-TNFα: 5′-GAG GCU GAG ACA UAG GCA C-dT-dT-3′.
Preparation of siRNA molecules. Modified and unmodified sense strands were dissolved in Tris buffer (50 mmol/l NaCl, 10 mmol/l Tris, pH 8.0) and annealed with equimolar amounts of the corresponding unmodified antisense strand dissolved in the same buffer. The resulting solution (0.1 ml) was heated at 90 °C and allowed to cool slowly within 2 hours. Then 10 µl of 3 mol/l sodium acetate were added and the resulting siRNAs were precipitated by addition of 0.275 ml of ethanol. The sample is kept in the freeze overnight and centrifuged (15 minutes, 13,400g, 4 °C). The pellet is dissolved in the appropriate buffer and used in the silencing experiments.
The anti-TNFα siRNA has previously been shown to efficiently downregulate murine TNFα mRNA.26 To save resources, we only present data from O-methyl modified scrambled siRNA as siRNA control (siControl) because it is representative of results obtained with unmodified, O-methyl, and O-methyl-propanediol scrambled siRNA in vitro and in vivo (Supplementary Figure S2).
siRNA degradation assay and RNA gel electrophoresis. One microgram duplex of each siRNA was incubated with 50% fetal bovine serum at 37 °C. Samples were incubated with 1% SDS prior to be analyzed after 0 minute, 1 hour, 4 hours, and 24 hours of serum incubation, by nondenaturing polyacrylamide gel electrophoresis. Double-stranded RNA was visualized using an Ethidium Bromide bath for 30 minutes. in order to determine the percentage of intact siRNA.
Cell culture, transfection, and cellular assays. HeLa cells were cultured under standard conditions. HeLa cells were transfected with 250 ng of the plasmid expressing murine TNFα plasmid using lipofectin (Invitrogen, Carlsbad, CA), following the manufacturer's instructions. One hour after transfection, TNFα expressing HeLa cells were transfected with 50 nmol/l of each siRNA, using oligofectamine (Invitrogen). The TNFα concentration was determined from cell culture supernatant by enzyme-linked immunoabsorbent assay kit (BenderMedSystems, Vienna, Austria) following the manufacturer's instructions.
Murine 4T1 cells were cultured under standard conditions. One hundred nmol/l of each siRNA duplex was incubated with lipofectamine 2000 before being added to the 4T1 cells. After 24 hours, the amount of TNFα produced by the cells was analyzed by enzyme-linked immunosorbent assay.
For peritoneal macrophage collection, healthy mice were sacrificed and 10-ml harvest medium was injected into their peritoneum. Fluid was collected after 10 minutes. The amount of harvested cells was adjusted (104 cells/well) and transfected with 100 nmol/l of siRNA using DOTAP (Roche, Mannheim, Germany), following the manufacturer's instructions. After 20 hours of transfection, cells were stimulated with lipopolysaccharide (10 ng/ml) for 10 hours and the TNFα released in the supernatant was measured by enzyme-linked immunoabsorbent assay.
PBMC were obtained from human blood using Ficoll density gradient separation. After 3 hours incubation at 37 °C, adherent cells were transfected with 10 nmol/l of siRNAs for 18 hours using DOTAP (Roche), following the manufacturer's instructions. Culture supernatants were then collected and human TNFα production upon immunostimulation was assessed by enzyme-linked immunoabsorbent assay (Bender MedSystems).
DSS-induced colitis model in C57BL/6 mice. Experiments were performed on female C57BL/6 mice (Harlan Iberica, Barcelona, Spain). Animals were housed under standard conditions at 22 °C and 70–80% relative humidity, in a 12 hours light/dark cycle. All animal procedures conformed to EU regulations and were approved by the local ethical committee.
Colitis was induced by addition of 5% DSS (MP Biomedicals, Santa Ana, CA; PM 36–50 kDa) to drinking water. Nine healthy animals (no DSS in drinking water) were used as noncolitic controls.
Food and drink were provided ad libitum and intake was monitored daily throughout the study.
In vivo administration of siRNAs. To create lipoplex siRNA preparations, 2 µl lipofectamine 2000 (Invitrogen) was mixed with 48 µl OptiMEM and incubated for 5 minutes, according to the manufacturer's instructions. Twenty nanomolar siRNA was suspended in 50 µl OptiMEM were then added to this mixture and incubated at room temperature for 20 minutes. This solution was immediately administered to rectums of anesthetized mice (4 nmol/mouse). All siRNA preparations were administered twice, on days 2 and 4. Each rectal administration consisted of a 20 µl solution of liposomal siRNA and was delivering using a P20 pipettor.
