Abstract
Ischemic heart disease (IHD) is one of the leading causes of death in Western countries. Prevention rather than treatment of heart disease can significantly improve patients’ quality of life and reduce health care costs. Flavonoids are widely distributed in vegetables, fruits and herbal medicines. Regularly consuming botanicals, especially those containing flavonoids has been associated with a reduction in cardiovascualar disease; thus, it is important to investigate how flavonoids improve cardiac resistance to heart disease and their related mechanisms of action. It has been shown that cardiomyocyte injury and death can result from ischemia-reperfusion, which is pathognomonic of ischemic heart disease. Massive reactive oxygen species (ROS) release at the onset of reperfusion produces cell injury and death. “Programming” the heart to either generate less ROS or to increase strategic ROS removal could reduce reperfusion response. Additionally, profuse nitric oxide (NO) release at reperfusion could be protective in “preconditioning” models. Botanical flavonoids induce preconditioning of the heart, thereby protecting against ischemia-reperfusion injury. In this article, we will discuss two herbs containing potent flavonoids, Scutellaria baicalensis and grape seed proanthocyanidin, which can potentially offer cardiac protection against ischemic heart disease.
Keywords: Cardiovascular Disease, Ischemic Heart Disease, Ischemia-Reperfusion Injury, Reactive Oxygen Species, Nitric Oxide, Flavonoids, Scutellaria baicalensis, Grape Seed Proanthocyanidin
Introduction
Cardiac injury following ischemia-reperfusion (I-R), clinically known as ischemic heart disease (IHD) or coronary artery disease, is one of the leading causes of death in the United States. The high mortality results from the massive myocardial injury that occurs when the ischemic heart is re-oxygenated, called reperfusion. Thus, preventing the reperfusion oxidant injury has been identified as the fundamental strategy for reducing the morbidity of acute cardiac injury (Ostadal, 2009).
A significant reduction in coronary artery disease incidence has been linked the consumption of herbal flavonoids (Keli et al., 1996; Arts et al., 2001; Mann et al., 2007), suggesting that the flavonoids may enhance tolerance to cardiac I-R injury. Flavonoids, which possess significant antioxident potential, are widely distributed in edible vegetables, fruits and many herbal medicines (Chao et al., 2009; Huang et al., 2009; Yook et al., 2010). Flavonoids’ protective effects against ischemic heart disease is based on several clinical studies that positively correlate flavonoid intake to a reduced incidence of the disease. Consuming flavonoids like catechin, which is present in plant seeds and teas, resulted in a 20% reduction in the incidence of the disease (Arts et al., 2001). A meta-analysis of prospective cohort studies concluded that high flavonoid intake from fruits, vegetables, tea, and red wine is associated with a reduced risk of ischemic heart disease (Huxley and Neil, 2003; Li et al., 2010). In a study of approximately 5,000 subjects, the intake of dietary flavonoids and tea was inversely associated with myocardial infarction (Geleijnse et al., 2002). It seems likely that flavonoid-containing botanicals have remarkable potential in protecting cardiac injury.
Scutellaria baicalensis extract (SbE) and grape seed proanthocyanidin extract (GSPE) contain flavonoid components which have been extensively tested for their antioxidant activity (Heim et al., 2002; Tong et al., 2009; Chan et al., 2010). In this article, after an introduction of the related background information, the role of SbE and GSPE in improving cardiac reserve against reperfusion injury will be reviewed.
Pathophysiology of Ischemia-Reperfusion Injury
By definition, reperfusion is the reestablisment of normoxic conditions after a period of hypoxia or ischemia. The hallmark of reperfusion is a significant burst of reactive oxygen species (ROS) which results in oxidant injury to myocardial tissue. Thus, interventions that reduce the injury could positively impact recovery and survival. The simplistic approach of administering antioxidants to reduce ROS-induced injury, however, has not always yielded beneficial results (Violi et al., 2004). The protective potential of an antioxidant depends on the scavenging of specific ROS species and its access to strategic intracellular sites (Becker, 2004; Yu et al., 2009). Alternatively, “programming” the heart to either generate less ROS or to increase strategic ROS removal by endogenous mechanisms could reduce reperfusion response.
