Abstract
It is generally accepted that butterfly wing color-patterns have ecological and behavioral functions that evolved through natural selection. However, particular wing color-patterns may be produced physiologically in response to environmental stress, and they may lack significant function. These patterns would represent an extreme expression of phenotypic plasticity and can eventually be fixed genetically in a population. Here, three such cases in butterflies are concisely reviewed, and their possible mechanisms of genetic assimilation are discussed. First, a certain modified color-pattern of Vanessa indica induced by temperature treatments resembles the natural color-patterns of its closely related species of the genus Vanessa (sensu stricto). Second, a different type of color-pattern modification can be induced in Vanessa cardui as a result of a general stress response. This modified pattern is very similar to the natural color-pattern of its sister species Vanessa kershawi. Third, a field observation was reported, together with experimental support, to show that the color-pattern diversity of a regional population of Zizeeria maha increased at the northern range margin of this species in response to temperature stress. In these three cases, modified color-patterns are unlikely to have significant functions, and these cases suggest that phenotypic plasticity plays an important role in butterfly wing color-pattern evolution. A neutral or non-functional trait can be assimilated genetically if it is linked, like a parasitic trait, with another functional trait. In addition, it is possible that environmental stress causes epigenetic modifications of genes related to color-patterns and that their transgenerational inheritance facilitates the process of genetic assimilation of a neutral or non-functional trait.
Keywords: butterfly wing, color-pattern, epigenetic modification, genetic assimilation, neutral or non-functional trait, phenotypic plasticity, speciation, stress response
Introduction
Butterfly wing color-patterns have long been appreciated for their beauty and diversity by lepidopterists worldwide. Partly for this reason, systematic classification has been well developed for this small group of Lepidoptera. Furthermore, lepidopterists have long known that aberrant forms of wing color-patterns are occasionally found spontaneously. Certain cases represent homeotic transformations or teratological abnormalities, whereas others are gynandromorphs (for example, see Sibatani, 1980 for homeosis). However, a significant proportion of these aberrant patterns are represented by globally simplified color-patterns with elongated or fused elements. Moreover, it is noteworthy that these organisms do not have significant abnormalities in other parts of the body (for example, see Fujioka, 1975 and Russwurm, 1978 for many individuals of spontaneously occurring aberrant types, and also see Sakaguti, 1979, 1981a for discussion). In the late nineteenth century, European lepidopterists, such as Standfuss, Dixey, and Merrifield, already knew that similar phenotypes can be produced by artificial temperature shocks (Sakaguti, 1981b). These phenotypes result from extreme expressions of phenotypic plasticity. However, the biological significance of this phenomenon remained elusive (Shapiro, 1984).
Nevertheless, Nijhout (1991) discussed the possible important contribution of aberrant phenotypes to color-pattern evolution by citing an interesting case of the form nigrosuffusa of Junonia coenia. The form nigrosuffusa occurs as a natural population in southern North America, and it is very similar to J. coenia individuals with a temperature-shocked phenotype. Furthermore, it also resembles a closely related species, J. genoveva. Nijhout (1991) argued that this coincidence could reflect the developmental evolutionary mechanisms of wing color-patterns. Nijhout (1984, 1991) also examined color-pattern modifications induced by cold-shock in butterflies from a developmental physiological point of view. In these two studies, Nijhout (1984, 1991) provided starting points for both the developmental physiology and the evolutionary biology of butterfly color-pattern changes.
Subsequently, a coincidence between the temperature-induced color-patterns of a given species, called the TS-type (TS for temperature shock), and the natural color-patterns of closely related species has been found in many instances (Otaki and Yamamoto, 2003, 2004a), suggesting that this phenomenon is widespread in butterflies. We now know that physiologically induced patterns can be assimilated genetically (Otaki et al., 2010), and this finding, together with other experimental results (Otaki, 2008a), makes us certain that phenotypic plasticity contributes significantly to the color-pattern evolution of butterflies. In addition to synthetic compilations of possible cases of plasticity-related evolution (Schlichting and Smith, 2002; West-Eberhard, 2003; Badyaev, 2005), results that demonstrate a role of phenotypic plasticity and genetic assimilation in evolution have also been reported recently from field studies of different organisms (Aubret and Shine, 2009; Buckley et al., 2010; Scoville and Pfrender, 2010; Muschick et al., 2011).
In many cases, it appears that color-patterns have evolved as a result of an environmental stress response. We have focused particularly on the genus Vanessa (Otaki and Yamamoto, 2004a,b; Otaki et al., 2006; Otaki, 2007a, 2008a,b,c) and, to a lesser extent, on the genus Junonia (Otaki et al., 2005; Otaki, 2007b, 2008a; Mahdi et al., 2011). In addition, we recently discovered an intriguing field case in which phenotypic plasticity in response to environmental stress contributes to the color-pattern evolution of the pale grass blue, Zizeeria maha (Otaki et al., 2010).
In this paper, we briefly review important information obtained from these studies and specify the topics that must be further examined to accurately understand this interesting and far-reaching phenomenon in biology. The possible contribution of epigenetic changes and their transgenerational inheritance to the evolution of color-patterns is also discussed.