Evaluation of the DAI. Throughout the experiment, animals were examined daily for the following variables: general state and appearance, weight loss, stool consistency, and food and drink intake. DAI scores were defined as follows: for weight: 0, no loss; 1, up to 5%; 2, 5%–10%; 3, 10%–15%; and 4, >15% weight loss; for stool: 0, normal; 1, soft stool; 2, semiliquid; 3, diarrhea but dry tail; and 4, diarrhea with wet tail; and for bleeding: 0, no blood; 1, presence in stool; 2, presence in anus; and 3, gross blood. The DAI parameter correlates well with the histopathological evaluation of inflammation and lesions in intestinal crypts.47
Colonic MPO activity. Neutrophil infiltration into tissue colon was quantified by measuring MPO activity as previously described48 for use in a 96-well plate. The change in optical density at 450 nm was measured at 3-minutes intervals.
One unit of MPO activity was defined as the amount that degraded 1.0 µmol of peroxide per minute at 25 °C. The results were expressed as U/mg wet tissue.
Histology and histological score. After flushing with cold phosphate-buffered saline, a whole-length longitudinal strip was fixed with paraformaldehyde 4%. Haematoxylin/eosin-stained sections were examined blindly and scored according to widely used criteria.49
Statistical analysis. All the data obtained were plotted and statistically analyzed using the software package GraphPad Prism version 5.0 for Windows. All treatment groups were compared using a one-way ANOVA and Bonferroni post-hoc test (*P < 0.05, **P < 0.01, and ***P < 0.001). Only significant differences among the groups are indicated in the charts.
Gene expression profiling. Sixteen hours after the second administration of siRNAs, the colon of treated and untreated animals was obtained for RNA isolation. Twenty-five nanogram total RNA was used for whole transcriptome amplification (Sigma, St Louis, MO) according to manufacturer's recommendations. Thirty milligram distal colon (0.8 cm from the rectum) from each of eight mice per group was used for RNA extraction using RNeasy minikit (Qiagen, Dusseldorf, Germany). Distal colon was quickly flash with phosphate-buffered saline before collection onto RNAlater before RNA extraction. Ten microgram complementary DNA were obtained after retrotranscription of mRNA, and subsequently fragmented, biotinylated, and hybridized (unpooled) to Mouse Gene ST 1.0 microarrays (Affymetrix), as described previously.50
Microarray data analysis. Significance analysis of microarrays and gene ontology functional classification were carried out as described in Supplementary Materials and Methods. All datasets from the array have been submitted to GEO (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE31906).
SUPPLEMENTARY MATERIAL Figure S1. Quality control of representative oligonucleotides and siRNAs. Figure S2. Silencing efficiency for various chemically modified siControl. Figure S3. Phenotypic changes in a DSS murine colitis model after specific siRNAs administration. Table S1. Differentially expressed genes. Materials and Methods.
Acknowledgments
We are grateful to Herbert Auer for performing Affymetrix array, and David Rosell and Evarist Planet for their guidance with Affymetrix array data analysis and statistics. Authors are in debt to the personnel at the “Scientific-Technical Services” of the Unitat de Bellvitge at the Universitat de Barcelona for their assistance. This study was supported by Ministerio de Ciencia e Innovación and FEDER (BFU2009-07506, BFU2007-63287, and CTQ2010-20541) and Marató TV3 (Grant No. 031633). The authors declared no conflict of interest.
Supplementary Material
Quality control of representative oligonucleotides and siRNAs.
Silencing efficiency for various chemically modified siControl.
Phenotypic changes in a DSS murine colitis model after specific siRNAs administration.
Differentially expressed genes.