During I/R, toxic ROS are released from within the cardiomyocytes and other locations (Zweier et al., 1987). While the non-cardiomyocyte sources, such as neutrophils, are equally important in producing oxidant injury, ROS generated within the cardiomyocytes probably inflict rapid and severe cell damage as evidenced in a cardiomyocyte model of simulated I/R and mitochondrial inhibition (Vanden Hoek et al., 2000; Yin et al., 2010). Conditions associated with oxygen deprivation such as ischemia and hypoxia predominantly result in mitochondrial ROS generation (Duranteau et al., 1998). With I/R, both mitochondrial and cytosolic sources release toxic ROS (Mohazzab et al., 1997).
Nitric oxide (NO) is an important trigger as well as mediator of delayed preconditioning. NO is released during ischemia and at the beginning of reperfusion by activation of the NOS enzyme, particularly cNOS. The effect of NO release on the outcome of I-R injury appears to depend on various factors such as species and study design, and ranges from beneficial to harmful (Schulz et al., 2004). Although increasing NO concentrations has demonstrated benefit, a simultaneous presence of excess ROS could result in the formation of peroxynitrite, a highly injurious reactive species. Thus, for NO to be beneficial, ROS levels during reperfusion need to be attenuated (Xie et al., 1998).
The protective effect of “preconditioning” the heart against I-R injury has been demonstrated in cardiomyocyte and animal models, and pharmacological agents can perform this preconditioning. The preconditioning effect can be seen in two windows: the early window is within minutes to 4 hours after the induction phase is initiated by receptor-ligand interaction while the delayed window is observed from 12 hours to 72 hours and requires de novo protein synthesis (Baxter and Ferdinandy, 2001). The delayed pharmacological preconditioning is a more promising phenomenon with considerable therapeutic potential. Clinical applicability of the preconditioning mechanism is profound given the significant statistics of mortality, morbidity and the associated health care expenses of IHD. Since ischemic preconditioning has demonstrated significant delayed preconditioning, studying the related cellular and molecular pathways evoked in these models would allow the definition of the characteristics of the protected prototype. Pharmacological agents, including herbal antioxidants, that activate similar mechanisms could be a more clinically practical method to inducing preconditioning. Scutellaria baicalensis extract (SbE) and grape seed proanthocyanidin extract (GSPE) contain flavonoid components which have been extensively tested for antioxidant activity (Heim et al., 2002; Shao et al., 2003a; Mehendale et al., 2007). The flavonoids from SbE and GSPE possess stimulating mechanisms that are protective against I-R injury during IHD.
Scutellaria baicalensis Extract (SbE)
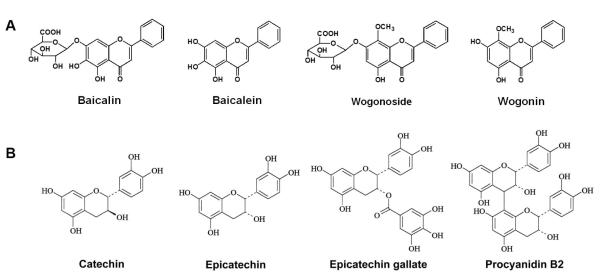
Scutellaria baicalensis Georgi (Labiatae) is widely used in the traditional medical systems of China and Japan for inflammatory diseases, hyperlipemia and arteriosclerosis (Gasiorowski et al., 2011). The major constituents of SbE are flavonoids like baicalin, baicalein, wogonoside and wogonin (Fig. 1A).
Figure 1.
Flavonoids in Scutellaria baicalensis extract (SbE) (A) and grape seed proanthocyanidin extract (GSPE) (B).
Flavonoids in SbE are effective scavengers of hydroxyl and peroxyl radicals and superoxide anions (Jovanovic et al., 1998). An example of the antioxidant reaction of these components is the scavenging of hydrated electrons (eaq− radicals) by baicalin. Eaq- radicals are formed when biological molecules are exposed to ultraviolet light and ionizing radiation such as ion beams or gamma-rays. Thus the eaq− scavenging abilities of flavonoids should be considered in the diet (Cai et al., 1999).
