Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9) has emerged as a therapeutic target for the reduction of low-density lipoprotein cholesterol (LDL-C). PCSK9 increases the degradation of the LDL receptor, resulting in high LDL-C in individuals with high PCSK9 activity. Here, we show that two locked nucleic acid (LNA) antisense oligonucleotides targeting PCSK9 produce sustained reduction of LDL-C in nonhuman primates after a loading dose (20 mg/kg) and four weekly maintenance doses (5 mg/kg). PCSK9 messenger RNA (mRNA) and serum PCSK9 protein were reduced by 85% which resulted in a 50% reduction in circulating LDL-C. Serum total cholesterol (TC) levels were reduced to the same extent as LDL-C with no reduction in high-density lipoprotein levels, demonstrating a specific pharmacological effect on LDL-C. The reduction in hepatic PCSK9 mRNA correlated with liver LNA oligonucleotide content. This verified that anti-PCSK9 LNA oligonucleotides regulated LDL-C through an antisense mechanism. The compounds were well tolerated with no observed effects on toxicological parameters (liver and kidney histology, alanine aminotransferase, aspartate aminotransferase, urea, and creatinine). The pharmacologic evidence and initial safety profile of the compounds used in this study indicate that LNA antisense oligonucleotides targeting PCSK9 provide a viable therapeutic strategy and are potential complements to statins in managing high LDL-C.
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9), a member of the proteinase K subfamily of subtilases,1 is involved in the regulation of circulating low-density lipoprotein cholesterol (LDL-C). PCSK9 regulates the level of circulating LDL-C through interaction with hepatic cell surface low-density lipoprotein receptors (LDLR),2,3 followed by internalization of the complex and lysosomal degradation of the LDLR.4 Studies on human genetic variations have shown that PCSK9 gain of function mutations are associated with hypercholesterolemia, i.e., high LDL-C, whereas loss of function mutations are associated with low LDL-C levels.5,6,7,8 High level of LDL-C is a major risk factor for development of atherosclerosis, which is the main cause of cardiovascular disease. Cardiovascular disease is the number one cause of death worldwide (WHO report The Global Burden of Disease: 2004 update).9 The PCSK9 loss of function mutations do not seem to cause any phenotypic changes in human subjects other than very low circulating LDL-C and the mutations are associated with a 47–88% reduction in the risk of developing cardiovascular disease.10,11,12 This suggests that PCSK9 is not essential for normal development, and validates PCSK9 as an attractive and specific therapeutic target for lowering circulating LDL-C. This is of particular interest in a subset of hypercholesterolemia patients where the current standard of care, statin therapy, fails to reduce LDL-C to intended target levels. Statins inhibit the rate-limiting step in cholesterol de novo synthesis, resulting in increased expression of liver LDLR and, eventually, increased uptake of LDL from the circulation. The same mechanism that leads to increased LDLR expression also increases liver expression of PCSK9. This has been suggested to limit the potency of statins, especially in patients with gain of function mutations of PCSK9.13
Several different approaches have been explored as a means to inhibit or reduce PCSK9, including antisense oligonucleotides,14,15 lipidoid nanoparticle (LNP) formulated short interfering RNA (siRNAs) directed against the PCSK9 messenger RNA (mRNA),16 antibodies directed against circulating PCSK9 protein17,18,19 and small peptides that block the PCSK9/LDLR interaction.20 Reduction of LDL-C by inhibition of PCSK9 in nonhuman primates has previously been demonstrated after a single dose of LNP-formulated siRNAs16 and single doses of monoclonal antibodies.17,19 Here, we report that single and multiple subcutaneous injections of two different anti-PCSK9 LNA antisense oligonucleotides produce potent and long-lasting reductions of LDL-C in nonhuman primates.
Results
In vitro characterization of compounds
Two PCSK9 specific LNA antisense oligonucleotides (SPC5001; a 14-mer and SPC4061; a 13-mer) were selected for the nonhuman primate pharmacology study after screening in vitro (Supplementary Figure S1 and S2). Both compounds potently reduced PCSK9 mRNA levels in treated cells. An unspecific control LNA oligonucleotide, SPC3088 (not complementary to PCSK9 mRNA in man, monkey, or mouse), was included in the in vitro experiments. SPC3088 had no significant effect on PCSK9 mRNA levels in vitro (Supplementary Figure S1 and S2).