REFERENCES
- Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E.et al. (1995A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion J Clin Invest 9555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D.et al. (1997A role for TNF-α and mucosal T helper-1 cytokines in the pathogenesis of Crohn's disease J Immunol 1596276–6282. [PubMed] [Google Scholar]
- Van Deventer SJ. Tumour necrosis factor and Crohn's disease. Gut. 1997;40:443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis KA., and, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- Prehn JL, Landers CJ., and, Targan SR. A soluble factor produced by lamina propria mononuclear cells is required for TNF-α enhancement of IFN-γ production by T cells. J Immunol. 1999;163:4277–4283. [PubMed] [Google Scholar]
- Funakoshi K, Sugimura K, Anezaki K, Bannai H, Ishizuka K., and, Asakura H. Spectrum of cytokine gene expression in intestinal mucosal lesions of Crohn's disease and ulcerative colitis. Digestion. 1998;59:73–78. doi: 10.1159/000007470. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Kobayashi D, Saito K, Furuya D, Yagihashi A, Araake H.et al. (2001Tumor necrosis factor-α in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR Clin Chem 471297–1301. [PubMed] [Google Scholar]
- Murch SH, Braegger CP, Walker-Smith JA., and, MacDonald TT. Location of tumour necrosis factor α by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woywodt A, Ludwig D, Neustock P, Kruse A, Schwarting K, Jantschek G.et al. (1999Mucosal cytokine expression, cellular markers and adhesion molecules in inflammatory bowel disease Eur J Gastroenterol Hepatol 11267–276. [DOI] [PubMed] [Google Scholar]
- Dieleman LA, Ridwan BU, Tennyson GS, Beagley KW, Bucy RP., and, Elson CO. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–1652. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Büschenfelde KH.et al. (1997Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice Eur J Immunol 271743–1750. [DOI] [PubMed] [Google Scholar]
- Gitter AH, Bendfeldt K, Schmitz H, Schulzke JD, Bentzel CJ., and, Fromm M. Epithelial barrier defects in HT-29/B6 colonic cell monolayers induced by tumor necrosis factor-α. Ann N Y Acad Sci. 2000;915:193–203. doi: 10.1111/j.1749-6632.2000.tb05242.x. [DOI] [PubMed] [Google Scholar]
- Marano CW, Lewis SA, Garulacan LA, Soler AP., and, Mullin JM. Tumor necrosis factor-α increases sodium and chloride conductance across the tight junction of CACO-2 BBE, a human intestinal epithelial cell line. J Membr Biol. 1998;161:263–274. doi: 10.1007/s002329900333. [DOI] [PubMed] [Google Scholar]
- Böcker U. Cytokines and cell homeostasis in the gastrointestinal tract. Lancaster. 2000.
- Myers KJ, Murthy S, Flanigan A, Witchell DR, Butler M, Murray S.et al. (2003Antisense oligonucleotide blockade of tumor necrosis factor-α in two murine models of colitis J Pharmacol Exp Ther 304411–424. [DOI] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB., and, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Bidlingmaier C, Siegmund B, Albrich S, Schulze J, Tschoep K.et al. (2000Specific type IV phosphodiesterase inhibitor rolipram mitigates experimental colitis in mice J Pharmacol Exp Ther 29222–30. [PubMed] [Google Scholar]
- Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD.et al. (2001Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent N Engl J Med 3451098–1104. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ., and, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5:119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- Brown SL, Greene MH, Gershon SK, Edwards ET., and, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151–3158. doi: 10.1002/art.10679. [DOI] [PubMed] [Google Scholar]
- Zuo L, Huang Z, Dong L, Xu L, Zhu Y, Zeng K.et al. (2010Targeting delivery of anti-TNFα oligonucleotide into activated colonic macrophages protects against experimental colitis Gut 59470–479. [DOI] [PubMed] [Google Scholar]
- Sidiropoulos P, Liu H, Mungre S, Anderson L, Thimmapaya B., and, Pope RM. Efficacy of adenoviral TNF α antisense is enhanced by a macrophage specific promoter. Gene Ther. 2001;8:223–231. doi: 10.1038/sj.gt.3301368. [DOI] [PubMed] [Google Scholar]
- Yacyshyn B, Bowen-Yacyshyn MB., and, Shanahan W. The clinical experience of antisense therapy to ICAM-1 in Crohn's disease. Curr Opin Mol Ther. 1999;1:332–335. [PubMed] [Google Scholar]
- Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA., and, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10:766–771. doi: 10.1261/rna.5239604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel C., and, Amiji M. Oral TNF-a gene silencing using a polymeric microsphere-based delivery system for the treatment of inflammatory bowel disease. J Control Release. 2011;150:77–86. doi: 10.1016/j.jconrel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen DR, Leirdal M., and, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- Aerschot AS-BT, Rozenski J, Hendrix C, Schepers D, Verhoeven G., and, Herdewijn P. Conjugation of oligonucleotides to 3'-polar moieties. Bull Soc Chim Belg. 1995;104:717–720. [Google Scholar]
