Abstract
Functional magnetic resonance imaging was used to explore the neural correlates of semantic judgments to Chinese characters. Adult participants were asked to indicate if character pairs were related in meaning that were arranged in a continuous variable according to association strength. This parametric manipulation allowed for a more precise determination of the role of the left inferior parietal lobule in processing meaning, which has not been reported in previous Chinese studies. Consistent with previous findings in English, participants showed activation in left inferior frontal gyrus (BA 47, 45) and left posterior middle temporal gyrus (BA 21). Characters with stronger semantic association elicited greater activation in left inferior parietal lobule (BA 39), suggesting stronger integration of highly related semantic features. By contrast, characters with weaker semantic association elicited greater activation in both an anterior ventral region (BA 47) and a mid-ventral region of left inferior frontal gyrus (BA 45), suggesting a controlled retrieval process and a selection process. Our findings of association strength are discussed in a proposed neuro-anatomical model of semantic processing.
Keywords: fMRI, Semantic, Meaning, Association strength
Introduction
Previous studies on visual word comprehension in English have shed light on the functional architecture of word recognition systems for printed words. These studies have identified brain regions for processing written language in both left inferior frontal gyrus (BAs 47, 45) and left posterior middle temporal gyrus (BA 21) (Booth et al. 2002; Chee et al. 1999; Howard et al. 1992; Shaywitz et al. 2001; Simos et al. 1999). These two regions are thought to be associated generally with comprehension of meaning in language processing in English.
The role of inferior frontal gyrus in semantic processing has been explored in English by comparing activation to semantic judgments involving closely related pairs (e.g., king–queen) versus distantly related pairs (e.g., net–ship). Distantly related pairs with weaker semantic association produced greater activation in left inferior frontal gyrus as compared to closely related pairs. Greater activation in left inferior frontal gyrus has been suggested to result from the difficulty of retrieving and/or selecting appropriate semantic features, as distantly related pairs share few semantic features (Fletcher et al. 2000). In support of this, many studies show greater inferior frontal gyrus activation in more difficult semantic tasks and in tasks with increased retrieval or selection demands. These studies include high versus low requirement for selection among alternatives (Thompson-Schill et al. 1997, 1999), weak versus strong association strength (Wagner et al. 2001; Chou et al. 2006a, b), naming low versus high familiarity objects (Whatmough et al. 2002), generating novel versus repeated base nouns (Seger et al. 2000), naming low versus high agreement pictures (Kan and Thompson-Schill 2004), deep versus shallow processing of words (Fujii et al. 2002) and producing words for pre-specified semantic categories versus over-learned letter sequences (Gurd et al. 2002).
A review article recently proposed different cognitive functions for sub-regions of the inferior frontal gyrus (Badre and Wagner 2007). The anterior ventral region of left inferior frontal gyrus (BA 47) may support controlled access to stored semantic representations, whereas the mid-ventral region of left inferior frontal gyrus (BA 45) may support a domain-general selection process among active representations. Previous studies using association strength have found greater activation related to weaker association in both the anterior ventral region and the mid-ventral region of left inferior frontal gyrus (BAs 47 and 45) (Badre et al. 2005; Chou et al. 2006a, b). For weaker association pairs, participants need controlled access to stored conceptual representations to seek for existing semantic associations in verbal semantic memory. Moreover, participants require a selection process that operates post-retrieval to resolve competition among active representations during semantic association judgment.
Distantly related pairs with weaker association in English have also been shown to produce greater activation in left posterior middle temporal gyrus (Chou et al. 2006a; Wible et al. 2006). Activation in this region has been implicated in the representation of verbal semantic information (Blumenfeld et al. 2006; Booth et al. 2002). Greater activation in this region for weaker association pairs may result from a more extensive access to semantic representations in order to identify distant relationships (Booth et al. 2007). In contrast, closely related pairs with stronger association in English produced greater activation in left inferior parietal lobule (Chou et al. 2006a, b; Raposo et al. 2006). Some studies have interpreted left inferior parietal lobule activation as related to feature integration and semantic categorization to form a coherent concept so that semantic relationships between words can be determined (Grossman et al. 2003; Smith 1995). Neuro-anatomical connectivity patterns also suggest that semantic-lexical integrative processes involve heteromodal association cortices in inferior parietal lobule (Mesulam 1998). Stronger association pairs may involve greater integration because there are more overlapping features between the words or because the shared features are more characteristic of each of the words (Chou, et al. 2006a, b). Greater integration for stronger association word pairs may account for the increase in left inferior parietal lobule activation with stronger association strength.
Despite differences between Chinese and English in the nature of mapping between orthography and semantics, neuroimaging studies have revealed substantial similarities across the two languages using a variety of semantic tasks in Chinese visual character/word comprehension. These studies include judging whether two characters are semantically related (Dong et al. 2005; Tan et al. 2001), semantic judgment of association strength (Booth et al. 2006), semantic categorization (Ding et al. 2003; Liu et al. 2006), silent word generation (Tan et al. 2000), and high-conflict versus low-conflict semantic judgment (Zhang et al. 2004) and matching characters in meaning to corresponding English words (Chee et al. 2000). These studies have reported common activated regions in left inferior frontal gyrus (BA 47, 45) and left posterior temporal areas (BA 21, 37) in Chinese. The importance of left inferior frontal gyrus in visual character/word comprehension is also supported by a study that taught English translations of Chinese characters to English speakers (Deng et al. 2008). None of the aforementioned neuroimaging studies of Chinese, however, found activation in left inferior parietal lobule. The lack of left inferior parietal lobule activation may be because these studies did not control for or systematically manipulate association strength of the Chinese characters. As noted previously, studies in English have shown that stronger association strength is correlated with greater activation in left inferior parietal lobule.
Our experiments were designed to evaluate whether association strength would modulate brain activation during semantic judgments on Chinese characters. We used association strength to explore semantic processing in Chinese, because no previous studies have systematically manipulated the degree of semantic association between pairs of Chinese characters. This parametric manipulation allows for a more precise determination of the brain regions critical for meaning based processing. We expected that stronger association would be correlated with greater activation in left inferior parietal lobule because the meanings of these character pairs can be more effectively integrated. In contrast, we expected that weaker association pairs would be correlated with greater activation in both the anterior ventral region and the mid-ventral region of left inferior frontal gyrus due to increased demands on controlled retrieval processes and on selection of lexical representations.