Using in vitro and in vivo models, the antioxidant effects have been demonstrated in both the extract and its active flavonoids (Yune et al., 2009). Hamada et al. investigated in vitro radical scavenging activities of baicalein and quantified superoxide and hydroxyl radicals by electron spin resonance spectrometry (Hamada et al., 1993). They reported that in a hypoxanthine-xanthine system, baicalein strongly reduced superoxide radicals. Electron paramagnetic resonance data showed that baicalin and baicalein scavenged hydroxyl radicals, DPPH radicals and alkyl radicals dose-dependently; wogonin and wogonoside had no effect on these radicals. This result suggests the need to study the actions of individual constituents of an extract to define the most effective constituent. When rats were pretreated with either oral or intraperitoneal SbE or its constituents, lipid peroxidation, a marker of oxidant injury, was attenuated, suggesting antioxidant protection (Gao et al., 1995).
It is believed that the antioxidant action of SbE is mediated by direct ROS scavenging, to which baicalein and wogonin contribute significantly (Bochorakova et al., 2003). Direct intracellular antioxidant activity has been shown from experiments in our group. SbE and baicalein attenuated oxidation of intracellular fluorescent probes in chick cardiomyocytes exposed to I/R (Shao et al., 2002). A rapid antioxidant protection by baicalein in the cardiomyocyte model was observed. As this system is devoid of other sources of ROS such as neutrophils or endothelial cells, the reduction of fluorescence clearly indicates rapid intracellular scavenging by baicalein. The flavonoid structure and a low-molecular weight endow such molecules with intracellular antioxidant properties.
When rats were pretreated with either oral or intraperitoneal SbE or its constituents, lipid peroxidation was attenuated, suggesting antioxidant protection (Kimura, 1982; Gao et al., 1995). SbE also prevented apoptosis by increasing the anti-apoptotic protein activity, demonstrating indirect antioxidant effects (Choi et al., 2002). It has been reported that SbE flavonoids also inhibit NO production (Kim et al., 2001).
Grape Seed Proanthocyanidin Extract (GSPE)
Grapevines are classified into the genus Vitis. A single Vitis species, V. vinifera, originated in Europe and has been thoroughly studied. Approximately 34 species have been characterized in North America and Central America, whereas more than 30 species are native to China (Vivier and Pretorius, 2002). GSPE possesses a broad spectrum of therapeutic properties (Ray et al., 2001) and is a popular herbal supplement with patients suffering from cardiovascular disease. Interest in grape seed as a possible cardioprotective agent peaked following demonstration of the “French paradox,” a positive correlation between the high intake of saturated fat and increased wine consumption, but a reduced risk of IHD (Renaud and de Lorgeril, 1992). Moderate consumption of wine, particularly red wine, has been associated with a reduced mortality from IHD (Rimm et al., 1991; Das, 1999).
GSPE is a potent ROS scavenger (Bagchi et al., 1997; Sato et al., 1999). In GSPE, the main polyphenolic oligomeric and polymeric proanthocyanidins are catechin, epicatechin, epicatechin gallate and procyanidin B2 (Fig. 1B) (Gonzalez-Paramas et al., 2004). Among these proanthocyanidins, procyanidin B2 has been shown to be the most effective compound in trapping oxygen free radicals (Da Silva et al., 1991). Proanthocyanidins and catechins are also found in wine (Das, 1999).
The capacity of the constituents of GSPE to act as antioxidants depends upon their molecular structure. The position of hydroxyl groups and other features are important for their free radical scavenging activities. Phenolic antioxidants (PPH) inhibit lipid peroxidation by a rapid donation of a hydrogen atom to the peroxyl radical (ROO.) resulting in formation of alkyl (aryl) hydroperoxide (ROOH), as illustrated in the following reaction: ROO. + PPH → ROOH + PP.. The polyphenol phenoxyl radical (PP.) produced can be stabilized by further donation of a hydrogen atom and formation of quinines (Stohs, 1995); or by reacting with another radical, including another phenoxyl radical, to generate new components (Hosny and Rosazza, 2002), thereby interrupting the initiation of a new chain reaction.
We reported direct, acute antioxidant effects in cardiomyocytes exposed to H2O2 and I/R (Shao et al., 2009). The studies suggested rapid bioavailability of GSPE inside the cardiomyocytes. Long-term consumption of GSPE has demonstrated cardiovascular benefits in animal models (Sato et al., 1999). Indirect antioxidant effects such as NADPH oxidase inhibition and reduced ROS-mediated apoptosis have been shown (Fitzpatrick et al., 2002). Other observed cardiovascular benefits from GSPE include reduced thrombosis and improved blood flow (Stein et al., 1999; Wollny et al., 1999). These effects are probably caused by the stimulated NO-release by various GSPE fractions in endothelium (Fitzpatrick et al., 2002). In chick cardiomyocytes, NO release is stimulated by low concentrations of GSPE and may trigger preconditioning (our unplished data).