Single-dose study, pharmacokinetics
A group of six monkeys received a single 10 mg/kg subcutaneous injection of SPC5001 with subsequent killing of single monkeys at day 4, 7, 14, 21, 28, and 56 after injection. Serum and liver tissue samples were collected at each time point for analysis of LDL-C and SPC5001 oligonucleotide content (Figure 1a). LDL-C levels decreased continuously over the first 3 weeks, with a maximum reduction of 50% at day 21. After day 21, the effect diminished slowly, and at day 56, LDL-C had returned to predose levels. The estimated half-life of the pharmacological LDL-C lowering effect was 24 days, calculated from day 21 to day 56. Liver SPC5001 content reached a maximum at day 7 (maximum tissue concentration (Cmax), 20 µg/g liver) (Figure 1a). The SPC5001 liver tissue half-life was calculated to be at least 6 days. The reduction in serum total cholesterol (TC) and apolipoprotein B (apoB) corresponded well to the reduction in LDL-C (Figure 1b) whereas HDL-C was reduced in the single animal killed at day 21 and increased at day 56, compared to respective predose level.
Figure 1.
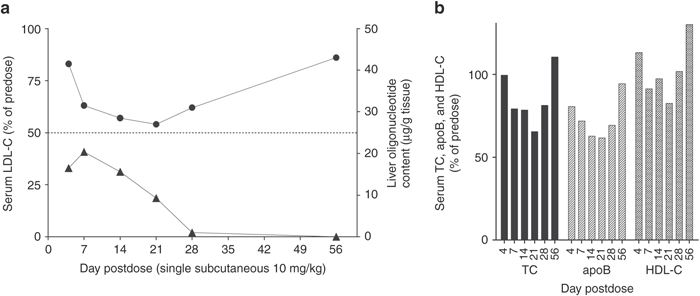
Single-dose study, pharmacokinetics. Monkeys were injected with a single 10 mg/kg subcutaneous dose of LNA antisense compound SPC5001 and analyzed at different time points after injection. (a) Serum LDL-C (circles) and liver oligonucleotide content (triangles), (b) serum total cholesterol (TC), apolipoprotein B (apoB), and HDL-C in corresponding monkeys. Each time point represents a single animal (n = 1). HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LNA, locked nucleic acid.
Multiple-dose study, treatment period
The potency of SPC5001 and SPC4061 was examined in a multiple-dose study, comprising an initial loading dose of 20 mg/kg, followed by four weekly maintenance doses of 5 mg/kg. Both compounds were administered subcutaneously as unformulated molecules in buffered saline. Forty-eight hours after last dose (day 30), three monkeys per group were killed and the remaining two monkeys were monitored for an additional 8-week recovery period. Serum was sampled weekly and analyzed for PCSK9 and LDL-C levels as well as for a range of biochemical and toxicological parameters (vide infra). Analyses of hepatic PCSK9, LDLR, and HMG-CoA reductase mRNA expression, LDLR protein, and liver lipid content were performed after killing. In addition, livers and kidneys were examined for histopathology changes.
Twenty-four hours after the first dose, both compounds produced a significant reduction in serum PCSK9 protein levels (Figure 2a). At day 7, SPC5001 produced a PCSK9 protein reduction of 85%, a level that was maintained for the duration of the treatment period (Figure 2a). SPC4061 was less potent, producing a maximum reduction of serum PCSK9 levels of 55% when injected at the same dose level as SPC5001 (Figure 2a). As expected, the reduction in circulating PCSK9 protein levels translated into a reduction in LDL-C (Figure 2b). The reduction in LDL-C was delayed compared to the effect on serum PCSK9, as there was no observed effect on LDL-C at 24 hours postinjection (Figure 2b). From day 4 and onwards, SPC5001 treatment resulted in a reduction in LDL-C to an average of 50% below predose levels, with a 70% reduction of LDL-C in the highest responder. Consistent with its lower potency, treatment with SPC4061 resulted in an average reduction of 35% of predose levels, with a 50% reduction of LDL-C in the highest responder (Figure 2b). For both compounds, the changes in circulating LDL-C levels corresponded to reductions in circulating apoB levels, which reached a sustained reduction of ~50 and 35% at day 4 and onwards (Figure 2c). HDL-C levels were not significantly changed at any point (Figure 2d), demonstrating that the reductions in total serum cholesterol (Figure 2e) were caused by a specific reduction of LDL-C. During the entire course of the study, neither the SPC5001 nor SPC4061-treated monkeys exhibited any significant changes in serum biochemical parameters, including toxicology markers (e.g., alanine aminotransferase, aspartate aminotransferase, urea, creatinine, glucose or triglyceride, data found in Supplementary Table S1).