Experiment 1
Materials and methods
Participants
Thirty-one native speakers of Chinese (mean age = 20.9, 14 females) in Taiwan participated in the functional magnetic resonance imaging (fMRI) study. They were given an informal interview to insure that they met the following inclusionary criteria: (1) right-handedness, (2) normal hearing and normal or corrected-to-normal vision, (3) free of neurological disease or psychiatric disorders, (4) not taking medication affecting the central nervous system, (5) no history of attention, reading, or oral-language deficits, and (6) no learning disability. After the administration of the informal interview, informed consent was obtained. The informed consent procedures were approved by the Institutional Review Board at the National Taiwan University Hospital.
Functional activation tasks
In the meaning judgment task, two visual Chinese characters were presented sequentially and the participant had to determine whether the character pair was related in meaning. Trials lasted 4,500 ms and consisted of a solid square (500 ms), followed by the first character (800 ms), a 200 ms blank interval, and the second character (3,000 ms). The participant was instructed to make a response during the presentation of the second character. Forty-eight character pairs were semantically related according to their free association values (mean = 0.14, SD = 0.13, ranging from 0.73 to 0.01) (Hue et al. 2005). Character pairs were arranged in a continuous variable according to association values. These character pairs included both closely related pairs with stronger association values and distantly related pairs with weaker association values. Because the distribution of these association values was skewed, we performed a logarithmic transformation on these values to get a symmetrical distribution (mean = 1.04, SD = 0.36, ranging from 1.86 to 0.30). Twenty-four character pairs were semantically unrelated with zero association values. The participants were instructed to quickly and accurately press with their right hand the yes button to the related pairs and the no button to the unrelated pairs.
The perceptual control condition had 24 pairs of non-characters. Non-characters were created by replacing radicals of real characters with other radicals that did not form real Chinese characters. For the perceptual controls, trials consisted of a solid square (500 ms), followed by the first non-character (800 ms), a 200 ms blank interval, and the second non-character (3,000 ms). Participants determined whether the pair of stimuli were identical or not by pressing a yes or no button. Non-characters were larger (50 font size) than real characters (40 font size). There were also 24 baseline events in which the participant was instructed to press a button when a solid square (1,300 ms) at the center of the visual field turned to a hollow square (3,000 ms) after a blank interval (200 ms). In order to control for visual-orthographic information between the first and the second characters, we compared the related or unrelated conditions to the perceptual control condition for the fMRI analyses.
Stimulus characteristics
Several lexical variables were controlled across the related and unrelated conditions (Table 1). First, all characters were monosyllabic. Second, the first and second character did not share radicals. Third, the first and second character together did not form a word (Wu and Liu 1987; Sinica Corpus 1998). Fourth, characters were matched for visual complexity (in terms of strokes per character) across conditions. Fifth, characters were matched for frequency across conditions (Wu and Liu 1987). Sixth, the number of nouns (48–50%), verbs (23%), and adjectives (21–27%), based on their most frequent usage in Academia Sinica balanced corpus (Sinica Corpus 1998), was matched across conditions. The correlation of character frequency or the measure semantic relation (Lee et al. in press) with association strength was not significant indicating that association effects should not be due to frequency or semantic relation differences.
Table 1.
Stimulus characteristics for first characters (first) and second characters (second) in the related and unrelated conditions
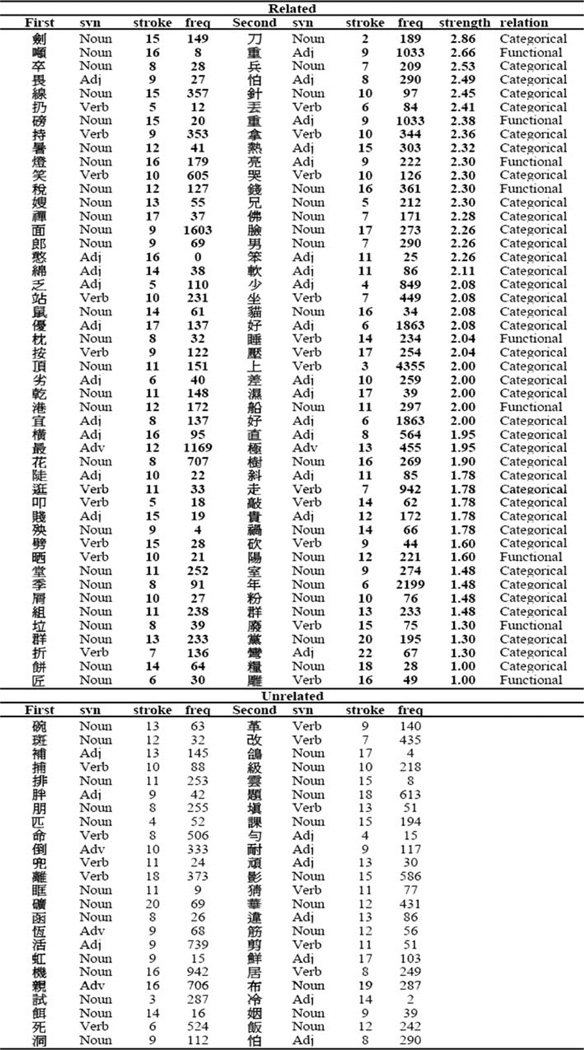 |
Syn syntactic category, adj adjective, adv adverb (Sinica Corpus 1998), stroke number of strokes, freq character frequency (Wu and Liu 1987), strength log transformed association strength for the related pairs (Hue et al. 2005), association strength was treated as a continuous variable in the statistical analyses, relation semantic relation (Lee et al. in press)
MRI data acquisition
Participants lay in the scanner with their head position secured. An optical response box was placed in the participants’ right hand. The head coil was positioned over the participants’ head. Participants viewed visual stimuli projected onto a screen via a mirror attached to the inside of the head coil. This study adopted an event-related design. Each participant performed two functional runs. Each of the two runs took 4.7 min. Each functional run had 135 image volumes.
