Abstract
Short interfering RNA (siRNA) is a potent activator of the mammalian innate immune system. When considering possible clinical applications of siRNA for humans, the adverse immunostimulatory effects must also be taken into account. Here, we show that atelocollagen-mediated systemic delivery of siRNA without chemical modifications did not cause any immunostimulation in both animals and human peripheral blood mononuclear cells (PBMCs), even if the siRNA harbored an interferon (IFN)-inducible sequence. In contrast, systemic delivery of immunostimulatory RNA (isRNA)-mediated by a cationic lipid (such as Invivofectamine) induced potent type-I IFNs and inflammatory cytokines. Regarding the mechanism by which the isRNA/atelocollagen complex avoided adverse effects on immunostimulation, we revealed that this complex was not incorporated into PBMCs. On the other hand, Invivofectamine delivered isRNA into PBMCs. The use of either atelocollagen or Invivofectamine as a vehicle elicited significant and undistinguishable therapeutic effects in a contact hypersensitivity (CHS) inflammatory model mouse, when we intravenously injected the siRNA targeting monocyte chemoattractant protein-1 as the complex. For the goal of realizing siRNA-based medicines for humans, atelocollagen is an excellent and promising delivery vehicle, and it has the useful advantage of evading detection by the “radar” of innate immunity.
Introduction
Short interfering RNAs (siRNAs) that mediate specific gene silencing through RNA interference (RNAi)1,2 are widely used to study gene function and are also being developed for therapeutic applications to human diseases.3 However, recent work demonstrating that there are unanticipated adverse effects associated with the use of siRNAs in mammals has raised concerns about the safe use of RNAi in humans.4,5,6 These undesirable effects principally consist of activation of the immune system, potentially harming the individual. Thus, synthetic siRNA duplexes can induce comparatively high levels of inflammatory cytokines and type-I interferons (IFNs) with sequence dependency, after systemic administration in mammals and primary human blood cell cultures.4,5,6,7 These responses are greatly potentiated by the use of delivery vehicles that facilitate cellular uptake of the siRNA. In almost all of the cases, such immune activation usually represents a significant undesirable adverse effect due to the toxicities associated with excessive cytokine release and associated inflammatory syndromes,3,7 despite the fact that the immunostimulatory effects of siRNAs may be harnessed therapeutically in oncological applications.8,9,10 Further, immunostimulation in animals often causes misinterpretation of the therapeutic effects of siRNAs.11 There is thus urgent need of a systemic delivery method of siRNAs to separate the original RNAi effect, which leads to the true therapeutic outcome, from the immunostimulation effect. A reliable method of this type is absolutely necessary to realize siRNA-based medicines for clinical study in humans. At the present time, the plenary method for preventing the immunostimulatory effect of siRNAs is to apply chemical modifications on the molecule at the 2′-hydroxy group.12,13 In this context, we aim to show whether delivery vehicle-mediated regulation of such immunostimulation in vivo can be achieved without any chemical modifications of the siRNA molecule itself.
We previously showed the efficacy of a biomaterial vehicle, atelocollagen, for systemically delivering siRNAs in vivo.14,15 Intravenously administered Bcl-xL (B-cell lymphoma-extra large) siRNA/atelocollagen complex successfully accumulated in a PC-3 (a human prostate cancer cell line) xenograft of nude mice, and exhibited a therapeutic antitumoral effect against the xenograft.14 We also successfully showed a therapeutic effect in a model of contact hypersensitivity (CHS), a type of delayed-allergy inflammatory disease, via systemic atelocollagen-mediated administration of an siRNA targeting monocyte chemoattractant protein-1 (MCP-1).15 Atelocollagen, which is prepared from the bovine dermis,16,17 contributes to increases in cellular uptake, nuclease resistance, and the prolonged release of siRNA molecules in various disease models in vivo.18,19,20,21 Thus, numerous previous studies by our group14,15,17,18,20,21 as well as those by other groups22,23,24,25,26,27 have already shown the clear therapeutic efficacy of atelocollagen-mediated in vivo delivery of siRNAs.
In our preliminary study, a cationic lipid-mediated delivery of immunostimulatory RNA (isRNA) induced a strong type-I IFN-response, whereas isRNA mixed with atelocollagen did not. isRNA is a double-stranded short RNA, which carries an IFN-inducible sequence (ref. 6 and Figure 1a). To elucidate the mechanism(s) of the effects of isRNA in greater detail, we intravenously injected isRNA mixed with various delivery vehicles (atelocollagen, N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethyl ammonium methylsulfate (DOTAP; Roche Diagnostics, Indianapolis, IN)), In vivo JET-PEI, or Invivofectamine) into mice, and found that only the mixture of isRNA and atelocollagen avoided the induction of IFN in serum. In this article, we describe the mechanism by why isRNA complexed with atelocollagen escapes the IFN-responsible radar in animals.
Figure 1.

isRNA mixed with atelocollagen did not induce any type-I IFNs in mice. (a) Structures of isRNA harboring the interferon (IFN)-inducible sequence (5′-UGUGU-3′). (b) Balb/c mice were intravenously injected with isRNA alone, atelocollagen alone, isRNA with atelocollagen, DOTAP alone, isRNA with DOTAP, In vivo JET-PEI alone, or isRNA with In vivo JET-PEI, respectively. The dose of isRNA was fixed at 50 µg per mouse. The levels of both IFN-α and IFN-β in mouse serum (6 hours postinjection) were evaluated using each specific ELISA. The results represent the means ± SD (n = 4). N.D., not detected. (c) Different mouse strains were examined. C57BL/6J, Balb/c, and ICR were intravenously injected with isRNA mixed with either atelocollagen or DOTAP as in b. The levels of both IFN-α and IFN-β in mouse serum (6 hours postinjection) were evaluated. The results represent the means ± SD (n = 4). N.D., not detected. ELISA, enzyme-linked immunosorbent assay; isRNA, immunostimulatory RNA.
Results
isRNA/atelocollagen complex did not induce any type-I IFNs in mice
For the immunostimulatory experiments, we used an isRNA molecule (Figure 1a) with an IFN-inducible sequence (5′-UGUGU-3′) as described by Judge et al.6 No induction of IFN-α or IFN-β was observed following the intravenous injection of the isRNA/atelocollagen complex into Balb/c mice, whereas injection of isRNA complexed with DOTAP, or isRNA complexed with In vivo JET-PEI both potently induced these IFNs (Figure 1b). Atelocollagen vehicle alone also did not induce IFN-α or IFN-β, although DOTAP, or In vivo JET-PEI vehicle alone did induce small amounts of both IFNs. isRNA alone without any vehicle showed no IFN-induction. The isRNA/atelocollagen complex did not induce IFN-α in any of the mouse strains examined (Figure 1c), but the isRNA/DOTAP complex induced IFN-α in all three strains (C57BL/6J, Balb/c, and ICR mice). The IFN-response in ICR mice was quite pronounced, but varied among the individual animals. For further study, we chose Balb/c mice due to the low level of individual differences.
isRNA/Invivofectamine complex induced large amounts of type-I IFNs in mice
Invivofectamine (Invitrogen, Carlsbad, CA) is a kind of cationic liposome especially for in vivo use. The isRNA/Invivofectamine complex induced a surprisingly large amount of IFN-α in mice (Figure 2a). The serum level exceeded 15,000 pg/ml. The isRNA/atelocollagen complex consistently did not induce IFNs. The isRNA/Invivofectamine complex also induced tumor necrosis factor-α (TNF-α) (Figure 2b). These results suggested that Invivofectamine emphasized the action of isRNA, compared with DOTAP, or In vivo JET-PEI, and thus Invivofectamine is convenient for use as a positive control of immunoresponse in mice. Indeed, when we intravenously injected the isRNA/DOTAP complex in mice, the level of TNF-α induction, if any, was below the limit of detection (data not shown). The IFN-α induction was increased by isRNA in a dose-dependent manner, and showed a peak at 3–6 hours postinjection (Figure 2c,d). The induction completely disappeared at 24 hours. In contrast, the isRNA/atelocollagen complex caused no induction of IFN-α up to 24 hours.