Figure 2.
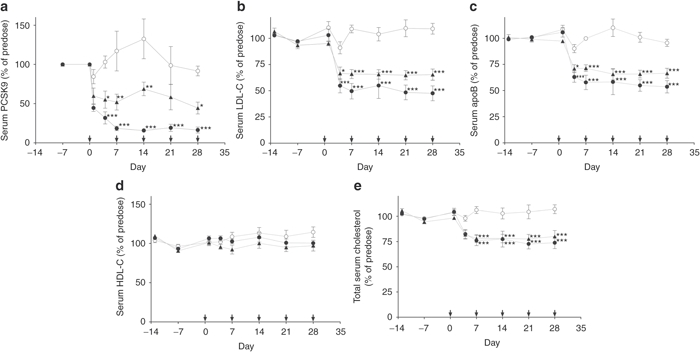
Multiple-dose study, long-term treatment. Treatment started with one subcutaneous 20 mg/kg dose, followed by a 5 mg/kg subcutaneous dose at day 7, 14, 21, and 28, as illustrated with arrows along the x-axis. Serum samples were collected days −13, −7, 1, 4, 7, 14, 21, and 28. Analyses were performed on (a) serum PCSK9, (b) LDL-C, (c) serum apoB, (d) HDL-C, and (e) total serum cholesterol. Data represent means ± SEM, n = 5, for saline control monkeys (white circles) and monkeys treated with SPC4061 (black triangles) and SPC5001 (black circles). *P < 0.05, **P < 0.005, ***P < 0.001, statistical analysis: one-way ANOVA with Bonferroni post-test. Note: There is only one predose value for serum PCSK9 and this is replotted at day 0 for illustrative purposes. ANOVA, analysis of variance; apoB, apolipoprotein B; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Three monkeys per group were killed 48 hours after the last injection of either oligonucleotide (day 30). Tissues were analyzed for oligonucleotide content, hepatic PCSK9, HMG-CoA reductase and LDLR mRNA levels, and histopathology. Although liver oligonucleotide content was similar (48 ± 9 and 58 ± 17 µg oligonucleotide/g liver for SPC4061 and SPC5001, respectively), reduction of PCSK9 mRNA expression compared to saline controls was 85% for SPC5001, but only 50% for SPC4061 (Figure 3a). The effect on circulating PCSK9 protein levels corresponded to the effect on hepatic PCSK9 mRNA expression, demonstrating an 85 and 50% reduction for SPC5001 and SPC4061, respectively (Figure 3a). Liver LDLR mRNA levels were not significantly affected by anti-PCSK9 treatment (Supplementary Table S2). Liver LDLR protein levels, analyzed by western blot, increased with 67% in SPC5001-treated monkeys compared to saline controls, whereas SPC4061 had no significant effect on liver LDLR protein levels (Supplementary Figure S3a,b). Livers were analyzed for cholesterol content to examine if the increased uptake of cholesterol-containing LDL-particles resulted in cholesterol accumulation. Neither of the oligonucleotide treatments led to cholesterol accumulation in the liver (Figure 3b); cholesterol content was lower in livers from treated monkeys, in particular in livers from monkeys treated with SPC4061. The reduced cholesterol content could not be explained by altered expression of HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis. Liver HMG-CoA reductase expression was not significantly affected in treated compared to control monkeys (Supplementary Table S2). Histopathology analysis of the livers and kidneys did not detect any degenerative changes in tissues from treated animals (data not shown).
Figure 3.
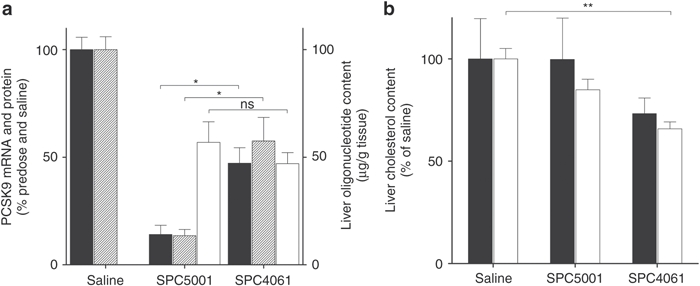
Multiple-dose study, killing. Treatment started with one subcutaneous 20 mg/kg dose, followed by a 5 mg/kg subcutaneous dose at day 7, 14, 21, and 28. Monkeys were killed 48 hours after last dose and liver was analyzed for (a) PCSK9 liver mRNA (black bars) and serum PCSK9 protein (striped bars) as percent of saline, as well as absolute liver oligonucleotide content (white bars), and (b) liver content of cholesterol esters (black bars) and free cholesterol (white bars). Data represent means ± SEM, n = 3. ns, not significant. *P < 0.05, **P < 0.005, ***P < 0.001, statistical analysis: one-way ANOVA with Bonferroni post-test. ANOVA, analysis of variance; mRNA, messenger RNA; ns, not significant.