All images were acquired using a 1.5 T Siemens scanner. Gradient-echo localizer images were acquired to determine the placement of the functional slices. For the functional imaging studies, a susceptibility weighted single-shot EPI (echo planar imaging) method with BOLD (blood oxygenation level-dependent) was used. Functional images were interleaved from bottom to top in a whole brain EPI acquisition. The following scan parameters were used: TE = 35 ms, flip angle = 90°, matrix size = 64 × 64, field of view = 24 cm, slice thickness = 5 mm, number of slices = 24; TR = 2,000 ms. In addition, a high resolution, T1 weighted 3D image was acquired (TR = 2,140 ms, TE = 4.8 ms, flip angle = 20°, matrix size = 256 × 256, field of view = 24 cm, slice thickness = 1 mm, gap = 0.5 mm, number of slices = 128). The orientation of the 3D image was identical to the functional slices. The task was administered in a pseudorandom order for all subjects, in which the order of related, unrelated, perceptual, and baseline trials was optimized for event-related design (Burock et al. 1998).
Image analysis
Data analysis was performed using SPM2 (Statistical Parametric Mapping) (http://www.fil.ion.ucl.ac.uk/spm). The functional images were corrected for differences in slice-acquisition time to the middle volume and were realigned to the first volume in the scanning session using affine transformations. No participant had more than 3 mm of movement in any plane. Co-registered images were normalized to the Montreal Neurological Institute (MNI) average template (12 linear affine parameters for brain size and position, eight non-linear iterations and 2 × 2 × 2 nonlinear basis functions). Statistical analyses were calculated on the smoothed data (10 mm isotropic Gaussian kernel), with a high pass filter (128 s cutoff period).
Data from each participant was entered into a general linear model using an event-related analysis procedure in SPM2 (Penny and Holmes 2003). Character pairs were treated as individual events for analysis and modeled using a canonical HRF (hemodynamic response function). There were four event types: related, unrelated, perceptual, and baseline. For the related pairs, association strength was an item-level parametric modulator in order to differentiate semantic relatedness as a continuous variable according to log transformed free association strength (Hue et al. 2005). The resulting model coefficients for individual subjects were entered into subsequent second-order random-effects analyses. Random-effects analysis using one-sample t tests across all participants was used to determine whether activation during a contrast was significant (i.e., parameter estimates were reliably greater than 0) in a whole brain analysis. First, we compared the related to perceptual control, unrelated to perceptual control, and related to unrelated conditions. The threshold was set to p < 0.01 FDR (false discovery rate) corrected at the voxel level with a cluster size greater than or equal to 10 voxels in order to get distinct clusters. Second, we examined the effects of the association strength for the related pairs. Positive effects indicated greater activation for related pairs with stronger association strength, whereas negative effects indicated greater activation for related pairs with weaker association strength. All reported areas of activation were significant using p < 0.05 FDR (false discovery rate) corrected at the voxel level with a cluster size greater than or equal to 10 voxels. Third, we also examined the effects of behavioral performance for related pairs, including reaction time in the scanner as a within-subject covariate in a whole brain analysis. Positive effects indicated greater activation for related pairs with slower reaction times, whereas negative effects indicated greater activation for related pairs with faster reaction times. All reported areas of activation were significant using p < 0.05 for FDR (false discovery rate) corrected at the voxel level with a cluster size greater than or equal to 10 voxels.
Results
Behavioral performance
The subject-based accuracy (mean ± SD) for the related and unrelated conditions was 94 ± 6% and 96 ± 4%, respectively, with no significant difference, a paired t(30) = 1.55, p = 0.13. The subject-based reaction times (mean ± SD) for the related and unrelated conditions were 967 ± 199 ms and 1,102 ± 188 ms, respectively, with the related condition being significantly faster than the unrelated condition, a paired t(30) = 5.37, p < 0.05. Because character pairs were arranged in a continuous variable according to association strength, we calculated correlation analyses between association strength for related pairs and behavioral performance. The item-based correlation between accuracy and association strength was not significant, r(48) = 0.28, p > 0.05; and the item-based correlation between reaction times and association strength was negative and significant, r(48) = −0.51, p < 0.01. The subject-based accuracy and reaction times (mean ± SD) for the perceptual control were 96 ± 4% and 838 ± 195 ms, respectively. The subject-based accuracy and reaction times (mean ± SD) for the baseline were 99 ± 1% and 654 ± 220 ms, respectively.
Brain activation patterns
The presentation of the results focuses on brain regions that have been implicated in previous studies of semantic processing, namely left inferior frontal gyrus, left middle temporal gyrus and left inferior parietal lobule. Because no significant differences were found between the analysis of correct responses alone and the analysis that includes all responses, only results from the analysis with all responses are presented to equate the statistical power between conditions with different accuracies (Bitan et al. 2007). All activation differences are reported in the Tables.
Table 2 shows greater activation for the related or unrelated conditions compared to the perceptual control condition. Both related and unrelated conditions produced greater activation in left inferior frontal gyrus (BA 47, 45) and left posterior middle temporal gyrus (BA 21) as compared to the perceptual control condition. The direct comparison of related and unrelated conditions produced greater activation in left inferior frontal gyrus (BA 47), left inferior parietal lobule (BA 39), and left posterior middle temporal gyrus (BA 21).
Table 2.