- Jackson AL., and, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- Corazza N, Brunner T, Buri C, Rihs S, Imboden MA, Seibold I.et al. (2004Transmembrane tumor necrosis factor is a potent inducer of colitis even in the absence of its secreted form Gastroenterology 127816–825. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cristofaro P, Silbermann R, Pusch O, Boden D, Konkin T.et al. (2006Engineering mucosal RNA interference in vivo Mol Ther 14336–342. [DOI] [PubMed] [Google Scholar]
- Herdewijn P, Saison-Behmoaras E, Van Aerschot A, Leserman L, Eritja R., and, Pfleiderer W. Antisense oligonucleotides as anticancer agents. Biomedical and Health Research. 1998;24:182–189. [Google Scholar]
- Eder PS, DeVine RJ, Dagle JM., and, Walder JA. Substrate specificity and kinetics of degradation of antisense oligonucleotides by a 3' exonuclease in plasma. Antisense Res Dev. 1991;1:141–151. doi: 10.1089/ard.1991.1.141. [DOI] [PubMed] [Google Scholar]
- Olson AD, DelBuono EA, Bitar KN., and, Remick DG. Antiserum to tumor necrosis factor and failure to prevent murine colitis. J Pediatr Gastroenterol Nutr. 1995;21:410–418. doi: 10.1097/00005176-199511000-00007. [DOI] [PubMed] [Google Scholar]
- Kojouharoff G, Hans W, Obermeier F, Männel DN, Andus T, Schölmerich J.et al. (1997Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice Clin Exp Immunol 107353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noti M, Corazza N, Mueller C, Berger B., and, Brunner T. TNF suppresses acute intestinal inflammation by inducing local glucocorticoid synthesis. J Exp Med. 2010;207:1057–1066. doi: 10.1084/jem.20090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Takagi T, Handa O, Ishikawa T, Nakagawa S, Yamaguchi T.et al. (2003Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-α deficient mice J Gastroenterol Hepatol 18560–569. [DOI] [PubMed] [Google Scholar]
- Kassiotis G., and, Kollias G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med. 2001;193:427–434. doi: 10.1084/jem.193.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Miyamoto S, Yasui Y, Sugie S., and, Tanaka T. Global gene expression analysis of the mouse colonic mucosa treated with azoxymethane and dextran sodium sulfate. BMC Cancer. 2007;7:84. doi: 10.1186/1471-2407-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Wagner AH, Fankhaenel S, Stojanovic T, Schweyer S, Panzner S.et al. (2005CD40 antisense oligonucleotide inhibition of trinitrobenzene sulphonic acid induced rat colitis Gut 5470–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M, Wu H, Aitken J, Kolachala VL, Neish AS, Sitaraman SV.et al. (2007Activation of toll-like receptor 3 protects against DSS-induced acute colitis Inflamm Bowel Dis 13856–864. [DOI] [PubMed] [Google Scholar]
- Cekaite L, Furset G, Hovig E., and, Sioud M. Gene expression analysis in blood cells in response to unmodified and 2'-modified siRNAs reveals TLR-dependent and independent effects. J Mol Biol. 2007;365:90–108. doi: 10.1016/j.jmb.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Choung S, Kim YJ, Kim S, Park HO., and, Choi YC. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem Biophys Res Commun. 2006;342:919–927. doi: 10.1016/j.bbrc.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Robbins M, Judge A, Liang L, McClintock K, Yaworski E., and, MacLachlan I. 2'-O-methyl-modified RNAs act as TLR7 antagonists. Mol Ther. 2007;15:1663–1669. doi: 10.1038/sj.mt.6300240. [DOI] [PubMed] [Google Scholar]
- Karikó K, Buckstein M, Ni H., and, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Salomon W, Bulock K, Lapierre J, Pavco P, Woolf T., and, Kamens J. Modified dsRNAs that are not processed by Dicer maintain potency and are incorporated into the RISC. Nucleic Acids Res. 2010;38:3771–3779. doi: 10.1093/nar/gkq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviñó A, Güimil Garcia R, Albericio F, Mann M, Wilm M, Neubauer G.et al. (1996New carbamate supports for the preparation of 3'-amino-modified oligonucleotides Bioorg Med Chem 41649–1658. [DOI] [PubMed] [Google Scholar]
- Cooper HS, Murthy SN, Shah RS., and, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- Krawisz JE, Sharon P., and, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG.et al. (1998Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines Clin Exp Immunol 114385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer H, Newsom DL, Nowak NJ, McHugh KM, Singh S, Yu CY.et al. (2007Gene-resolution analysis of DNA copy number variation using oligonucleotide expression microarrays BMC Genomics 8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality control of representative oligonucleotides and siRNAs.
Silencing efficiency for various chemically modified siControl.
Phenotypic changes in a DSS murine colitis model after specific siRNAs administration.
Differentially expressed genes.