Multiple-dose study, recovery
After the final injections (day 28), two monkeys from each group were monitored for a recovery period of 8 weeks before final killing at day 84. During this period, circulating LDL-C was analyzed weekly (day 35, 42, 49, and 56) or biweekly (day 70 and 84). In monkeys treated with SPC5001, LDL-C levels increased gradually to 65% of the predose levels during the first 4 weeks of the recovery period (Figure 4). For monkeys treated with SPC4061, LDL-C levels returned to predose levels by the end of the recovery period whereas they were still lower compared to predose levels in monkeys treated with SPC5001 (Figure 4). Pharmacokinetic analysis of the plasma half-life for of SPC5001 in the recovery period gave an estimated half-life of at least 7 days. This is similar to the estimated tissue half-life that was found in the single-dose study. Tissue analysis after termination of the recovery period demonstrated liver PCSK9 mRNA expression at predose levels and no histological changes in kidneys or livers of the treated monkeys (data not shown).
Figure 4.
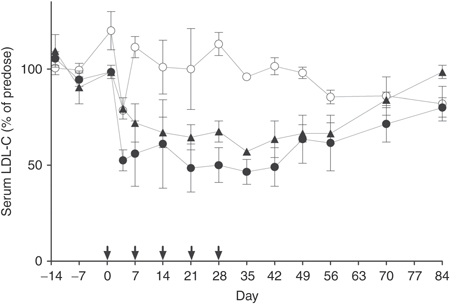
Multiple-dose study, recovery. Treatment started with one subcutaneous 20 mg/kg dose, followed by a 5 mg/kg subcutaneous dose at day 7, 14, 21, and 28, as illustrated with arrows along the x-axis. For two monkeys per group, serum samples were collected throughout treatment (days −13, −7, 1, 4, 7, 14, 21, and 28) and during an 8-week recovery period (days 35, 42, 49, 56, 77, and 84). Serum LDL-C was analyzed for saline control monkeys (white circles) and monkeys treated with SPC4061 (black triangles) and SPC5001 (black circles). Data represent means with error bars displaying maximum and minimum value, n = 2. LDL-C, low-density lipoprotein cholesterol.
Discussion
Hepatic PCSK9 mRNA expression was inhibited in Macaca fascicularis (cynomolgus monkey) by two LNA antisense oligonucleotides with two or more LNAs at both ends flanking a DNA sequence, a molecular design with high serum stability and affinity for target mRNA.21 SPC4061 and SPC5001 are a total of 13 and 14 nucleotides in length, respectively, an LNA antisense oligonucleotide size which previously been shown to be highly potent in vivo, in both rodents and nonhuman primates.22 SPC4061, which targets a sequence in PCSK9 that is conserved from mice to man, has recently been reported to reduce PCSK9 mRNA/protein expression and hepatic LDLR levels in mice.15 In cultured human cells, SPC5001 (which target a sequence in man and monkeys that is not conserved in mice), demonstrated equal potency to SPC4061 but the compound had better and longer lasting in vivo potency as measured by different parameters in nonhuman primates.