Figure 2.
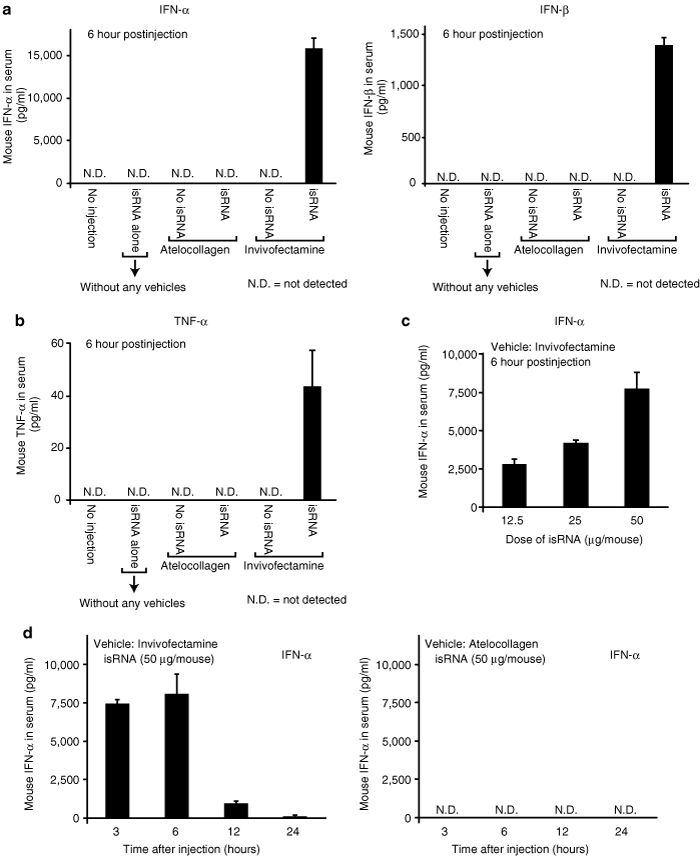
isRNA mixed with Invivofectamine but not with atelocollagen potently induced type-I IFNs and an inflammatory cytokine, TNF-α, in mice. (a,b) Balb/c mice were intravenously injected with various reagents as indicated in the figures. The dose of isRNA was fixed at 50 µg per mouse. The levels of (a) IFN-α, IFN-β and (b) TNF-α were evaluated at 6 hours following the injection using each specific ELISA. The results represent the means ± SD (n = 4). (c) The relationship between isRNA dose and IFN-response in mice. isRNA (12.5, 25, or 50 µg) mixed with Invivofectamine was intravenously injected into mice. First, the isRNA/Invivofectamine complex (50 µg) was prepared, and then it was diluted with 5% glucose. Each IFN-α level in mouse serum was evaluated at 6 hours following the injection as in a. The results represent the means ± SD (n = 4). (d) Time-course experiments. Each IFN-α level in mouse serum at 3, 6, 12, and 24 hours postinjection was determined by the specific ELISA. Two kinds of formulations (isRNA with Invivofectamine and isRNA with atelocollagen) were examined. The results represent the means ± SD (n = 4). N.D., not detected. ELISA, enzyme-linked immunosorbent assay; IFN, interferon; isRNA; immunostimulatory RNA; TNF, tumor necrosis factor.
The isRNA/Invivofectamine complex but not the isRNA/atelocollagen complex induced secretion of IFN-α in whole blood cultures in vitro
We hypothesized that there should be some responsible cell(s) to react with isRNA/Invivofectamine in the bloodstream, and to secrete IFNs. We successfully established an in vitro experimental system to evaluate this possibility (Figure 3a). We examined six kinds of test reagents to react with mouse whole blood or human whole blood. Only the isRNA/Invivofectamine complex induced secretion of IFN-α in whole blood from both mice and humans (Figure 3b,c), suggesting that some component cell(s) in whole blood were involved in IFN-induction. Thus, we prepared peripheral blood mononuclear cells (PBMCs) from human whole blood, and performed the next experiment.
Figure 3.
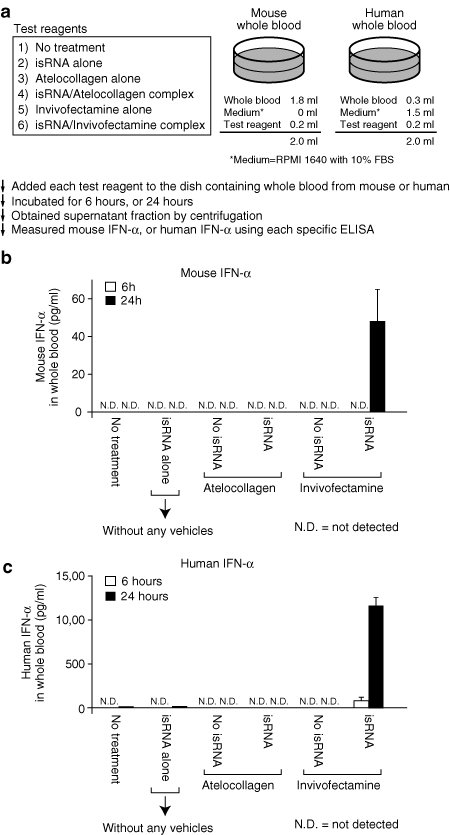
isRNA with Invivofectamine but not with atelocollagen induced secretion of IFN-α from whole blood. (a) The experimental procedures, including information on the mixing of test reagents. A dose of isRNA was fixed at 50 µg/35 mm dish. The information on the test reagents was provided in the box. Each test reagent was mixed with whole blood from a (b) mouse or a (c) human. (b,c) Each sample was collected at 6 or 24 hours after the mixing, and the serum fraction was separated by centrifugation. The level of IFN-α was determined by each specific ELISA. The results represent the means ± SD (n = 3). ELISA, enzyme-linked immunosorbent assay; IFN, interferon; isRNA; immunostimulatory RNA.
The isRNA/Invivofectamine complex but not the isRNA/atelocollagen complex induced IFN-α, and inflammatory cytokines in human PBMC cultures
Human PBMCs treated with the isRNA/Invivofectamine complex secreted IFN-α, TNF-α, IL-1β, IL-6, and IL-12 (Figure 4). In contrast, the isRNA/atelocollagen complex did not show any such inductions in PBMC cultures. As shown in Supplementary Figure S1a, both the isRNA/JET-PEI complex and the isRNA/DOTAP complex showed IFN-α induction in PBMCs. Further, JET-PEI alone, DOTAP alone, or Invivofectamine alone all induced a small amount of IFN-α, whereas atelocollagen alone did not (Figure 4a, Supplementary Figure S1a). JET-PEI-FluoF and Megafectin20 (red fluorescence-labeled DOTAP) showed some interaction with PBMCs via confocal microscope analysis, suggesting direct immunostimulation by the vehicles alone. On the other hand, fluorescein isothiocyanate -labeled atelocollagen alone did not show any interaction with PBMCs (Supplementary Figure S1c). These results are consistent with the animal experiment shown in Figure 1b.