Experiment 1: greater activation for the related or unrelated conditions compared to the perceptual control condition
| Condition | Regions | H | BA | z test | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Related-perceptual | Inferior frontal gyrus | L | 47 | 6.68 | 832 | −48 | 30 | −3 |
| Inferior frontal gyrus | L | 45 | −47 | 24 | 12 | |||
| Medial frontal gyrus | L | 6 | 5.37 | 66 | −6 | 36 | 39 | |
| Middle temporal gyrus | L | 21 | 5.26 | 356 | −57 | −46 | −3 | |
| Unrelated-perceptual | Inferior frontal gyrus | L | 47 | 5.87 | 594 | −48 | 30 | −3 |
| Inferior frontal gyrus | L | 45 | −45 | 24 | 14 | |||
| Middle temporal gyrus | L | 21 | 4.44 | 69 | −60 | −45 | 0 | |
| Related–unrelated | Inferior parietal lobule | L | 39 | 5.44 | 139 | −45 | −63 | 30 |
| Inferior frontal gyrus | L | 47 | 5.18 | 98 | −42 | 36 | −6 | |
| Medial frontal gyrus | L | 9 | 4.60 | 277 | −9 | 42 | 30 | |
| Middle temporal gyrus | L | 21 | 4.43 | 68 | −60 | −42 | −3 | |
| Precuneus | L | 7 | 4.15 | 19 | −3 | −57 | 51 |
The direct comparison between the related and unrelated conditions is also presented
H hemisphere, L left, R right, BA Brodmann’s area. Coordinates of activation peak(s) within a region based on a z test are given in the MNI stereotactic space (x, y, z). Voxels number of voxels in cluster at p < 0.01 FDR (false discovery rate) corrected, only clusters greater than or equal to 10 are presented
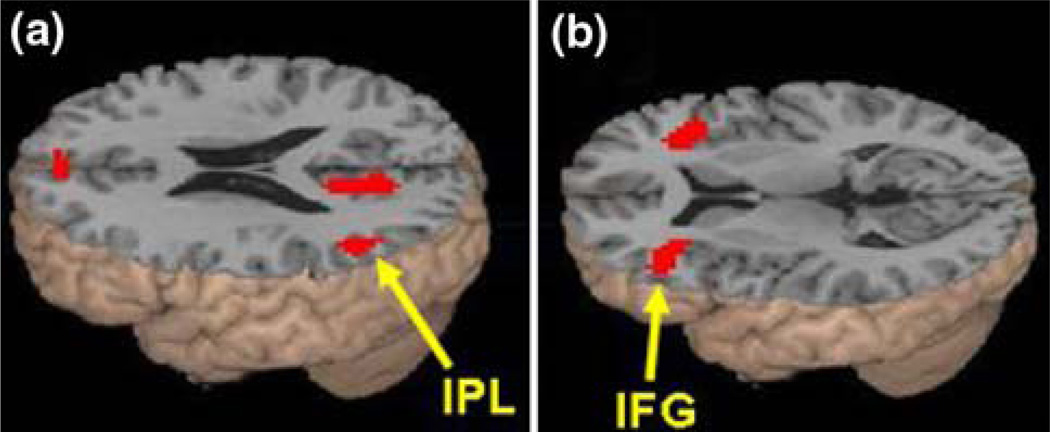
The effect of semantic association strength is shown in Table 3. Stronger association produced greater activation in left inferior parietal lobule (IPL, BA 39) (see Fig. 1a). Weaker association produced greater activation in the ventral regions of left inferior frontal gyrus (IFG, BA 45, 47) (see Fig. 1b). We further examined the effect of the continuous variable of reaction time for the related pairs as an item-based (within-subject) covariate. Slower reaction times produced greater activation in medial frontal gyrus (BA 9, MNI coordinates [−3, 39, 36], Z = 4.73, voxels = 62,) and right inferior frontal gyrus (BA 47, MNI coordinates [36, 30, −12], Z = 4.19, voxels = 46). No significant effect was found for faster reaction times. Thus, reaction time differences should not be responsible for the observed effects of semantic association strength in IPL and IFG.
Table 3.
Experiment 1: effects of semantic association for related pairs
| Modulation | Regions | H | BA | z test | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Stronger association | Precuneus | L | 31/7 | 4.65 | 466 | −6 | −42 | 33 |
| Medial frontal gyrus | R | 10 | 3.92 | 94 | 12 | 57 | 18 | |
| Inferior parietal lobule | L | 39 | 3.70 | 42 | −48 | −60 | 29 | |
| Weaker association | Inferior frontal gyrus | R | 47 | 4.66 | 213 | 42 | 27 | −3 |
| Inferior frontal gyrus | L | 45 | 4.64 | 415 | −54 | 24 | 10 | |
| Inferior frontal gyrus | L | 47 | −40 | 24 | 0 | |||
| Middle temporal gyrus | L | 21 | 3.68 | 15 | −48 | −48 | −9 |
See Table 2 note. Voxels number of voxels in cluster at p < 0.05 FDR (false discovery rate) corrected, only clusters greater than or equal to 10 are presented
Fig. 1.
Association strength effects in Experiment 1. a Stronger association was correlated with greater activation for the related pairs in left inferior parietal lobule (IPL, BA 39). b Weaker association was correlated with greater activation for the related pairs in the ventral portions of left inferior frontal gyrus (IFG, BA 45, 47)
Experiment 2
Materials and methods
In Experiment 2 we tested different participants in a different scanner to determine if we could replicate the semantic association effects. Experiment 2 also used a 3 T rather than the 1.5 T MRI as used in Experiment 1.
Participants
Thirty-two native speakers of Chinese (mean age = 20.8, 18 females) in Taiwan participated in the fMRI study. They were given an informal interview to insure that they met the same inclusionary criteria as Experiment 1. After the administration of the informal interview, informed consent was obtained. The informed consent procedures were approved by the Institutional Review Board at the National Taiwan University Hospital.
Functional activation tasks
The tasks were the same as Experiment 1.
Stimulus characteristics
The stimuli were the same as Experiment 1.
MRI data acquisition
The experimental procedures were the same as Experiment 1 except for the following information. The following scan parameters were used: TE = 24 ms, flip angle = 90°, matrix size = 64 by 64, field of view = 25.6 cm, slice thickness = 3 mm, number of slices = 34; TR = 2,000 ms. In addition, a high resolution, T1 weighted 3D image was acquired (TR = 1,560 ms, TE = 3.68 ms, flip angle = 15°, matrix size = 256 by 256, field of view = 25.6 cm, slice thickness = 1 mm, number of slices = 192). Each functional run had 136 image volumes.