The single-dose experiment (10 mg/kg) suggests that maximal reduction of LDL-C is obtained after maximal concentration of SPC5001 is reached in the liver. For both compounds, maximal reduction of PCSK9 protein in serum also preceded the maximal pharmacological effects on LDL-C. A similar effect has been observed with anti-PCSK9 antibodies where the maximal effect on LDL-C was not reached until day 10, despite free serum PCSK9 being rapidly depleted by the monoclonal antibodies.17,18 Comparing the two LNA antisense oligonucleotides, SPC4061 and SPC5001 exhibited the same onset of effect rate but SPC5001 exhibited a higher pharmacological activity already after the first dose. At killing, serum PCSK9 protein and liver PCSK9 mRNA were more reduced by SPC5001 than by SPC4061 despite similar liver oligonucleotide content, demonstrating that SPC5001 had almost twice the activity/µg compound compared to SPC4061. A single dose of SPC5001 (10 mg/kg) reduced LDL-C to 50% of predose levels, an effect that lasted at least 3 weeks. A similar effect has been reported for a single dose of LPN-formulated anti-PCSK9 siRNA (5 mg/kg), where LDL-C was reduced with 40–60% for up to 14 days, and had returned to 80% of baseline level at day 21.16
The LNA antisense oligonucleotides produced only a modest (for SPC5001) or no significant (for SPC4061) increase in total liver LDLR protein at termination of treatment. LDLR protein levels in whole liver homogenate may not fully represent the amount of LDLR activity on the hepatocyte cell surface, and the increase in LDLR in livers of treated monkeys was sufficient to significantly reduce LDL-C. However, it should be noted that the effect of anti-PCSK9 oligonucleotide treatment on liver LDLR levels was small compared to the effect reported in a previous study with SPC4061 in mice, where liver LDLR protein levels were three times higher in treated than in control mice.15
Multiple doses of both SPC4061 and SPC5001 reduced LDL-C levels with no associated HDL-C reduction, i.e., the decrease in TC corresponded to a specific reduction in LDL-C. The same degree of lipoprotein class specificity has been observed with lipid-formulated siRNA targeting PCSK9 mRNA,16 whereas it has been reported that an anti-PCSK9 monoclonal antibody induce a significant reduction in HDL-C.17 Anti-PCSK9-induced reduction of serum LDL-C is caused by increased LDLR-mediated LDL uptake, which may in theory lead to increased accumulation of tissue cholesterol, particularly in the liver. However, we were not able to detect increased cholesterol content in the livers of the treated monkeys. A possible explanation for this could be that cholesterol synthesis was reduced as a consequence of the increased liver uptake of LDL-C, even though there was no significant effect on mRNA expression of HMG-CoA reductase (rate-limiting enzyme for cholesterol synthesis). An alternative possibility is that bile excretion was enhanced to prevent cholesterol accumulation.
In humans, serum PCSK9 levels have a significant linear correlation with serum glucose.23,24 However, it has been reported that PCSK9 knock-out mice, at 4 months of age, are hyperglycemic and glucose intolerant.25 This suggests that both absence and high levels of serum PCSK9 are associated with high serum glucose levels. Serum glucose levels in LNA antisense-treated monkeys were monitored throughout the treatment period, and there were no detectable changes in blood glucose levels even with the 85% reduction in serum PCSK9 found in animals treated with SPC5001. It may be that total elimination of PCSK9, as is the case in knock-out mice,25 is necessary for the reported glucose intolerance.
In the present study, we have shown safety and pharmacological proof-of-concept in nonhuman primates (cynomolgus monkeys) of LDL-C reduction by LNA antisense oligonucleotides targeting PCSK9 mRNA. The cholesterol-lowering effect is LDL-C specific. The most potent compound, SPC5001, produced an 85% reduction in both PCSK9 liver mRNA and serum protein levels which correlated with a long-lasting 50% reduction in LDL-C. Taken together, anti-PCSK9 LNA antisense oligonucleotides offer the appropriate preclinical properties for becoming attractive LDL-C lowering drugs alone, or in combination with current therapies (i.e., statins).
Materials and Methods
Animals and conditions. The nonhuman primate study was conducted by a certified contract organization (AAALAC accredited and approved by the National Ministry of Agriculture) in accordance with the testing facility's standard operating procedure. The study was performed as non-GLP in treatment-naive male cynomolgus monkeys, where standard operating procedures were in accordance with OECD Good Laboratory Practice Regulations. The study complied with ICH guideline M3 (R2), 11th June 2009 and ICH guideline S3A, 27th October 1994. The monkey age at initiation of treatment was 2–3 years, and body weights were between 1.8–3.5 kg. The monkeys were subjected to a 12-hour light/dark cycle. They received an expanded complete commercial primate diet (special diet services: OWN (E) short SQC, 100 g/monkey/day), in addition, they received a fruit or vegetable a day. Water was accessed ad libitum.