Figure 4.
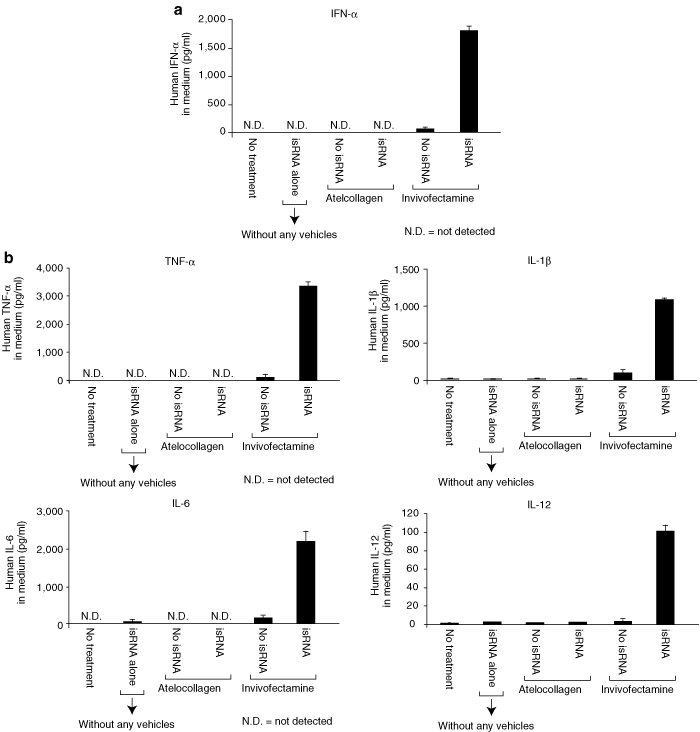
isRNA with Invivofectamine but not with atelocollagen induced IFN-α and inflammatory cytokines in human PBMCs in vitro. (a,b) Human PBMCs were treated with isRNA alone, atelocollagen alone, isRNA with atelocollagen, Invivofectamine alone, or isRNA with Invivofectamine, respectively. The isRNA concentration was kept constant at 100 nmol/l. Twenty-four hours later, the culture supernatants were collected by centrifugation. The secretion levels of (a) IFN-α, or (b) TNF-α, IL-1β, IL-6, and IL-12 were determined. The results represent the means ± SD (n = 4). IFN, interferon; isRNA; immunostimulatory RNA; PBMC, peripheral blood mononuclear cell.
The expression levels of IFN-response genes in human PBMCs
The induction levels of four IFN-response genes28,29,30 (OAS1, 2′-5′-oligoadenylate synthetase 1; OAS2, 2′-5′-oligoadenylate synthetase 2; IRF9, interferon regulatory factor 9; and IFIT1, interferon-induced protein with tetratricopeptide repeats 1) in PBMCs were determined by real-time reverse transcriptase-PCR. All four of the genes were dramatically induced in PBMCs, when treated with the isRNA/Invivofectamine complex (Figure 5). The isRNA/Invivofectamine complex induced these genes beginning at 3 hours after the treatment (Supplementary Figure S2). In contrast, the isRNA/atelocollagen complex did not induce any expression of these genes at all (Figure 5). It was of interest that Invivofectamine vehicle alone suddenly induced these genes 24 hours after the treatment (Figure 5), but the reason for this finding was not clear.
Figure 5.
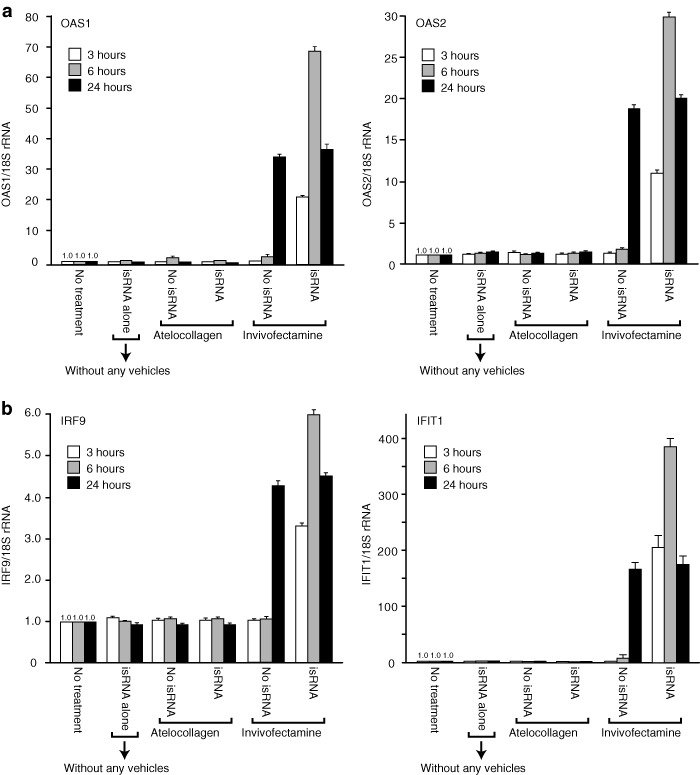
The expression levels of IFN-response genes in human PBMCs treated with isRNA with atelocollagen or Invivofectamine. (a,b) Human PBMCs were treated with isRNA with atelocollagen or Invivofectamine. At the time point of 3, 6, or 24 hours after the treatment, each total RNA was extracted. All of the total RNA were processed for quantitative real-time RT-PCR to determine the expression of (a) OAS1 and OAS2, (b) IRF9 and IFIT1. 18S rRNA was used as a control. The results represent the means ± SD (n = 4). IFN, interferon; isRNA; immunostimulatory RNA; PBMC, peripheral blood mononuclear cell; rRNA, ribosomal RNA; RT-PCR, reverse transcriptase-PCR.
isRNA/atelocollagen was not incorporated into PBMCs
We have already established a method for quantifying incorporated siRNA into the tissues/cells.14 The method utilizes a fluorescence-labeled oligoribonucleotide probe specific for the antisense or sense strand of the target siRNA and reversed-phase high-performance liquid chromatography for detecting the hybridized oligoribonucleotides.14 This system enables us to measure the level of intracellularly incorporated siRNA with high accuracy.14 These procedures are summarized in Supplementary Figure S3. Our results showed that the isRNA/Invivofectamine complex was incorporated into PBMCs, whereas no isRNA/atelocollagen complex was incorporated (Figure 6a). The confocal microscope analysis using a Cy3-labeled isRNA gave results consistent with those above (Figure 6b,c).
Figure 6.