Comparing and Contrasting SbE and GSPE
The constituent flavonoids of these two extracts differ structurally at very specific sites (Fig. 1 A and B). Flavonoids in GSPE possess the 3′-4′ catechol structure in the B-ring, and an OH group in position 3 which makes the GSPE flavonoids extremely potent antioxidants (C-ring). While these features are absent in the SbE flavonoids, the presence of a 4-oxo and 2-3 double bond (C-ring) imparts them with antioxidant activity, in addition to a pro-oxidant activity (Heim et al., 2002).
The flavonoids are potent ROS scavengers but paradoxically are capable of producing ROS depending upon experimental conditions (Chang et al., 2009; Wang et al., 2009; Lu et al., 2010). Flavonoids in SbE, particularly baicalein, may stimulate ROS production from the mitochondria (Hodnick et al., 1994). In the presence of excess ROS, baicalein acts as a scavenger and forms stable semiquinone radicals. The stability of the radical prevents pro-oxidant toxicity except in conditions of high flavonoid concentrations for extended intervals (Heim et al., 2002). GSPE is also known to be a pro-oxidant at concentrations that are significantly higher than the commonly used dose (Shao et al., 2003b). The ROS-scavenging by flavonoids may affect ROS-dependent signaling and the biological effects will represent the sum total of these disparate properties.
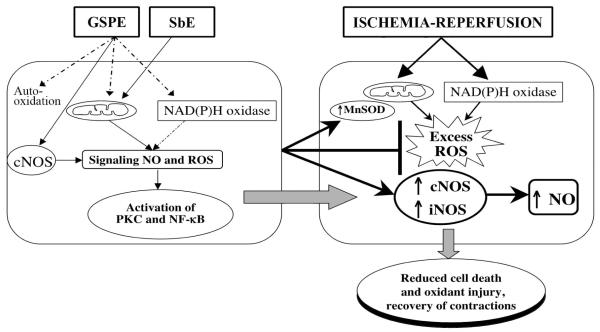
With respect to NO, flavonoids in SbE such as wogonin and baicalein, suppress its release by inhibiting NOS/guanylate cyclase (Kim et al., 2001). In normal tissue GSPE stimulates NO release via a purinergic pathway (Fitzpatrick et al., 2002; Mendes et al., 2003). GSPE has shown a reduced NO release, although only in models of inflammation, which could be an indirect effect of suppression of iNOS upregulating cytokines (Roychowdhury et al., 2001). Our unplished data suggest that cardiomyocytes show a non-toxic ROS response to SbE treatment but NO release with GSPE treatment during the induction phase. It is appears that the two extracts may induce two distinct preconditioning mechanisms (Fig. 2).
Fig. 2.
Potential mechanisms of Scutellaria baicalensis extract (SbE) and grape seed proanthocyanidin extract (GSPE) in delayed preconditioning in cardiomyocytes. NO-nitric oxide; ROS-reactive oxygen species; PKC-protein kinase C; MnSOD-manganese superoxide dismutase; cNOS-constitutive nitric oxide synthase; iNOS-inducible NOS.
The flavonoid constituents are absorbed rapidly and are bioavailable after oral consumption (Yamashita et al., 2002; Lai et al., 2003). In a cardiomyocyte model, rapid intracellular access of both baicalein and GSPE have been demonstrated as well (Shao et al., 2002). In cultured cells, the low molecular weight compounds of GSPE enter via paracellular pathways, with the more lipophilic compounds diffusing across membranes (Konishi et al., 2003). Thus, effects of the flavonoids in a cellular model should reflect those in intact animals.
Summary
Based on the compelling epidemiological data linking flavonoid use to reduced myocardial injury, the ongoing flavonoid studies should significantly advance our understanding of the cellular mechanisms involved in the protective actions of the heart. Evidence suggests that the use of herbal flavonoids has cardioprotective effects while the use of antioxidant vitamins does not reduce the incidence of IHD (Gavagan, 2002; Vivekananthan et al., 2003). Herbal flavonoids, such as those found in SbE and GSPE, may play a vital role in modulating cardiomyocyte response against reperfusion injury in a novel paradigm. Herbal extracts, by virtue of being composed of a mixture of active compounds, could act through more than one mechanism, and hence could potentially exert multiple beneficial effects. Exploration of pathophysiological mechanisms of SbE and GSPE in modulating cardiovascular function will promote safe and effective use of botanical flavonoids in the treatment and prevention of coronary heart disease.