Image analysis
The data analysis was the same as Experiment 1, except that for the effects of semantic association we used an inclusive mask of the related versus unrelated (p < 0.005 uncorrected) for stronger association or the related versus perceptual (p < 0.005 uncorrected) for weaker association (Chou et al. 2006b).
Results
Behavioral performance
The subject-based accuracy (mean ± SD) for the related and unrelated conditions was 96 ± 4% and 97 ± 3%, respectively, with no significant difference, a paired t(31) = 1.98, p = 0.06. The subject-based reaction times (mean ± SD) for the related and unrelated conditions were 827 ± 175 ms and 873 ± 173 ms, respectively, with the related condition being significantly faster than the unrelated condition, a paired t(31) = 2.32, p < 0.05. Because character pairs were arranged in a continuous variable according to association strength, we calculated correlation analyses between association strength for related pairs and behavioral performance. The item-based correlation between accuracy and association strength was not significant, r(48) = 0.28, p > 0.05; and the item-based correlation between reaction times and association strength was negative and significant, r(48) = −0.44, p < 0.01. The subject-based accuracy and reaction times (mean ± SD) for the perceptual control were 99 ± 1% and 628 ± 121 ms, respectively. The subject-based accuracy and reaction times (mean ± SD) for the baseline were 98 ± 3% and 574 ± 127 ms, respectively.
Brain activation patterns
Table 4 shows greater activation for the related or unrelated conditions compared to the perceptual control condition. Both related and unrelated conditions produced greater activation in left inferior frontal gyrus (BA 47, 45) and left posterior middle temporal gyrus (BA 21) as compared to the perceptual control condition. The direct comparison of related and unrelated conditions produced greater activation in left inferior frontal gyrus (BA 47, 45), left inferior parietal lobule (BA 39, 40), and left posterior middle temporal gyrus (BA 21).
Table 4.
Experiment 2: greater activation for the related or unrelated conditions compared to the perceptual control condition
| Condition | Regions | H | BA | z test | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Related-perceptual | Inferior frontal gyrus | L | 45 | 6.28 | 1,449 | −48 | 27 | 15 |
| Inferior frontal gyrus | L | 47 | −42 | 27 | −3 | |||
| Middle frontal gyrus | L | 46 | −45 | 18 | 27 | |||
| Medial frontal gyrus | L | 6 | 5.81 | 737 | −3 | 12 | 48 | |
| Middle temporal gyrus | L | 21 | 5.79 | 663 | −57 | −42 | 0 | |
| Inferior frontal gyrus | R | 47 | 5.22 | 100 | 40 | 24 | −3 | |
| Cerebellum | L | – | 4.94 | 105 | −6 | −45 | −9 | |
| Unrelated-perceptual | Inferior frontal gyrus | L | 47 | 6.15 | 997 | −42 | 24 | −3 |
| Middle frontal gyrus | L | 46 | −45 | 15 | 26 | |||
| Inferior frontal gyrus | L | 45 | −51 | 28 | 12 | |||
| Medial frontal gyrus | L | 6 | 5.77 | 319 | −3 | 12 | 54 | |
| Middle temporal gyrus | L | 21 | 4.17 | 46 | −54 | −48 | 0 | |
| Insula | R | 13 | 3.68 | 18 | 33 | 21 | 3 | |
| Related–unrelated | Inferior frontal gyrus | L | 47 | 5.67 | 305 | −42 | 33 | −3 |
| Inferior frontal gyrus | L | 45 | −48 | 30 | 8 | |||
| Inferior parietal lobule | L | 39 | 5.59 | 1,009 | −42 | −66 | 33 | |
| Inferior parietal lobule | L | 40 | −51 | −51 | 30 | |||
| Medial frontal gyrus | L | 8 | 4.85 | 1,090 | −12 | 39 | 45 | |
| Middle frontal gyrus | L | 6 | −33 | 9 | 57 | |||
| Posterior cingulate gyrus | L | 31 | 4.82 | 545 | −9 | −48 | 36 | |
| Middle temporal gyrus | L | 21 | 4.49 | 439 | −66 | −42 | −3 |
The direct comparison between the related and unrelated conditions is also presented
See Table 2 note. Voxels number of voxels in cluster at p < 0.01 FDR (false discovery rate) corrected, only clusters greater than or equal to 10 are presented
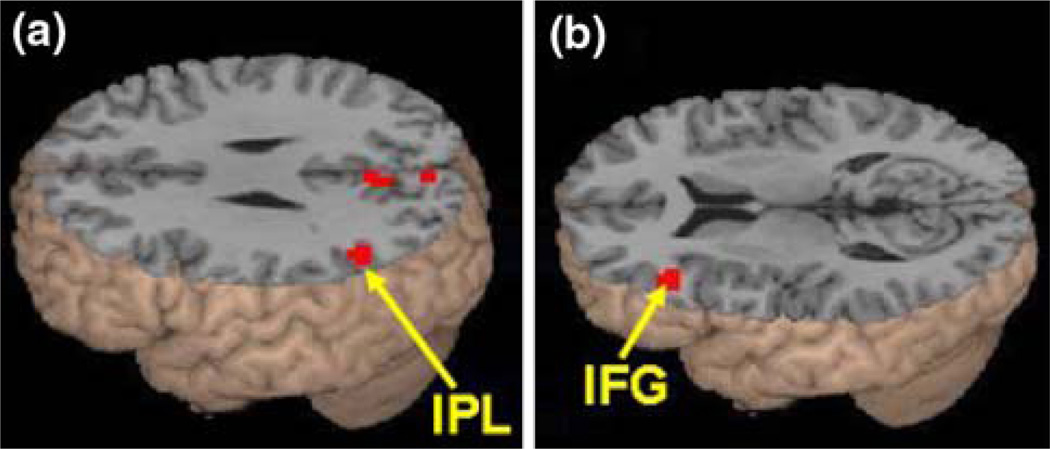
The effects of semantic association strength for the related pairs are shown in Table 5. Stronger association produced greater activation in left inferior parietal lobule (IPL, BA 39), (see Fig. 2a). Weaker association produced greater activation in mid left inferior frontal gyrus (IFG, BA 45) (see Fig. 2b). We further examined the effect of the continuous variable of reaction time for the related pairs as an item-based (within-subject) covariate. Slower reaction times produced greater activation in medial frontal gyrus (BA 6, MNI coordinates [−3, 12, 57], Z = 4.26, voxels = 317). No significant effect was found for faster reaction times. Thus, reaction time differences should not be responsible for the observed effects of semantic association strength in IPL and IFG.