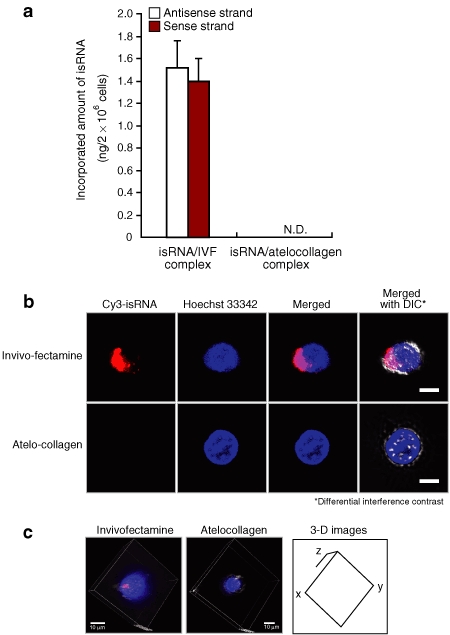
isRNA with Invivofectamine but not with atelocollagen was incorporated into human PBMCs in vitro. (a) Human PBMCs were treated with the isRNA/atelocollagen or isRNA/Invivofectamine complex. Six hours later, the cells were collected by centrifugation, and each total RNA was extracted. The amount of isRNA was determined by hybridizing with sequence-specific fluorescence-labeled oligoribonucleotide probes, followed by quantification with reversed-phase HPLC. The results represent the means ± SD (n = 4). N.D., not detected. (b) Human PBMCs were treated with the Cy3-labeled isRNA/atelocollagen, or Cy3-labeled isRNA/Invivofectamine complex. All of the cells were photographed using a confocal microscope system (Nikon A1 Rsi; Nikon, Tokyo, Japan). Hoechst 33342 was used for nuclear staining. Differential interference contrast (DIC) images were also obtained, and then merged with the fluorescence images. Bars, 10 µm. (c) Three-dimensional images were constructed using the NIS-Elements C software package (Nikon) according to the instruction manual. HPLC, high-performance liquid chromatography; isRNA; immunostimulatory RNA; PBMC, peripheral blood mononuclear cell.
Systemic administration of MCP-1 siRNA with either atelocollagen, or Invivofectamine showed a significant anti-inflammatory effect in a mouse CHS model
CHS is a common skin disease, presenting clinically as allergic contact dermatitis.31,32 At inflammatory sites in a typical CHS model in the mouse ear, elevated expression of MCP-1 was observed.15,31 In a previous report,15 we have already shown that systemic administration of a siRNA targeting MCP-1 (termed MCP-1 siRNA) mixed with atelocollagen elicited a significant therapeutic effect against a mouse CHS model. In Figure 7b, systemic injection of the MCP-1 siRNA/Invivofectamine complex to CHS model significantly reduced ear swelling (anti-inflammatory effect), compared with systemic injection of the MCP-1 siRNA-SCR/Invivofectamine complex. The statistical analysis revealed that the therapeutic effect of MCP-1 siRNA/Invivofectamine complex injection was equivalent to that of systemic injection of the MCP-1 siRNA/atelocollagen complex (Figure 7b). Both the MCP-1 siRNA/Invivofectamine and the MCP-1 siRNA/atelocollagen complex significantly reduced the amount of MCP-1 protein in CHS ears (Figure 7c). On the other hand, MCP-1 siRNA/DOTAP and MCP-1 siRNA/In vivo JET-PEI achieved almost no reduction in the amount of MCP-1 protein, which was consistent with their inconclusive therapeutic effects (Figure 7b,c). Each delivery vehicle alone, as well as MCP-1 siRNA alone (without any vehicles) or MCP-1 siRNA-SCR alone (without any vehicles), exhibited no therapeutic effects or reduction in the amount of MCP-1 protein (Figure 7b,c). During the therapy period, the blood levels of MCP-1 protein were slightly detectable in the groups receiving MCP-1 siRNA/Invivofectamine, MCP-1 siRNA-SCR/Invivofectamine, and Invivofectamine alone, whereas all of the other groups showed undetectable levels of MCP-1 protein (data not shown).
Figure 7.
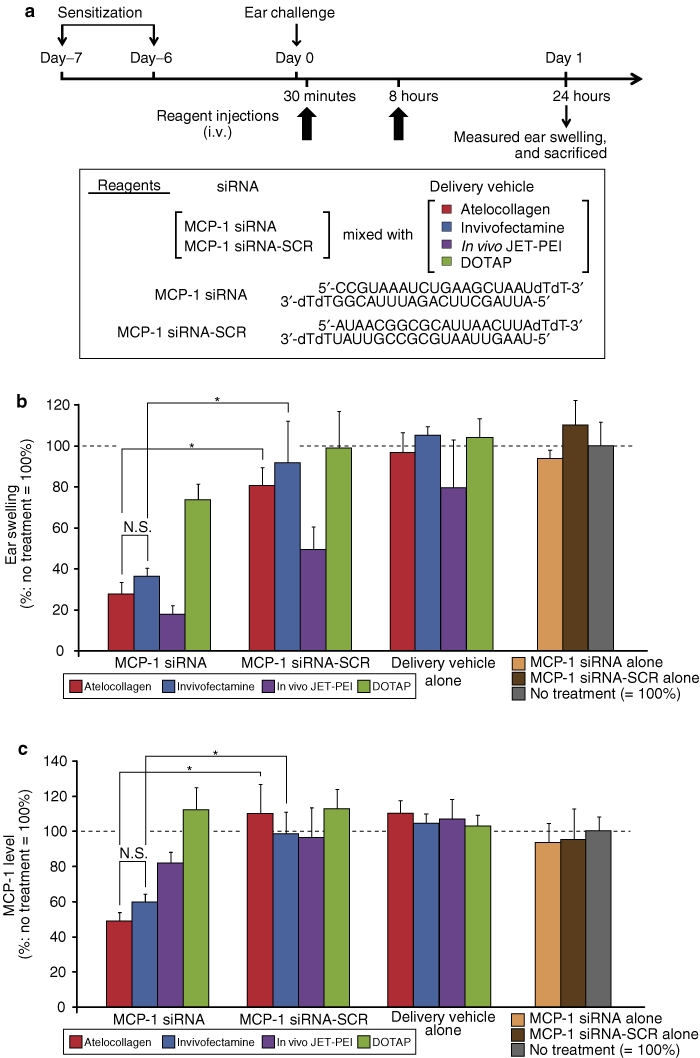
Therapeutic effect of MCP-1 siRNA mixed with four different delivery vehicles on the CHS model. (a) The therapeutic procedures in the CHS model targeting MCP-1. Sensitized mice were intravenously injected with MCP-1 siRNA or MCP-1 siRNA-SCR mixed with various delivery vehicles (atelocollagen, Invivofectamine, In vivo JET-PEI, or DOTAP). Mice were treated with two injections (30 minutes and 8 hours) after the ear challenge. The dose of the siRNA was constantly fixed at 50 µg/injection. Ear swelling was determined 24 hours after the ear challenge, and the ears were collected (sacrificed). (b) The results of ear swelling. The results represent percent ratios toward no treatment (NT). The results also represent the means ± SE (n = 6). *P < 0.05. (c) The excised ears at 24 hours after the ear challenge were homogenized and then centrifuged. The amount of MCP-1 in each supernatant was measured by a specific ELISA for mouse MCP-1. The results represent the means ± SE (n = 6). *P < 0.05. CHS, contact hypersensitivity; ELISA, enzyme-linked immunosorbent assay; MCP-1, monocyte chemoattractant protein-1; siRNA, short interfering RNA.
The level of macrophage infiltration in the CHS ears treated with MCP-1 siRNA/Invivofectamine complex was dramatically lower than in those treated with MCP-1 siRNA-SCR/Invivofectamine complex (Supplementary Figure S4). The MCP-1 siRNA/atelocollagen complex showed a similar reduction of infiltrated macrophages. The recruitment of T cells (CD3-positive cells) in the treated CHS ears also showed consistent results (Supplementary Figure S4). Hematoxylin and eosin staining revealed that ear swelling and inflammatory cell infiltration were inhibited in the CHS ears treated with MCP-1 siRNA/Invivofectamine, or MCP-1 siRNA/atelocollagen complex, compared with their scrambled control siRNA complexes (Supplementary Figure S4).