Study layout. Pretest samples from all monkeys were collected 13 and 7 days before the first administration of study compounds (day −13 and −7). The monkeys were fasted overnight before sampling for clinical laboratory determinants and necropsy. Blood samples were collected from the femoral veins. Study compounds were formulated in sterile physiological saline (0.9% NaCl) and injected subcutaneously. The monkeys were divided into four groups. Monkeys in the first group (n = 6), used for the pharmacokinetic study, received a single 10 mg/kg subcutaneous dose of SPC5001. One individual monkey was thereafter killed at different time points after injection. The additional three groups (n = 5 per group) received multiple doses of vehicle (saline, control group) or study compound SPC5001 or SPC4061. Each study compound was delivered as an initial 20 mg/kg subcutaneous loading dose, followed by weekly 5 mg/kg subcutaneous maintenance doses over 4 weeks, or five administrations in total (at days 0, 7, 14, 21, and 28). Weekly blood samples were drawn before each subsequent dose. Three monkeys from each multiple dosing group were killed 48 hours after the last dose (day 30). The additional two monkeys per group were left treatment-free for an additional 8 weeks and were killed at day 84. Liver and kidney tissue from all monkeys was collected in RNAlater (Ambion, Austin, TX) or snap frozen in liquid nitrogen.
Oligonucleotides design and synthesis. The study compounds SPC5001 and SPC4061 are complementary to human (accession #NM174936) and Macaca fascicularis (cynomolgus monkey) PCSK9 mRNA, and have the following sequences: TGCtacaaaacCCA and GTctgtggaaGCG (uppercase LNA, lowercase DNA), respectively. SPC3088, used for comparison in the in vitro experiments, have the sequence CGTcagtatgcgAATc. SPC3088 is not complementary to PCSK9 mRNA in mouse, monkey, or man, and was used as unspecific control. All oligonucleotides contained phosphorothioate internucleoside linkages. The oligonucleotides were synthesized using standard phosphoramidite protocols on an ÄKTA Oligopilot (GE Healthcare, Brondby, Denmark) at 130 µmol–8 mmol scales employing custom-made polystyrene primer supports. The DNA monomers were obtained from Proligo (Sigma-Aldrich, St Louis, MO) and the LNA monomers and solid support were produced by Santaris Pharma A/S (commercially available from Exiqon, Vedbaek, Denmark). After synthesis, the oligonucleotides were cleaved from the support using aqueous ammonia at 65 °C overnight. The oligonucleotides were purified by ion-exchange and desalted using a Millipore-membrane and were finally characterized by liquid chromatography-mass spectrometry (reverse phase and electrospray ionization-mass spectrometry).
Serum biochemical analysis. All serum biochemical parameters were analyzed on an Olympus AU 640 fully automated analyzer (Olympus, Hamburg, Germany). The reagents sets used were the following: TC (Olympus ref: OSR 6116), HDL cholesterol (Randox ref: CH 2652), LDL cholesterol (Randox ref: CH 2657), apoB (Randox ref: LP2117), glucose (Olympus ref: OSR 6121), urea (Olympus ref: OSR 6134), triglycerides (Olympus ref: OSR 61118), creatinine (Olympus ref: OSR 6178), aspartate aminotransferase (Olympus ref: OSR 6109), and alanine aminotransferase (Olympus ref: OSR 6107).
Histopathology. Histopathology examinations were performed for liver and kidneys on site by the contract organization performing the study. Analyses were performed in accordance with the testing facility's standard operating procedure. The tissue samples were fixed and preserved in 10% neutral formalin. Slides were stained with hematoxylin and eosin.
Tissue quantitative PCR analysis. Total RNA was extracted from liver tissue homogenates using RNeasy mini kit (Qiagen, Valencia, CA) spin columns according to the manufacturer's instructions. mRNA quantification of selected genes was carried out using commercially available TaqMan assays (Applied Biosystems, Foster City, CA). First strand synthesis of complementary DNA was generated from total RNA by a reverse transcription reaction using random decamers, 0.5 µg total RNA, and the M-MLV RT enzyme (Ambion) according to manufacturer's instructions. An Applied Biosystems 7500 Fast Real-Time PCR instrument was used for amplification. Data were analyzed and quantified using the 7500 Fast SDS software (Applied Biosystems, Naerum, Denmark). PCSK9 mRNA levels were normalized to β-actin and presented relative to saline controls as specified in the figures.
Serum PCSK9 ELISA. Serum PCSK9 protein content was analyzed with CircuLex human PCSK9 ELISA Kits in accordance with the manufacturer's instructions (MBL, Woburn, MA). The assay was validated with serum from control monkeys (Macaca fascicularis) before commencing analyses.