Table 5.
Experiment 2: effects of semantic association for related pairs
| Modulation | Regions | H | BA | z test | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Stronger association | Inferior parietal lobule | L | 40 | 3.82 | 54 | −51 | −60 | 46 |
| Inferior parietal lobule | L | 39 | −48 | −57 | 30 | |||
| Precuneus | L | 31/7 | 3.81 | 90 | −9 | −48 | 39 | |
| Precuneus | L | 7 | 3.65 | 14 | −2 | −81 | 30 | |
| Weaker association | Inferior frontal gyrus | L | 45 | 3.67 | 50 | −42 | 9 | 24 |
| Inferior frontal gyrus | L | 45 | 3.65 | 36 | −48 | 24 | 12 | |
| Medial frontal gyrus | R | 8 | 3.37 | 12 | 12 | 18 | 48 |
See Table 2 note. Voxels number of voxels in cluster at p < 0.05 FDR (false discovery rate) corrected, only clusters greater than or equal to 10 are presented. An inclusive mask of the related versus unrelated (p < 0.005 uncorrected) was used for stronger association or of the related versus perceptual (p < 0.005 uncorrected) for weaker association
Fig. 2.
Association strength effects in Experiment 2. a Stronger association was correlated with greater activation for the related pairs in left inferior parietal lobule (IPL, BA 39). b Weaker association was correlated with greater activation for the related pairs in the mid ventral portion of left inferior frontal gyrus (IFG, BA 45)
Discussion
The neural correlates of semantic processing to visually presented Chinese characters were examined with a task requiring association judgments as to whether character pairs were related in meaning. Our finding that both the related and unrelated Chinese character pairs activated left posterior middle temporal gyrus (BA 21) is consistent with English findings. Some argue that left posterior middle temporal gyrus is involved in the representation of verbal semantic information (Blumenfeld et al. 2006; Booth et al. 2002; Martin 2001). Several studies also suggest that the best candidate for the storage of lexical representations is left posterior MTG (Hickok and Poeppel 2004, 2007; Martin 2007).
To the best of our knowledge, this is the first study to examine the effect of association strength between characters on brain activation when processing Chinese. Parametric manipulations allowed us to more precisely investigate brain regions that are involved in semantic processing. We found that stronger semantic association produced greater activation in left inferior parietal lobule (BA 39). Activation in this region has previously been identified in semantic tasks in English, including associative judgments (Binder et al. 1997; Chou et al. 2006a, b), similarity judgments (Price et al. 1999), category judgments (Pugh et al. 1996) and concrete versus abstract word judgments (Chee et al. 1999). Greater activation in left inferior parietal lobule has been interpreted as evidence of semantic integration (Thompson et al. 2007), as supported by a larger N400 wave over the parietal cortex following errors of semantic integration during ERP (event-related potential) measures (Hagoort et al. 1999). Stronger association pairs may involve greater integration because there are more overlapping features between the characters or because the shared features are more characteristic of each of the characters (Chou et al. 2006a, b). Greater integration for stronger association pairs in Chinese may account for the greater left inferior parietal lobule activation with stronger association strength. This is the first study to demonstrate the role of left inferior parietal lobule in semantic processing in Chinese.
We also found that weaker semantic association in Chinese produced greater activation in left inferior frontal gyrus (BA 47, 45). Previous studies in English suggest that left inferior frontal gyrus is involved in effortful semantic processing, particularly when there is increased demands on the process of selecting relevant semantic knowledge or when comparing words along semantic features (Blumenfeld et al. 2006; Fletcher et al. 1998; Thompson-Schill et al. 1997, 1999; Whatmough et al. 2002). The inferior frontal gyrus has also been implicated in conditions with increased retrieval demands (Kikyo et al. 2002; Kirchhoff et al. 2000; Seger et al. 2000; Wagner et al. 2001). Of particular relevance to the current study, semantic judgments in English to weaker association pairs produced greater activation in left inferior frontal gyrus as compared to stronger association pairs (Fletcher et al. 2000). As with English, greater activation for weaker association pairs in Chinese could result from increased demands on the retrieval or selection of appropriate semantic features.
The subparts of left inferior frontal gyrus have been associated with distinct cognitive processes involved in language tasks. In general, the dorsal region of left inferior frontal gyrus is thought to be specialized for processing phonological representations, while the ventral region of left inferior frontal gyrus is proposed to be specialized for manipulating semantic representations (Poldrack et al. 1999). A further study has shown a neural dissociation of controlled retrieval and selection mechanisms in the ventral prefrontal region (Badre et al. 2005). The anterior ventral region (BA 47) is thought to be sensitive to increased demands on the top-down retrieval of semantic knowledge. In contrast, the mid ventral region (BA 45) is thought be involved in the selection of relevant semantic knowledge over irrelevant competing information. In the current study, greater activation for weaker semantic association was found in both the anterior ventral region (BA 47) and the mid-ventral region (BA 45). Greater activation in the anterior ventral region (BA 47) may be due to increased demands on the controlled retrieval of semantic knowledge for distantly related pairs in left middle temporal gyrus. When association strength between two Chinese characters is weaker, the anterior ventral region may be involved in a demanding search of posterior representations to seek for existing semantic associations stored in verbal semantic memory. Greater activation in the mid-ventral region (BA 45) may be due to increased demands on a selection process. When association strength between two Chinese characters is weaker, the mid-ventral region may be involved in selecting relevant semantic features over irrelevant semantic features.