Acknowledgements
This work was supported in part by the NIH/NCCAM grants AT003255, AT004418, and AT005362.
References
- Arts IC, Hollman PC, Feskens EJ, Bueno de Mesquita HB, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am. J. Clin. Nutr. 2001;74:227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997;95:179–189. [PubMed] [Google Scholar]
- Baxter GF, Ferdinandy P. Delayed preconditioning of myocardium: current perspectives. Basic Res. Cardiol. 2001;96:329–344. doi: 10.1007/s003950170041. [DOI] [PubMed] [Google Scholar]
- Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc. Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Bochorakova H, Paulova H, Slanina J, Musil P, Taborska E. Main flavonoids in the root of Scutellaria baicalensis cultivated in Europe and their comparative antiradical properties. Phytother. Res. 2003;17:640–644. doi: 10.1002/ptr.1216. [DOI] [PubMed] [Google Scholar]
- Cai Z, Li X, Katsumura Y. Interaction of hydrated electron with dietary flavonoids and phenolic acids: rate constants and transient spectra studied by pulse radiolysis. Free Radic. Biol. Med. 1999;27:822–829. doi: 10.1016/s0891-5849(99)00118-5. [DOI] [PubMed] [Google Scholar]
- Chan E, Wong CY, Wan CW, Kwok CY, Wu JH, Ng KM, So CH, Au AL, Poon CC, Seto SW, Kwan YW, Yu PH, Chan SW. Evaluation of anti-oxidant capacity of root of Scutellaria baicalensis Georgi, in comparison with roots of Polygonum multiflorum Thunb and Panax ginseng CA Meyer. Am. J. Chin. Med. 2010;38:815–827. doi: 10.1142/S0192415X10008263. [DOI] [PubMed] [Google Scholar]
- Chang TN, Huang GJ, Ho YL, Huang SS, Chang HY, Chang YS. Antioxidant and antiproliferative activities of Crossostephium chinensis (L.) Makino. Am. J. Chin. Med. 2009;37:797–814. doi: 10.1142/S0192415X09007259. [DOI] [PubMed] [Google Scholar]
- Chao J, Lee MS, Amagaya S, Liao JW, Wu JB, Ho LK, Peng WH. Hepatoprotective effect of shidagonglao on acute liver injury induced by carbon tetrachloride. Am. J. Chin. Med. 2009;37:1085–1097. doi: 10.1142/S0192415X0900751X. [DOI] [PubMed] [Google Scholar]
- Choi J, Conrad CC, Malakowsky CA, Talent JM, Yuan CS, Gracy RW. Flavones from Scutellaria baicalensis Georgi attenuate apoptosis and protein oxidation in neuronal cell lines. Biochim. Biophys. Acta. 2002;1571:201–210. doi: 10.1016/s0304-4165(02)00217-9. [DOI] [PubMed] [Google Scholar]
- Da Silva JMR, Darmon N, Fernandez Y, Mitjavila S. Oxygen free radical scavenger capacity in aqueous models of different procyanidins from grape seeds. J. Agric. Food Chem. 1991;39:1549–1552. [Google Scholar]
- Das DK, Sato M, Ray PS, Maulik G, Engelman RM, Bertelli AA, Bertelli A. Cardioprotection of red wine: role of polyphenolic antioxidants. Drugs Exp. Clin. Res. 1999;25:115–120. [PubMed] [Google Scholar]
- Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J. Biol. Chem. 1998;273:11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick DF, Bing B, Maggi DA, Fleming RC, O’Malley RM. Vasodilating procyanidins derived from grape seeds. Ann. N. Y. Acad. Sci. 2002;957:78–89. doi: 10.1111/j.1749-6632.2002.tb02907.x. [DOI] [PubMed] [Google Scholar]
- Gao D, Sakurai K, Chen J, Ogiso T. Protection by baicalein against ascorbic acid-induced lipid peroxidation of rat liver microsomes. Res. Commun. Mol. Pathol. Pharmacol. 1995;90:103–114. [PubMed] [Google Scholar]
- Gasiorowski K, Lamer-Zarawska E, Leszek J, Parvathaneni K, Yendluri BB, Blach-Olszewska Z, Aliev G. Flavones from root of Scutellaria baicalensis Georgi: drugs of the future in neurodegeneration? CNS Neurol. Disord. Drug Targets. 2011;10:184–191. doi: 10.2174/187152711794480384. [DOI] [PubMed] [Google Scholar]