Tissue oligonucleotide content. Oligonucleotide content in tissue was analyzed as previously described.22 In brief, snap frozen tissue samples were homogenized in a 25 mmol/l Tris buffer (pH 8.0) containing 0.5% Igepal CA-630 and 1 mg/ml of proteinase K (Sigma-Aldrich). Control samples were spiked with respective oligonucleotide at 5–250 µg/g tissue for use as standard curves. Oligonucleotides were extracted with phenol-chloroform-isoamyl alcohol (25:24:1) saturated with 10 mmol/l Tris (pH 8.0). Oligonucleotide content was determined with ELISA specific for either compound22; streptavidin-coated strips were incubated with a biotinylated capture probe (a 6-mer LNA phosphorodiester oligonucleotide complementary to the 5′ end of SPC4061 and a 7-mer complementary to the 5′ end of SPC5001, respectively). Wells were washed and aspired. Oligonucleotide extracts were diluted to picomolar range, added to respective strip, and incubated for 0.5 hours. Wells were washed and aspired, and a 5′-digitoxin conjugated (Dig) detection probe was added. The Dig probes were 7-mer LNA phosphorodiester oligonucleotides complementary to the 3′ end of SPC4061 or SPC5001, respectively. Wells were incubated with respective Dig probe for 1 hour, washed, aspired, and incubated for 1 hour with anti-Dig POD Fab fragments diluted 1:4,000 (Roche Applied Science, Indianapolis, IN). Wells were washed and aspired and signal was developed with TMB+Substrate-Chromogen (Dako, Glostrup, Denmark) before addition of stop solution. Signal intensity was analyzed by spectrophotometer at 450 nm. Additional analyses are described in Supplementary Materials and Methods.
Statistical analysis. Statistical analysis was performed using one-way analysis of variance with Bonferroni's multiple comparison test as post-test to determine the exact nature of the differences if the data followed a Gaussian distribution. Otherwise, the nonparametric Kruskal–Wallis test with Dunn's multiple comparison test was used as a post-test. P values of <0.05 were considered to be statistically significant. The Provantis data acquisition system was used to analyze the primate data. The primate pharmacodynamic data (LDL-C and oligonucleotide concentration in liver) were analyzed using an inhibitory effect Emax model (model 103) in the WinNonLin program (version 5.2.1; Pharsight, Munich, Germany).
SUPPLEMENTARY MATERIAL Figure S1. PCSK9 mRNA levels in HepG2 cells after 5 days of gymnotic delivery of LNA oligonucleotides at 0.4, 2.0, and 10 µmol/l concentrations. Figure S2. PCSK9 mRNA levels in HepG2 cells 24 hours after transfection with LNA oligonucleotides at 0.4, 2.0, and 10 nmol/l concentrations. Figure S3. Liver LDLR protein levels. Table S1. Biochemical parameters in nonhuman primate study. Table S2. qPCR analysis of liver samples. Materials and Methods.
Acknowledgments
The authors wish to thank Helle Knudsen for skillful technical assistance. All authors are employees of Santaris Pharma A/S. All the presented work was designed, conducted and/or sponsored by Santaris Pharma A/S. The LNA oligonucleotide SPC5001 has entered the stage of clinical testing.
Supplementary Material
PCSK9 mRNA levels in HepG2 cells after 5 days of gymnotic delivery of LNA oligonucleotides at 0.4, 2.0, and 10 µmol/l concentrations.
PCSK9 mRNA levels in HepG2 cells 24 hours after transfection with LNA oligonucleotides at 0.4, 2.0, and 10 nmol/l concentrations.
Liver LDLR protein levels.
Biochemical parameters in nonhuman primate study.
qPCR analysis of liver samples.
REFERENCES
- Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S.et al. (2003The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation Proc Natl Acad Sci USA 100928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M.et al. (2003Mutations in PCSK9 cause autosomal dominant hypercholesterolemia Nat Genet 34154–156. [DOI] [PubMed] [Google Scholar]
- Horton JD, Cohen JC., and, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci. 2007;32:71–77. doi: 10.1016/j.tibs.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD.et al. (2007Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation J Biol Chem 28218602–18612. [DOI] [PubMed] [Google Scholar]
- Cariou B, Le May C., and, Costet P. Clinical aspects of PCSK9. Atherosclerosis. 2011;216:258–265. doi: 10.1016/j.atherosclerosis.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Abifadel M, Rabès JP, Devillers M, Munnich A, Erlich D, Junien C.et al. (2009Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease Hum Mutat 30520–529. [DOI] [PubMed] [Google Scholar]
- Horton JD, Cohen JC., and, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50 ( suppl.:S172–S177. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotowski IK, Pertsemlidis A, Luke A, Cooper RS, Vega GL, Cohen JC.et al. (2006A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol Am J Hum Genet 78410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The global burden of disease: 2004 update . < http://www.who.int/healthinfo/global_ burden_disease/2004_report_update/en/.2008 >.