The MCP-1 siRNA/atelocollagen complex showed cell-specific delivery in the inflammatory CHS ear
To identify the cells in which MCP-1 messenger RNA was knocked down in the treated CHS ear by the MCP-1 siRNA/atelocollagen complex, we performed laser microdissection to pick up the desired cells, complementary DNA preparation, and quantitative real-time reverse transcriptase-PCR for mouse MCP-1. MCP-1 knockdown was determined in keratinocytes (stained with Toluidine blue) and macrophages (stained with anti-F4/80 antibody), suggesting that our siRNA delivery method via atelocollagen was cell-specific, at least toward those cells (Supplementary Figure S5). The increased expression of MCP-1 in keratinocytes and macrophages in the inflammatory CHS ears has already been reported.33,34 Thus, our delivery method is a reasonable means of achieving therapeutic effects via MCP-1 knockdown.
Endo180 is a candidate receptor protein for delivery of the siRNA/atelocollagen complex into inflammatory macrophages
Endo180 (CD280), a member of the mannose receptor family, is constitutively recycled between clathrin-coated pits on the cell surface and intracellular endosomes.35,36 Endo180 is also known as a receptor (a binding protein) for type I collagen.36,37 IFN-γ stimulation produces activated macrophages (M1 designation as classically activated macrophages, ref. 38) from normal-state macrophages, and further, Endo180 is expressed on such stimulated macrophages.39 In Supplementary Figure S6, we showed the expression of Endo180 protein on the cell surface of the IFN-γ-mediated activated RAW264.7 macrophages via immunostaining and immunoblotting methods. In contrast, no expression of the protein was observed in unstimulated RAW264.7 macrophages (Supplementary Figure S6). We also observed that the Cy3-labeled siRNA/atelocollagen complex was delivered only into the activated RAW264.7 macrophages (Y. Takei and S. Inaba, unpublished results). Thus, Endo180 is a candidate receptor protein for delivery of the siRNA/atelocollagen into inflammatory macrophages, but not into normal-state, unstimulated macrophages (or monocytes).
Estimation of liver enzyme and renal function in CHS model mice
Liver and renal functions were tested at days 1, 4, and 7 in the CHS model mouse after treatment with MCP-1 siRNA with atelocollagen, DOTAP, In vivo JET-PEI, or Invivofectamine (Supplementary Figure S7). Only the MCP-1 siRNA/In vivo JET-PEI complex showed quite increased levels of aspartate aminotransferase, and alanine aminotransferase on day 4.
Discussion
Activation of innate immunity and the production of IFNs have direct effects in modulating viral replication, tumor growth, angiogenesis, and inflammatory and other immunological processes.11,40 To avoid the potential of misinterpreting the therapeutic efficacy caused by siRNA-mediated immunostimulation for a specific RNAi effect in animal models, we investigators have to appreciate the capacity of siRNAs to activate such a response and to fully characterize the immunostimulatory potential of active siRNA sequences in particular.
In the present study, we used isRNA6 because it has an active sequence to immune response, and showed that the isRNA/atelocollagen complex did not induce any immune response in both animals and PBMC cultures in vitro (Figures 1,2,3,4). On the other hand, the isRNA/Invivofectamine complex potently induced type-I IFNs and TNF-α (Figures 2,3,4). We showed that the isRNA/atelocollagen complex was not incorporated into PBMCs at all, whereas the isRNA/Invivofectamine complex was massively incorporated into them (Figure 6). These results suggested that the isRNA delivered into PBMCs via Invivofectamine triggered the mouse immune response at an organismal level through endosomal Toll-like receptors (TLR), particularly TLR7 and TLR8.41,42 In contrast, the absence of incorporation of isRNA into PBMCs via atelocollagen led to an immunostimulatory-null effect in mice. Turning to the therapeutic effect on the CHS model, MCP-1 siRNA with atelocollagen and MCP-1 siRNA with Invivofectamine had almost the same level of anti-inflammatory effect. Here, MCP-1 siRNA did not harbor an IFN-inducible sequence. Nevertheless, the MCP-1 siRNA/Invivofectamine complex induced a low level of IFN-α in mice, whereas the MCP-1 siRNA/atelocollagen complex did not (data not shown). From these results, we conclude that atelocollagen is superior to Invivofectamine as a vehicle from the viewpoint of the null-adverse effect on immunostimulation. Thus, the use of atelocollagen as a vehicle allows us to prevent the immunostimulatory effect of the siRNA in vivo without any chemical modifications, even if it harbors IFN-inducible sequences. These results demonstrated that atelocollagen vehicle-mediated regulation of immunostimulation of siRNA without chemical modifications was actually possible. This concept overturns the common sense notion that chemical modifications are absolutely necessary to prevent such immunostimulatory effects of siRNA.12,13,28
PBMCs from whole blood include plasmacytoid dendritic cells, which are the first reactor cells for isRNA to produce IFN-α. The incorporated isRNA stimulates TLR7 on the endosomes of PBMCs to secrete IFN-α. IFN-α produced by plasmacytoid dendritic cells acts on surrounding monocytes and macrophages, causing the production of high levels of inflammatory cytokines including IL-6, IL-1β, and TNF-α,7 consistent with our results. Systemic release of IFN-α, IL-6, and IL-1β induces symptoms of fever, rigors, and chills.7 Cytokines released into the blood cause activation of vascular endothelial cells, resulting in a cascade of effects on blood cells including (i) margination, (ii) pavementing, (iii) emigration, and (iv) hemorrhage, exacerbating lymphopenia and thrombocytopenia. Activation of the cells by siRNA, either directly or via the action of cytokines, can upregulate cyclooxygenase 2 and nitric oxide synthase enzyme pathways, resulting in eicosanoid (prostaglandins, etc.) and nitric oxide production, and contributing to the symptoms of toxicity, including pain.7 IL-1β and TNF-α acting on the vascular endothelium result in the development of hypotension. To prevent all of these adverse immunostimulatory effects of siRNAs in clinical applications for humans, our atelocollagen-mediated systemic method is a promising approach.
The intravenously injected isRNA/Invivofectamine complex was also incorporated into the liver, spleen, kidney, and lung in normal mice (Supplementary Figure S8). On the other hand, the isRNA/atelocollagen complex was not incorporated into these organs. Indeed, the isRNA/Invivofectamine complex induced IFIT1 in the liver, spleen, kidney, and lung in mice, whereas the isRNA/atelocollagen complex did not (Supplementary Figure S8). According to our previous results, the intravenously administered Cy3-labeled siRNA/atelocollagen complex is not accumulated into normal tissue, but is accumulated into tumors or inflammatory tissues where the tissue permeability is elevated,14,15 and this is finally incorporated into the interior of the cells. It is of note that the siRNA/atelocollagen complex was not incorporated into either plasmacytoid dendritic cells or other blood cells in the bloodstream or into normal tissues/organs, and that this lack of incorporation led to the null-IFN responses in mice. In the present study, we showed that atelocollagen-mediated systemic siRNA delivery occurred in a cell-specific manner (at least in keratinocytes and macrophages) in the inflammatory CHS ears. Furthermore, we showed that Endo180 is a candidate receptor protein for delivery of the siRNA/atelocollagen complex into activated macrophages. Thus, we found a clue to clarify the mechanism(s) underlying our atelocollagen-mediated cell-specific delivery method, although numerous experiments will be needed to fully elucidate the mechanisms. Endo180 is expressed in tumors, and acts to promote tumor growth in vivo.43 In brief, Endo180 is expected to become a key protein to explain the mechanism(s) of specific delivery of siRNAs to inflammatory regions or tumors via atelocollagen-mediated methods.