- Gavagan T. Cardiovascular disease. Prim. Care. 2002;29:323–338. doi: 10.1016/s0095-4543(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam study. Am. J. Clin. Nutr. 2002;75:880–886. doi: 10.1093/ajcn/75.5.880. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Paramas AM, Esteban-Ruano S, Santos-Buelga C, de Pascual-Teresa S, Rivas-Gonzalo JC. Flavanol content and antioxidant activity in winery byproducts. J. Agric. Food Chem. 2004;52:234–238. doi: 10.1021/jf0348727. [DOI] [PubMed] [Google Scholar]
- Hamada H, Hiramatsu M, Edamatsu R, Mori A. Free radical scavenging action of baicalein. Arch. Biochem. Biophys. 1993;306:261–266. doi: 10.1006/abbi.1993.1509. [DOI] [PubMed] [Google Scholar]
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Hodnick WF, Duval DL, Pardini RS. Inhibition of mitochondrial respiration and cyanide- stimulated generation of reactive oxygen species by selected flavonoids. Biochem. Pharmacol. 1994;47:573–580. doi: 10.1016/0006-2952(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Hosny M, Rosazza JP. Novel oxidations of (+)-catechin by horseradish peroxidase and laccase. J. Agric. Food. Chem. 2002;50:5539–5545. doi: 10.1021/jf020503j. [DOI] [PubMed] [Google Scholar]
- Huang X, Kojima-Yuasa A, Xu S, Kennedy DO, Hasuma T, Matsui-Yuasa I. Combination of Zizyphus jujuba and green tea extracts exerts excellent cytotoxic activity in HepG2 cells via reducing the expression of APRIL. Am. J. Chin. Med. 2009;37:169–179. doi: 10.1142/S0192415X09006758. [DOI] [PubMed] [Google Scholar]
- Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2003;57:904–908. doi: 10.1038/sj.ejcn.1601624. [DOI] [PubMed] [Google Scholar]
- Jovanovic SV, Steenken S, Simic MG, Hara Y. Antioxidant properties of flavonoids: reduction potentials and electron transfer reactions of flavonoid radicals. Antioxid. Health Dis. 1998;7:137–161. [Google Scholar]
- Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch. Intern. Med. 1996;156:637–642. [PubMed] [Google Scholar]
- Kim H, Kim YS, Kim SY, Suk K. The plant flavonoid wogonin suppresses death of activated C6 rat glial cells by inhibiting nitric oxide production. Neurosci. Lett. 2001;309:67–71. doi: 10.1016/s0304-3940(01)02028-6. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Okuda H, Tani T, Arichi S. Studies on Scutellaria Radix. VI. Effects of flavanone compounds on lipid peroxidation in rat liver. Chem. Pharm. Bull. 1982;30:1792–1795. doi: 10.1248/cpb.30.1792. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Kobayashi S, Shimizu M. Tea polyphenols inhibit the transport of dietary phenolic acids mediated by the monocarboxylic acid transporter (MCT) in intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2003;51:7296–7302. doi: 10.1021/jf034894t. [DOI] [PubMed] [Google Scholar]
- Lai MY, Hsiu SL, Tsai SY, Hou YC, Chao PD. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J. Pharm. Pharmacol. 2003;55:205–209. doi: 10.1211/002235702522. [DOI] [PubMed] [Google Scholar]
- Li J, Liu H, Ramachandran S, Waypa GB, Yin JJ, Li CQ, Han M, Huang HH, Sillard WW, Vanden Hoek TL, Shao ZH. Grape seed proanthocyanidins ameliorate Doxorubicin-induced cardiotoxicity. Am. J. Chin. Med. 2010;38:569–584. doi: 10.1142/S0192415X10008068. [DOI] [PubMed] [Google Scholar]
- Lu YX, Zhang Q, Li J, Sun YX, Wang LY, Cheng WM, Hu XY. Antidiabetic effects of total flavonoids from Litsea Coreana leve on fat-fed, streptozotocin-induced type 2 diabetic rats. Am. J. Chin. Med. 2010;38:713–725. doi: 10.1142/S0192415X10008184. [DOI] [PubMed] [Google Scholar]