- Abifadel M, Rabès JP, Jambart S, Halaby G, Gannagé-Yared MH, Sarkis A.et al. (2009The molecular basis of familial hypercholesterolemia in Lebanon: spectrum of LDLR mutations and role of PCSK9 as a modifier gene Hum Mutat 30E682–E691. [DOI] [PubMed] [Google Scholar]
- Peterson AS, Fong LG., and, Young SG. PCSK9 function and physiology. J Lipid Res. 2008;49:1152–1156. doi: 10.1194/jlr.E800008-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD.et al. (2006Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote Am J Hum Genet 79514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G., and, Konrad RJ. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res. 2010;51:2714–2721. doi: 10.1194/jlr.M008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MJ, Lemonidis KM, Whipple CP, Subramaniam A, Monia BP, Crooke ST.et al. (2007Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice J Lipid Res 48763–767. [DOI] [PubMed] [Google Scholar]
- Gupta N, Fisker N, Asselin MC, Lindholm M, Rosenbohm C, Ørum H.et al. (2010A locked nucleic acid antisense oligonucleotide (LNA) silences PCSK9 and enhances LDLR expression in vitro and in vivo PLoS ONE 5e10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A.et al. (2008Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates Proc Natl Acad Sci USA 10511915–11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JC, Piper DE, Cao Q, Liu D, King C, Wang W.et al. (2009A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates Proc Natl Acad Sci USA 1069820–9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni YG, Condra JH, Orsatti L, Shen X, Di Marco S, Pandit S.et al. (2010A proprotein convertase subtilisin-like/kexin type 9 (PCSK9) C-terminal domain antibody antigen-binding fragment inhibits PCSK9 internalization and restores low density lipoprotein uptake J Biol Chem 28512882–12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni YG, Di Marco S, Condra JH, Peterson LB, Wang W, Wang F.et al. (2011A PCSK9-binding antibody that structurally mimics the EGF(A) domain of LDL-receptor reduces LDL cholesterol in vivo J Lipid Res 5278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer-Smith H., and, Basak A. Regulatory effects of peptides from the pro and catalytic domains of proprotein convertase subtilisin/kexin 9 (PCSK9) on low-density lipoprotein receptor (LDL-R) Curr Med Chem. 2010;17:2168–2182. doi: 10.2174/092986710791299948. [DOI] [PubMed] [Google Scholar]
- Hansen JB, Fisker N, Westergaard M, Kjaerulff LS, Hansen HF, Thrue CA.et al. (2008SPC3042: a proapoptotic survivin inhibitor Mol Cancer Ther 72736–2745. [DOI] [PubMed] [Google Scholar]
- Straarup EM, Fisker N, Hedtjärn M, Lindholm MW, Rosenbohm C, Aarup V.et al. (2010Short locked nucleic acid antisense oligonucleotides potently reduce apolipoprotein B mRNA and serum cholesterol in mice and non-human primates Nucleic Acids Res 387100–7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoski SG, Lagace TA, Cohen JC, Horton JD., and, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537–2543. doi: 10.1210/jc.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc G, Tremblay M, Paré G, Jacques H, Hamelin J, Benjannet S.et al. (2010A new method for measurement of total plasma PCSK9: clinical applications J Lipid Res 51140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbikay M, Sirois F, Mayne J, Wang GS, Chen A, Dewpura T.et al. (2010PCSK9-deficient mice exhibit impaired glucose tolerance and pancreatic islet abnormalities FEBS Lett 584701–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCSK9 mRNA levels in HepG2 cells after 5 days of gymnotic delivery of LNA oligonucleotides at 0.4, 2.0, and 10 µmol/l concentrations.
PCSK9 mRNA levels in HepG2 cells 24 hours after transfection with LNA oligonucleotides at 0.4, 2.0, and 10 nmol/l concentrations.
Liver LDLR protein levels.
Biochemical parameters in nonhuman primate study.
qPCR analysis of liver samples.