Several reports have shown that a “bifunctional” siRNA is useful to treat various cancers, including melanomas.9,10 A bifunctional siRNA induces innate immunity through TLRs and simultaneously inhibits the target gene expression via an RNAi effect, two mutually reinforcing effects that operate like the dual edges of a sword.8 However, the concept is open to discussion, especially with respect to clinical application, although it is of much interest as basic life science. In that context, we have an opinion to separate the immunostimulating activity from the gene silencing activity of RNAi, and then use it for humans. More recently, short-hairpin RNAs (shRNAs) delivered by lentiviral vector transduction were shown to trigger RIG-I-mediated IFN activation in a sequence- and 5′-triphosphate-dependent manner.44 Thus, the shRNA-lentiviral vector also results in undesirable immunostimulation, although viral vector-mediated siRNA/shRNA delivery might be essentially inadequate for clinical use.
In conclusion, we demonstrated that atelocollagen-mediated systemic siRNA delivery did not cause any immunostimulations in animals, even if the siRNA harbors the IFN-inducible sequence. In order to actually realize a siRNA-based medicine for humans, atelocollagen is an excellent and promising delivery vehicle, and it has the useful advantage of evading detection by the “radar” of innate immunity.
Materials and Methods
siRNAs. All of the siRNAs were synthesized by Dharmacon (Lafayette, CO). isRNA is a double-stranded short RNA with an IFN-inducible sequence (5′-UGUGU-3′) and overhangs as UU. isRNA was originally termed β-gal 728,6 and its structure is shown in Figure 1a. MCP-1 siRNA and MCP-1 siRNA-SCR (a scrambled control of MCP-1 siRNA) were previously reported,15 and their nucleotide sequences are shown in Figure 7a.
isRNA/atelocollagen complex preparation. For preparing the isRNA/atelocollagen complex, equal volumes of atelocollagen (0.1%) in phosphate-buffered saline and isRNA (stock: 20 mg/ml) solution were mixed by rotating for 20 minutes at 4 °C.14 Thus, the final concentration of atelocollagen became 0.05%. For in vivo mouse study, the isRNA concentration was constantly 250 µg/ml (50 µg/mouse body, and 200 µl injection volume/mouse). For the PBMC experiments, the final concentration of isRNA was 100 nmol/l (50 pmol = 0.66 µg/24-well plate). MCP-1 siRNA and MCP-1 siRNA-SCR were mixed with atelocollagen using the method described above and in a previous report.15
isRNA/Invivofectamine complex preparation. Invivofectamine (Invitrogen) was used according to the manufacturer's protocol. Thus, isRNA solution (20 mg/ml) was combined with Invivofectamine reagent (1.0 ml) and diluted in 15 ml of 5% glucose. The mixed complex was concentrated using an Amicon Ultra-15 centrifugal filter tube (Millipore, Jaffrey, NH). The concentrated complex was collected and brought the volume to 4 ml by adding 5% glucose. For in vivo mouse study, the isRNA concentration was constantly 250 µg/ml (50 µg/mouse body, and 200 µl injection volume/mouse). For PBMC experiments, the final concentration of isRNA was 100 nmol/l (50 pmol = 0.66 µg/24-well plate).
isRNA/In vivo JET-PEI complex preparation. In vivo JET-PEI is a product of a synthetic polymer from Polyplus transfection (Illkirch-Graffenstaden, France). Preparation of the isRNA/In vivo JET-PEI complex (N/P ratio 8) was performed according to the manufacturer's instructions. Thus, isRNA (50 µg) in water (50 µl) was mixed with 10% glucose (50 µl) to prepare solution A. Eight microliters of In vivo JET-PEI in water (42 µl) was mixed with 10% glucose (50 µl) to prepare solution B. Finally, solution A and B were combined, left for 15 minutes at room temperature, and vortexed. For the in vivo mouse studies, the isRNA concentration was kept constant at 250 µg/ml (50 µg/mouse body, and 200 µl injection volume/mouse).
isRNA/DOTAP complex preparation. The isRNA/DOTAP complex was prepared as described in previous reports.5,15 In brief, DOTAP (30 µg) was mixed with Opti-MEM (70 µl). The solution was combined with 50 µg of isRNA resuspended in 100 µl of Opti-MEM (Invitrogen, Carlsbad, CA), and then incubated for 15 minutes. Finally, the mixture (200 µl) was intravenously injected. For the in vivo mouse studies, the isRNA concentration was kept constant at 250 µg/ml (50 µg/mouse body, and 200 µl injection volume/mouse).
Animals. Eight-week-old female Balb/c, C57BL/6J, and ICR mice were purchased from SLC Japan (Hamamatsu, Japan). The animal experiments were performed in compliance with the guidelines of the Institute for Laboratory Animal Research, Nagoya University Graduate School of Medicine.
Measurement of IFN and inflammatory cytokine induction. The levels of mouse IFN-α and IFN-β were determined with a mouse IFN-α or IFN-β ELISA kit (PBL Biomedical Laboratories, Piscataway, NJ). The level of mouse TNF-α was determined with a Quantikine mouse TNF-α ELISA kit (R&D Systems, Minneapolis, MN). The levels of human IFN-α or IFN-β were determined with a human IFN-α or IFN-β ELISA kit (PBL Biomedical Laboratories). The levels of TNF-α, IL-6, IL-12, and IL-1β in human PBMCs were simultaneously determined by a Bio-Plex Suspension Array System using a Bio-Plex Pro Human cytokine Group I (Bio-Rad Laboratories, Hercules, CA).
Whole blood experiment. Blood samples from humans were collected in heparinized tubes. Informed consent was obtained from all of the donors for blood collection. Mouse whole blood was also collected with tuberculin syringes containing ~0.02 ml of 20 U of heparin by cardiac puncture. Each whole blood sample was mixed with either isRNA/atelocollagen complex, atelocollagen alone, isRNA/Invivofectamine complex, Invivofectamine alone, or isRNA alone as described in Figure 3a. The isRNA concentration was kept constant at 50 µg/200 µl. After 6 and 24 hours of incubation at 37 °C in 5% CO2, we prepared supernatant fractions by centrifugation of each whole blood sample reacted, and measured the IFN-α levels by enzyme-linked immunosorbent assay.
PBMCs. Human PBMCs were isolated from whole blood from healthy donors by a standard Ficoll-hypaque density centrifugation technique.45 Isolated PBMCs were seeded at a density of 1 × 106 cells/24-well (medium volume: 0.5 ml/well) and cultured in RPMI1640 with 10% fetal bovine serum at 37 °C in 5% CO2. The PBMCs were treated with isRNA/atelocollagen complex, atelocollagen alone, isRNA/Invivofectamine complex, Invivofectamine alone, or isRNA alone. In all tests, the isRNA concentration was 100 nmol/l (1.33 µg/ml). Each supernatant was collected after 24 hours of incubation.