- Mann GE, Rowlands DJ, Li FY, de Winter P, Siow RC. Activation of endothelial nitric oxide synthase by dietary isoflavones: role of NO in Nrf2-mediated antioxidant gene expression. Cardiovasc. Res. 2007;75:261–274. doi: 10.1016/j.cardiores.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Mehendale S, Aung H, Wang CZ, Tong R, Foo A, Xie JT, Yuan CS. Scutellaria baicalensis and a constituent flavonoid, baicalein, attenuate ritonavir-induced gastrointestinal side-effects. J. Pharm. Pharmacol. 2007;59:1567–1572. doi: 10.1211/jpp.59.11.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes A, Desgranges C, Cheze C, Vercauteren J, Freslon JL. Vasorelaxant effects of grape polyphenols in rat isolated aorta. Possible involvement of a purinergic pathway. Fundam. Clin. Pharmacol. 2003;17:673–681. doi: 10.1046/j.1472-8206.2003.00198.x. [DOI] [PubMed] [Google Scholar]
- Mohazzab HK, Kaminski PM, Wolin MS. Lactate and PO2 modulate superoxide anion production in bovine cardiac myocytes: potential role of NADH oxidase. Circulation. 1997;96:614–620. doi: 10.1161/01.cir.96.2.614. [DOI] [PubMed] [Google Scholar]
- Ostadal B. The past, the present and the future of experimental research on myocardial ischemia and protection. Pharmacol. Rep. 2009;61:3–12. doi: 10.1016/s1734-1140(09)70002-7. [DOI] [PubMed] [Google Scholar]
- Ray SD, Parikh H, Hickey E, Bagchi M, Bagchi D. Differential effects of IH636 grape seed proanthocyanidin extract and a DNA repair modulator 4-aminobenzamide on liver microsomal cytochrome 4502E1-dependent aniline hydroxylation. Mol. Cell Biochem. 2001;218:27–33. doi: 10.1023/a:1007272611915. [DOI] [PubMed] [Google Scholar]
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- Roychowdhury S, Wolf G, Keilhoff G, Bagchi D, Horn T. Protection of primary glial cells by grape seed proanthocyanidin extract against nitrosative/oxidative stress. Nitric. Oxide. 2001;5:137–149. doi: 10.1006/niox.2001.0335. [DOI] [PubMed] [Google Scholar]
- Sato M, Maulik G, Ray PS, Bagchi D, Das DK. Cardioprotective effects of grape seed proanthocyanidin against ischemic reperfusion injury. J. Mol. Cell. Cardiol. 1999;31:1289–1297. doi: 10.1006/jmcc.1999.0961. [DOI] [PubMed] [Google Scholar]
- Schulz R, Kelm M, Heusch G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004;61:402–413. doi: 10.1016/j.cardiores.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Becker LB, Vanden Hoek TL, Schumacker PT, Li CQ, Zhao D, Wojcik K, Anderson T, Qin Y, Dey L, Yuan CS. Grape seed proanthocyanidin extract attenuates oxidant injury in cardiomyocytes. Pharmacol. Res. 2003a;47:463–469. doi: 10.1016/s1043-6618(03)00041-0. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Vanden Hoek TL, Qin Y, Becker LB, Schumacker PT, Li CQ, Dey L, Barth E, Halpern H, Rosen GM, Yuan CS. Baicalein attenuates oxidant stress in cardiomyocytes. Am. J. Physiol. Heart. Circ. Physiol. 2002;282:999–1006. doi: 10.1152/ajpheart.00163.2001. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Vanden Hoek TL, Xie J, Wojcik K, Chan KC, Li CQ, Hamann K, Qin Y, Schumacker PT, Becker LB, Yuan CS. Grape seed proanthocyanidins induce pro-oxidant toxicity in cardiomyocytes. Cardiovasc. Toxicol. 2003b;3:331–339. doi: 10.1385/ct:3:4:331. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Wojcik KR, Dossumbekova A, Hsu C, Mehendale SR, Li CQ, Qin Y, Sharp WW, Chang WT, Hamann KJ, Yuan CS, Hoek TL. Grape seed proanthocyanidins protect cardiomyocytes from ischemia and reperfusion injury via Akt-NOS signaling. J. Cell Biochem. 2009;107:697–705. doi: 10.1002/jcb.22170. [DOI] [PubMed] [Google Scholar]
- Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999;100:1050–1055. doi: 10.1161/01.cir.100.10.1050. [DOI] [PubMed] [Google Scholar]
- Stohs SJ. The role of free radicals in toxicity and disease. J. Basic Clin. Physiol. Pharmacol. 1995;6:205–228. doi: 10.1515/jbcpp.1995.6.3-4.205. [DOI] [PubMed] [Google Scholar]
- Tong R, Mehendale SR, Wang CZ, Shao Z, Yuan CS. Comparison of antioxidant effects of various Scutellaria baicalensis fractions and the potential role of catalase upregulation. Am. J. Chin. Med. 2009;37:621–623. doi: 10.1142/S0192415X09007107. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek T, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ. Res. 2000;86:541–548. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- Violi F, Cangemi R, Sabatino G, Pignatelli P. Vitamin E for the treatment of cardiovascular disease: is there a future? Ann. N. Y. Acad. Sci. 2004;1031:292–304. doi: 10.1196/annals.1331.029. [DOI] [PubMed] [Google Scholar]
- Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- Vivier MA, Pretorius IS. Genetically tailored grapevines for the wine industry. Trends Biotechnol. 2002;20:472–478. doi: 10.1016/s0167-7799(02)02058-9. [DOI] [PubMed] [Google Scholar]
- Wang W, Zu Y, Fu Y, Reichling J, Suschke U, Nokemper S, Zhang Y. In vitro antioxidant, antimicrobial and anti-herpes simplex virus type 1 activity of Phellodendron amurense Rupr. from China. Am. J. Chin. Med. 2009;37:195–203. doi: 10.1142/S0192415X09006655. [DOI] [PubMed] [Google Scholar]
- Wollny T, Aiello L, Di Tommaso D, Bellavia V, Rotilio D, Donati MB, de Gaetano G, Iacoviello L. Modulation of haemostatic function and prevention of experimental thrombosis by red wine in rats: a role for increased nitric oxide production. Br. J. Pharmacol. 1999;127:747–755. doi: 10.1038/sj.bjp.0702586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YW, Kaminski PM, Wolin MS. Inhibition of rat cardiac muscle contraction and mitochondrial respiration by endogenous peroxynitrite formation during posthypoxic reoxygenation. Circ. Res. 1998;82:891–897. doi: 10.1161/01.res.82.8.891. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Sakane T, Harada M, Sugiura N, Koda H, Kiso Y, Sezaki H. Absorption and metabolism of antioxidative polyphenolic compounds in red wine. Ann. N. Y. Acad. Sci. 2002;957:325–328. doi: 10.1111/j.1749-6632.2002.tb02934.x. [DOI] [PubMed] [Google Scholar]
- Yin YY, Li WP, Gong HL, Zhu FF, Li WZ, Wu GC. Protective effect of astragaloside on focal cerebral ischemia/reperfusion injury in rats. Am. J. Chin. Med. 2010;38:517–527. doi: 10.1142/S0192415X10008020. [DOI] [PubMed] [Google Scholar]
- Yook HS, Kim KH, Park JE, Shin HJ. Antioxidative and antiviral properties of flowering cherry fruits (Prunus serrulata L. var. spontanea) Am. J. Chin. Med. 2010;38:937–948. doi: 10.1142/S0192415X10008366. [DOI] [PubMed] [Google Scholar]
- Yu T, Chen QE, Chen ZW, Xiong Z, Ye M. Protective effects of total flavones of rhododendra against global cerebral ischemia reperfusion injury. Am. J. Chin. Med. 2009;37:877–887. doi: 10.1142/S0192415X09007284. [DOI] [PubMed] [Google Scholar]
- Yune TY, Lee JY, Cui CM, Kim HC, Oh TH. Neuroprotective effect of Scutellaria baicalensis on spinal cord injury in rats. J. Neurochem. 2009;110:1276–1287. doi: 10.1111/j.1471-4159.2009.06214.x. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. U. S. A. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]