A neuro-anatomical model of semantic processing in the left hemisphere has recently been proposed, including posterior middle temporal gyrus, angular gyrus in inferior parietal cortex, anterior (ventral) inferior frontal gyrus, and posterior (mid ventral) inferior frontal gyrus (Lau et al. 2008). Left posterior middle temporal gyrus is suggested to mediate the long-term storage of and access to information associated with lexical representations. This ‘lexical’ information serves as input to higher-order semantic processes. Left inferior parietal cortex has been suggested to support the integration of lexical input into the larger units for semantic processing. Left anterior inferior frontal gyrus is proposed to mediate controlled retrieval of lexical representations based on top-down information, and left posterior inferior frontal gyrus is proposed to mediate selection between activated candidate representations. Our Chinese findings of greater activation for stronger association in left inferior parietal lobule and greater activation for weaker association in both the anterior ventral region and the mid-ventral region of inferior frontal gyrus are consistent with this proposed neuro-anatomical model (Lau et al. 2008).
In conclusion, this study is the first to demonstrate that stronger semantic association between Chinese characters produces greater activation in left inferior parietal lobule, suggesting that closely related pairs allow for more complete semantic integration. We also showed that weaker association between Chinese characters produced greater activation in both the anterior ventral region and mid-ventral region of left inferior frontal gyrus suggesting that distantly related pairs required controlled retrieval processes and selection of appropriate semantic features.
Acknowledgments
This research was supported by National Science Council of Taiwan (NSC 95-2413-H-002-030) to Tai-Li Chou. This research was also supported by grants from the National Institute of Child Health and Human Development (HD042049) to James R. Booth. This work was also supported in part by the Department of Medical Imaging and 3T MRI Lab in National Taiwan University Hospital.
Contributor Information
Tai-Li Chou, Email: tlchou25@ntu.edu.tw, Department of Psychology, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei 106, Taiwan; Neurobiology and Cognitive Science Center, National Taiwan University, Taipei, Taiwan.
Chih-Wei Chen, Department of Psychology, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei 106, Taiwan.
Mei-Yao Wu, Department of Psychology, National Taiwan University, No. 1, Sec. 4, Roosevelt Road, Taipei 106, Taiwan.
James R. Booth, Department of Communication Sciences and Disorders, Northwestern University, Evanston, USA
References
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17(1):353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Chou TL, Lu D, Cone NE, Cao F, Bigio JD, Booth JR. The interaction between orthographic and phonological information in children: an fMRI study. Hum Brain Mapp. 2007;28(9):880–891. doi: 10.1002/hbm.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld HK, Booth JR, Burman DD. Diffierential prefrontal-temporal neural correlates of semantic processing in children. Brain Lang. 2006;99(3):226–235. doi: 10.1016/j.bandl.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Modality independence of word comprehension. Hum Brain Mapp. 2002;16(4):251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Lu D, Burman DD, Chou TL, Jin Z, Peng DL, Zhang L, Ding GS, Deng Y, Liu L. Specialization of phonological and semantic processing in Chinese word reading. Brain Res. 2006;1071(1):197–207. doi: 10.1016/j.brainres.2005.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Bebko G, Burman DD, Bitan T. Children with reading disorder show modality independent brain abnormalities during semantic tasks. Neuropsychologia. 2007;45(4):775–783. doi: 10.1016/j.neuropsychologia.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuro-Report. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Chee MW, O’Craven KM, Bergida R, Rosen BR, Savoy RL. Auditory and visual word processing studied with fMRI. Hum Brain Mapp. 1999;7(1):15–28. doi: 10.1002/(SICI)1097-0193(1999)7:1<15::AID-HBM2>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Weekes B, Lee KM, Soon CS, Schreiber A, Hoon JJ, Chee M. Overlap and dissociation of semantic processing of Chinese characters, English words, and pictures: evidence from fMRI. Neuroimage. 2000;12(4):392–403. doi: 10.1006/nimg.2000.0631. [DOI] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Bitan T, Burman DD, Bigio JD, Cone NE, Lu D, Cao F. Developmental and skill effects on the neural correlates of semantic processing to visually presented words. Hum Brain Mapp. 2006a;27:915–924. doi: 10.1002/hbm.20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Burman DD, Bitan T, Bigio JD, Lu D, Cone NE. Developmental changes in the neural correlates of semantic processing. Neuroimage. 2006b;29:1141–1149. doi: 10.1016/j.neuroimage.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Deng Y, Booth JR, Chou TL, Ding G, Peng D. Item-specific and generalization effects on brain activation when learning Chinese characters. Neuropsychologia. 2008;46:1864–1876. doi: 10.1016/j.neuropsychologia.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Perry C, Peng D, Ma L, Li D, Xu S, Luo Q, Xu D, Yang J. Neural mechanisms underlying semantic and orthographic processing in Chinese-English bilinguals. NeuroReport. 2003;14(12):1557–1562. doi: 10.1097/00001756-200308260-00003. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nakamura K, Okada T, Hanakawa T, Fukuyama H, Mazziotta JC, Shibasaki H. Neural mechanisms underlying the processing of Chinese words: an fMRI study. Neurosci Res. 2005;52(2):139–145. doi: 10.1016/j.neures.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. The functional roles of prefrontal cortex in episodic memory. I. Encoding. Brain. 1998;121(Pt 7):1239–1248. doi: 10.1093/brain/121.7.1239. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ. “Sculpting the response space”–an account of left prefrontal activation at encoding. Neuroimage. 2000;12(4):404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- Fujii T, Okuda J, Tsukiura T, Ohtake H, Suzuki M, Kawashima R, Itoh M, Fukuda H, Yamadori A. Encoding-related brain activity during deep processing of verbal materials: a PET study. Neurosci Res. 2002;44(4):429–438. doi: 10.1016/s0168-0102(02)00160-8. [DOI] [PubMed] [Google Scholar]