RNA extraction and quantitative real-time reverse transcriptase-PCR. Total RNA was extracted from the PBMCs using an RNeasy Mini kit (Qiagen, Hilden, Germany). Each total RNA (400 ng) was reverse transcribed with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Quantitative PCR was performed using the TaqMan Gene Expression Assays and 7500 Real-time PCR System (Applied Biosystems, Foster City, CA). The expression levels of OAS1 (Assay ID, Hs00242943_m1), OAS2 (Assay ID, Hs00942643_m1), IRF9 (Assay ID, Hs00196051_m1), and IFIT1 (Assay ID, Hs00356631_g1) were normalized to the eukaryotic 18S ribosomal RNA.
Quantification of the isRNA incorporated into human PBMCs. Human PBMCs were cultured at 1 × 106 cells/24-well with isRNA/atelocollagen, or isRNA/Invivofectamine complex. Six hours after the incubation, the cells were collected by centrifugation and vigorously washed twice with phosphate-buffered saline (−). Total RNA was extracted using a mirVana miRNA Isolation Kit (Ambion, Austin, TX) according to the manufacturer's protocol. A fluorescence-labeled oligoribonucleotide specific for the antisense strand (5′-CUACACAAAUCAGCGAUUU-3′) or the sense strand (5′-GAUGUGUUUAGUCGCUAAA-3′) of the isRNA was added to the total RNA fraction to allow hybridization (Supplementary Figure S3). The incorporated amount of the isRNA was quantitated as described in our previous report.14
CHS model. The CHS model was prepared by our previously described method15 as summarized in Figure 7a. Ear thickness was measured with a thickness gauge (Peacock, Tokyo, Japan) before and 24 hours after the ear challenge. The ear swelling was determined by subtracting the thickness before the ear challenge. After the measurement of ear swelling, we killed the mice and excised the ears. The amount of MCP-1 protein in each ear was determined by an enzyme-linked immunosorbent assay specific for mouse MCP-1 (R&D Systems), and normalized with the protein concentration as described previously.15
Statistical analysis. The data were analyzed using the Mann–Whitney U test, and probability values of less than 0.05 were considered to indicate significant differences.
SUPPLEMENTARY MATERIAL Figure S1. JET-PEI vehicle alone and DOTAP vehicle alone interacted with the human PBMCs in vitro. Figure S2. Time-course experiment of the expression levels of IFN-response genes such as OAS1, OAS2, IRF9, and IFIT1. Figure S3. Procedures for quantitating isRNA incorporated into PBMCs. Figure S4. Decreased infiltration of macrophages and T cells in the treated CHS ears. Figure S5. MCP-1 mRNA was downregulated in keratinocytes and macrophages in the treated CHS ears by the MCP-1 siRNA/atelocollagen complex. Figure S6. Endo180 on the macrophages activated by stimulation with IFN-γ is a candidate receptor for binding with the siRNA/atelocollagen complex. Figure S7. Examination of adverse effects on liver enzymes, or renal function, when MCP-1 siRNA mixed with various delivery vehicles was intravenously injected in the CHS model mouse. Figure S8. Tissue distribution of isRNA intravenously delivered with Invivofectamine or atelocollagen in normal Balb/c mice and IFIT1 induction in the liver and spleen.
Acknowledgments
Support was received in the form of Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (17016030) and from the Japan Society for the Promotion of Science (17790185, 19590273, and 21590305). We thank Drs Kazuo Kita, Takayuki Okubo, Ryoichi Ioka, and Takuya Kato for their helpful suggestions regarding the experiments and the manuscript. We also thank Yuriko Sawa for her excellent technical assistance. Finally, the authors wish to acknowledge Ikuyo Mizuguchi and the Division for Medical Research Engineering, Nagoya University Graduate School of Medicine, for their technical support with the confocal microscope system (Nikon A1 Rsi).
Supplementary Material
JET-PEI vehicle alone and DOTAP vehicle alone interacted with the human PBMCs in vitro.
Time-course experiment of the expression levels of IFN-response genes such as OAS1, OAS2, IRF9, and IFIT1.
Procedures for quantitating isRNA incorporated into PBMCs.
Decreased infiltration of macrophages and T cells in the treated CHS ears.
MCP-1 mRNA was downregulated in keratinocytes and macrophages in the treated CHS ears by the MCP-1 siRNA/atelocollagen complex.
Endo180 on the macrophages activated by stimulation with IFN-γ is a candidate receptor for binding with the siRNA/atelocollagen complex.
Examination of adverse effects on liver enzymes, or renal function, when MCP-1 siRNA mixed with various delivery vehicles was intravenously injected in the CHS model mouse.
Tissue distribution of isRNA intravenously delivered with Invivofectamine or atelocollagen in normal Balb/c mice and IFIT1 induction in the liver and spleen.
REFERENCES
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE., and, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K., and, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Castanotto D., and, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JT., and, Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23:1399–1405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S.et al. (2005Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7 Nat Med 11263–270. [DOI] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K., and, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Judge A., and, MacLachlan I. Overcoming the innate immune response to small interfering RNA. Hum Gene Ther. 2008;19:111–124. doi: 10.1089/hum.2007.179. [DOI] [PubMed] [Google Scholar]
- Schlee M, Hornung V., and, Hartmann G. siRNA and isRNA: two edges of one sword. Mol Ther. 2006;14:463–470. doi: 10.1016/j.ymthe.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS.et al. (20085'-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma Nat Med 141256–1263. [DOI] [PubMed] [Google Scholar]
- Besch R, Poeck H, Hohenauer T, Senft D, Häcker G, Berking C.et al. (2009Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells J Clin Invest 1192399–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins M, Judge A, Ambegia E, Choi C, Yaworski E, Palmer L.et al. (2008Misinterpreting the therapeutic effects of small interfering RNA caused by immune stimulation Hum Gene Ther 19991–999. [DOI] [PubMed] [Google Scholar]
- Karikó K, Buckstein M, Ni H., and, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Sioud M, Furset G., and, Cekaite L. Suppression of immunostimulatory siRNA-driven innate immune activation by 2'-modified RNAs. Biochem Biophys Res Commun. 2007;361:122–126. doi: 10.1016/j.bbrc.2007.06.177. [DOI] [PubMed] [Google Scholar]
- Mu P, Nagahara S, Makita N, Tarumi Y, Kadomatsu K., and, Takei Y. Systemic delivery of siRNA specific to tumor mediated by atelocollagen: combined therapy using siRNA targeting Bcl-xL and cisplatin against prostate cancer. Int J Cancer. 2009;125:2978–2990. doi: 10.1002/ijc.24382. [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Takei Y, Yuzawa Y, Hanai K, Nagahara S, Tarumi Y.et al. (2008Downregulation of monocyte chemoattractant protein-1 involving short interfering RNA attenuates hapten-induced contact hypersensitivity Mol Ther 16387–395. [DOI] [PubMed] [Google Scholar]
- Ochiya T, Takahama Y, Nagahara S, Sumita Y, Hisada A, Itoh H.et al. (1999New delivery system for plasmid DNA in vivo using atelocollagen as a carrier material: the Minipellet Nat Med 5707–710. [DOI] [PubMed] [Google Scholar]
- Takei Y., and, Kadomatsu K. In vivo delivery technique of nucleic acid compounds using atelocollagen: its use in cancer therapeutics targeted at the heparin-binding growth factor midkine. Gene Ther Mol Biol. 2005;9:257–264. [Google Scholar]
- Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S., and, Muramatsu T. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res. 2004;64:3365–3370. doi: 10.1158/0008-5472.CAN-03-2682. [DOI] [PubMed] [Google Scholar]
- Minakuchi Y, Takeshita F, Kosaka N, Sasaki H, Yamamoto Y, Kouno M.et al. (2004Atelocollagen-mediated synthetic small interfering RNA delivery for effective gene silencing in vitro and in vivo Nucleic Acids Res 32e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno H, Takei Y, Muramatsu T, Komori K., and, Kadomatsu K. Controlled release of small interfering RNA targeting midkine attenuates intimal hyperplasia in vein grafts. J Vasc Surg. 2006;44:633–641. doi: 10.1016/j.jvs.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Takei Y, Kadomatsu K, Goto T., and, Muramatsu T. Combinational antitumor effect of siRNA against midkine and paclitaxel on growth of human prostate cancer xenografts. Cancer. 2006;107:864–873. doi: 10.1002/cncr.22068. [DOI] [PubMed] [Google Scholar]
- Takeshita F, Minakuchi Y, Nagahara S, Honma K, Sasaki H, Hirai K.et al. (2005Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo Proc Natl Acad Sci USA 10212177–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma K, Iwao-Koizumi K, Takeshita F, Yamamoto Y, Yoshida T, Nishio K.et al. (2008RPN2 gene confers docetaxel resistance in breast cancer Nat Med 14939–948. [DOI] [PubMed] [Google Scholar]
- Takeshita F, Patrawala L, Osaki M, Takahashi RU, Yamamoto Y, Kosaka N.et al. (2010Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes Mol Ther 18181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogawa M, Yuasa T, Kimura S, Tanaka M, Kuroda J, Sato K.et al. (2005Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer J Clin Invest 115978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata E, Ashihara E, Kimura S, Takenaka K, Sato K, Tanaka R.et al. (2008Administration of PLK-1 small interfering RNA with atelocollagen prevents the growth of liver metastases of lung cancer Mol Cancer Ther 72904–2912. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Nakashiro K, Tanaka H, Azuma K, Goda H, Hara S.et al. (2010Knockdown of Akt isoforms by RNA silencing suppresses the growth of human prostate cancer cells in vitro and in vivo Biochem Biophys Res Commun 39979–83. [DOI] [PubMed] [Google Scholar]
- Bridge AJ, Pebernard S, Ducraux A, Nicoulaz AL., and, Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat Genet. 2003;34:263–264. doi: 10.1038/ng1173. [DOI] [PubMed] [Google Scholar]
- Sledz CA, Holko M, de Veer MJ, Silverman RH., and, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- Sadler AJ., and, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebeler M, Trautmann A, Voss A, Bröcker EV, Toksoy A., and, Gillitzer R. Differential and sequential expression of multiple chemokines during elicitation of allergic contact hypersensitivity. Am J Pathol. 2001;158:431–440. doi: 10.1016/s0002-9440(10)63986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui G, Mitsui K, Hirano T, Ohara O, Kato M., and, Niwano Y. Kinetic profiles of sequential gene expressions for chemokines in mice with contact hypersensitivity. Immunol Lett. 2003;86:191–197. doi: 10.1016/s0165-2478(03)00017-8. [DOI] [PubMed] [Google Scholar]
- Grabbe S., and, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- Sebastiani S, Albanesi C, De PO, Puddu P, Cavani A., and, Girolomoni G. The role of chemokines in allergic contact dermatitis. Arch Dermatol Res. 2002;293:552–559. doi: 10.1007/s00403-001-0276-9. [DOI] [PubMed] [Google Scholar]
- Sheikh H, Yarwood H, Ashworth A., and, Isacke CM. Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor. J Cell Sci. 2000;113 (Pt 6):1021–1032. doi: 10.1242/jcs.113.6.1021. [DOI] [PubMed] [Google Scholar]
- Wienke D, MacFadyen JR., and, Isacke CM. Identification and characterization of the endocytic transmembrane glycoprotein Endo180 as a novel collagen receptor. Mol Biol Cell. 2003;14:3592–3604. doi: 10.1091/mbc.E02-12-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EK, Nakamura M, Wienke D, Isacke CM, Pozzi A., and, Liang P. Endo180 binds to the C-terminal region of type I collagen. J Biol Chem. 2005;280:22596–22605. doi: 10.1074/jbc.M501155200. [DOI] [PubMed] [Google Scholar]
- Mosser DM., and, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Xing Q, Ren Y, Harmsen MC., and, Bank RA. Endo180 and MT1-MMP are involved in the phagocytosis of collagen scaffolds by macrophages and is regulated by interferon-gamma. Eur Cell Mater. 2010;20:197–209. doi: 10.22203/ecm.v020a16. [DOI] [PubMed] [Google Scholar]
- Robbins M, Judge A., and, MacLachlan I. siRNA and innate immunity. Oligonucleotides. 2009;19:89–102. doi: 10.1089/oli.2009.0180. [DOI] [PubMed] [Google Scholar]
- Robbins M, Judge A, Liang L, McClintock K, Yaworski E., and, MacLachlan I. 2'-O-methyl-modified RNAs act as TLR7 antagonists. Mol Ther. 2007;15:1663–1669. doi: 10.1038/sj.mt.6300240. [DOI] [PubMed] [Google Scholar]
- Sioud M. Single-stranded small interfering RNA are more immunostimulatory than their double-stranded counterparts: a central role for 2'-hydroxyl uridines in immune responses. Eur J Immunol. 2006;36:1222–1230. doi: 10.1002/eji.200535708. [DOI] [PubMed] [Google Scholar]
- Wienke D, Davies GC, Johnson DA, Sturge J, Lambros MB, Savage K.et al. (2007The collagen receptor Endo180 (CD280) is expressed on basal-like breast tumor cells and promotes tumor growth in vivo Cancer Res 6710230–10240. [DOI] [PubMed] [Google Scholar]
- Kenworthy R, Lambert D, Yang F, Wang N, Chen Z, Zhu H.et al. (2009Short-hairpin RNAs delivered by lentiviral vector transduction trigger RIG-I-mediated IFN activation Nucleic Acids Res 376587–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooper MM, Wassink L, M'Rabet L., and, Graus YM. The modulatory effects of prostaglandin-E on cytokine production by human peripheral blood mononuclear cells are independent of the prostaglandin subtype. Immunology. 2002;107:152–159. doi: 10.1046/j.1365-2567.2002.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
JET-PEI vehicle alone and DOTAP vehicle alone interacted with the human PBMCs in vitro.
Time-course experiment of the expression levels of IFN-response genes such as OAS1, OAS2, IRF9, and IFIT1.
Procedures for quantitating isRNA incorporated into PBMCs.
Decreased infiltration of macrophages and T cells in the treated CHS ears.
MCP-1 mRNA was downregulated in keratinocytes and macrophages in the treated CHS ears by the MCP-1 siRNA/atelocollagen complex.
Endo180 on the macrophages activated by stimulation with IFN-γ is a candidate receptor for binding with the siRNA/atelocollagen complex.
Examination of adverse effects on liver enzymes, or renal function, when MCP-1 siRNA mixed with various delivery vehicles was intravenously injected in the CHS model mouse.
Tissue distribution of isRNA intravenously delivered with Invivofectamine or atelocollagen in normal Balb/c mice and IFIT1 induction in the liver and spleen.