- Grossman M, Koenig P, Glosser G, DeVita C, Moore P, Rhee J, Detre J, Alsop D, Gee J. Neural basis for semantic memory difficulty in Alzheimer’s disease: an fMRI study. Brain. 2003;126(Pt 2):292–311. doi: 10.1093/brain/awg027. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles K, Marshall JC, Fink GR. Posterior parietal cortex is implicated in continuous switching between verbal fluency tasks: an fMRI study with clinical implications. Brain. 2002;125(Pt 5):1024–1038. doi: 10.1093/brain/awf093. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Brown C, Osterhout L. The neurocognition of syntactic processing. In: Brown C, Hagoort P, editors. The neurocognition of language. New York: Oxford University Press; 1999. pp. 273–316. [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K, Wise R, Brown WD, Friston K, Weiller C, Frackowiak R. The cortical localization of the lexicons Positron emission tomography evidence. Brain. 1992;115(Pt 6):1769–1782. doi: 10.1093/brain/115.6.1769. [DOI] [PubMed] [Google Scholar]
- Hue CW, Kao CH, Lo M. Association norms for 600 Chinese characters. Taiwan: Taiwanese Psychological Association; 2005. [Google Scholar]
- Kan IP, Thompson-Schill SL. Effect of name agreement on prefrontal activity during overt and covert picture naming. Cogn Affect Behav Neurosci. 2004;4(1):43–57. doi: 10.3758/cabn.4.1.43. [DOI] [PubMed] [Google Scholar]
- Kikyo H, Ohki K, Miyashita Y. Neural correlates for feeling-of-knowing: an fMRI parametric analysis. Neuron. 2002;36(1):177–186. doi: 10.1016/s0896-6273(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20(16):6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D. A cortical network for semantics: (de)constructing the N400. Nat Rev Neurosci. 2008;9(12):920–933. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- Lee SH, Chen SY, Chou TL. Effects of vocabulary sizes on semantic processing to Chinese characters between fifth graders and adults. Formosa J Mental Health. (in press) [Google Scholar]
- Liu CL, Hue CW, Chen CC, Chuang KH, Liang KC, Wang YH, Wu CW, Chen JH. Dissociated roles of the middle frontal gyri in the processing of Chinese characters. NeuroReport. 2006;17(13):1397–1401. doi: 10.1097/01.wnr.0000233090.00463.35. [DOI] [PubMed] [Google Scholar]
- Martin M. Functional neuroimaging of semantic memory. In: Cabeza RKA, editor. Handbook of functional neuroimaging of cognition. Cambridge: MIT Press; 2001. pp. 153–186. [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes A. Random effects analysis. In: Frackowiak RSJ, Friston KJ, Frith CD, editors. Human brain function. 2nd edn. San Diego: Academic Press; 2003. pp. 843–850. [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ, Mummery CJ, Moore CJ, Frakowiak RS, Friston KJ. Delineating necessary and sufficient neural systems with functional imaging studies of neuropsychological patients. J Cogn Neurosci. 1999;11(4):371–382. doi: 10.1162/089892999563481. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, et al. Cerebral organization of component processes in reading. Brain. 1996;119(Pt 4):1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Raposo A, Moss HE, Stamatakis EA, Tyler LK. Repetition suppression and semantic enhancement: an investigation of the neural correlates of priming. Neuropsychologia. 2006;44(12):2284–2295. doi: 10.1016/j.neuropsychologia.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Seger CA, Desmond JE, Glove GH, Gabrieli J. Functional magnetic resonance imaging evidence for right-hemisphere involvement in processing unusual semantic relationships. Neuropsychology. 2000;14(3):361–369. doi: 10.1037//0894-4105.14.3.361. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Marchione KE, Fletcher JM, Klorman R, et al. The functional neural architecture of components of attention in language-processing tasks. Neuroimage. 2001;13(4):601–612. doi: 10.1006/nimg.2000.0726. [DOI] [PubMed] [Google Scholar]
- Simos PG, Papanicolaou AC, Breier JI, Wheless JW, Constantinou JE, Gormley WB, Maggio WW. Localization of language-specific cortex by using magnetic source imaging and electrical stimulation mapping. J Neurosurg. 1999;91(5):787–796. doi: 10.3171/jns.1999.91.5.0787. [DOI] [PubMed] [Google Scholar]
- Sinica Corpus. Academia Sinica balanced corpus (version) Taiwan, Taipei: 1998. [Google Scholar]
- Smith EE. Concepts and categorization. In: Kosslyn SM, Osherson DN, editors. An invitation to cognitive science. 2nd edn. Cambridge: MIT Press; 1995. pp. 1–25. [Google Scholar]
- Tan LH, Spinks JA, Gao J-H, Liu H-L, Perfetti CA, Xiong J, Stofer KA, Pu Y, Liu Y, Fox PT. Brain activation in the processing of Chinese characters and words: a functional MRI study. Hum Brain Mapp. 2000;10(1):16–27. doi: 10.1002/(SICI)1097-0193(200005)10:1<16::AID-HBM30>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, Gao JH. The neural system underlying Chinese logograph reading. Neuroimage. 2001;13(5):836–846. doi: 10.1006/nimg.2001.0749. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Bonakdarpour B, Fix SC, Blumenfeld HK, Parrish TB, Gitelman DR, Mesulam MM. Neural correlates of verb argument structure processing. J Cogn Neurosci. 2007;19(11):1753–1767. doi: 10.1162/jocn.2007.19.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23(3):513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31(2):329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Whatmough C, Chertkow H, Murtha S, Hanratty K. Dissociable brain regions process object meaning and object structure during picture naming. Neuropsychologia. 2002;40(2):174–186. doi: 10.1016/s0028-3932(01)00083-5. [DOI] [PubMed] [Google Scholar]
- Wible CG, Han SD, Spencer MH, Kubicki M, Niznikiewicz MH, Jolesz FA, McCarley RW, Nestor P. Connectivity among semantic associates: an fMRI study of semantic priming. Brain Lang. 2006;97(3):294–305. doi: 10.1016/j.bandl.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Wu JT, Liu IM. Exploring the phonetic and semantic features of Chinese words. Taiwan National Science Council: Technical Report NSC75-0301-H002-024. 1987
- Zhang JX, Zhuang J, Ma L, Yu W, Peng D, Ding G, Zhang Z, Weng X. Semantic processing of Chinese in left inferior prefrontal cortex studied with reversible words. Neuroimage. 2004;23(3):975–982. doi: 10.1016/j.neuroimage.2004.07.008. [DOI] [PubMed] [Google Scholar]