Three Cases of Plasticity-Related Evolution
Bidirectional evolution in the genus Vanessa
The first systematic study of the TS-type and its possible contribution to evolution has been conducted in the genus Vanessa (sensu stricto). The examination of the color-patterns of several Vanessa species shows that the width of the orange area compared with the entire wing area is dependent on the species. One can linearly arrange Vanessa species from those with the narrowest orange area to those with the widest (Otaki and Yamamoto, 2004b). Subsequent molecular phylogenetic analysis revealed that there are two Vanessa groups: the atalanta group and the indica group (Otaki et al., 2006). This finding was also validated by a more thorough molecular analysis of Vanessa (sensu lato; Wahlberg and Rubinoff, 2011). Both the atalanta and indica groups show similar increases and decreases in the orange area (Otaki and Yamamoto, 2004b; Otaki et al., 2006; Otaki, 2008c). This result means that the increase and decrease in the orange area occurred independently from the phylogeny and geographic locations within this genus. These bidirectional color-pattern changes are not seen in other groups of Vanessa (sensu lato), such as the Cynthia group and Bassaris group, and the bidirectional changes are thus potentially “programmed” to evolve with the emergence of the Vanessa (sensu stricto) group.
In contrast, the increase and decrease of the orange area can be induced experimentally by subjecting V. indica to temperature treatments (Otaki and Yamamoto, 2004a; Otaki, 2008b). It is important to note that the color-patterns of the modified individuals resemble the non-treated natural color-patterns of other related species of the indica group (Otaki, 2008b,c). Is this resemblance only a simple coincidence? Most species of the indica group, except V. indica, inhabit the islands of Indonesia and are found in relatively restricted mountainous areas, where the temperature is relatively low or temperature fluctuations in a day are relatively high. In contrast, V. indica is widely distributed in Asia. It has been speculated that the ancestral species of the Indonesian Vanessa were exposed to a natural “temperature treatment,” showing color-pattern modifications as a side-effect, and subsequently adapted to those environments (Otaki, 2008c). A similar hypothesis can be proposed in the atalanta group, in which V. tameamea is endemic to mountainous areas of the Hawaiian islands, whereas its sister V. atalanta is widely distributed in Europe and North America (Otaki et al., 2006; Otaki, 2008c).
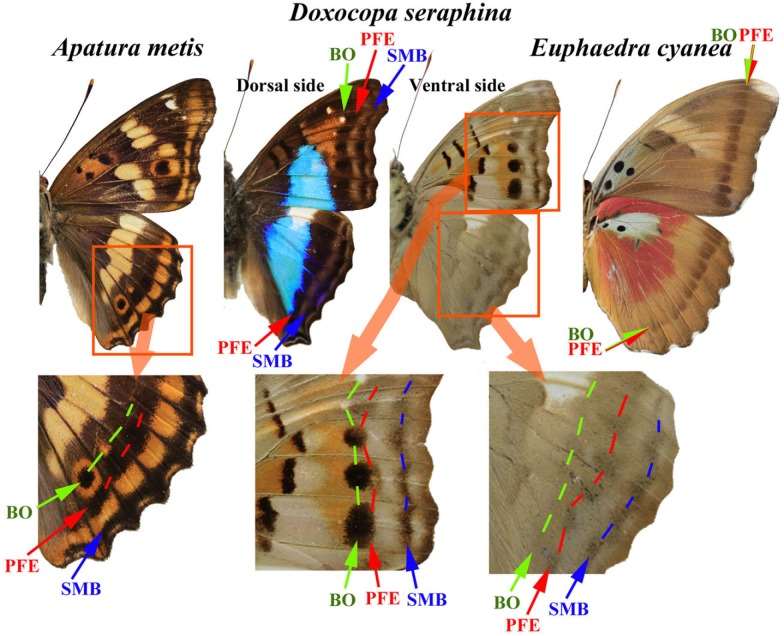
The Vanessa case is highly informative in that there may be an environmental role in the color-pattern evolution of nymphalid butterflies beyond Vanessa. A distinct feature of the TS-type modifications is a simplified overall color-pattern, especially a compromised eyespot and parafocal element (Nijhout, 1984; Otaki, 1998, 2009; Otaki and Yamamoto, 2004a). Similar phenotypes can be observed to occur relatively widely in the natural color-patterns of nymphalid butterflies (Figure 1).
Figure 1.
Examples of nymphalid butterflies that exhibit three features of the TS-type modifications: (1) the merging of the border ocellus (BO) with the parafocal element (PFE) that accompanies the dislocation of the PFE and the miniaturization of the BO; (2) the triangular PFE that points at the focus of the BO; and (3) the simplification of the overall color-pattern on the wings and the blurring of elemental boundaries. In the hindwing of Apatura metis, one BO is clearly identifiable in a wing compartment where the PFE and the submarginal band (SMB) are also clearly observed. In the adjacent compartments, the BOs are compromised and merge with the PFEs. In Doxocopa seraphina, a similar merging of the BO and the PFE is observed both on the dorsal side and on the ventral side of the fore- and hindwings. In the hindwing, the PFEs are triangular. In addition, the overall color-patterns are relatively simple. In Euphaedra cyanea, the BO and the PFE are not distinguishable. This species shows further simplified color-patterns with blurred elemental boundaries or non-existent elements throughout the wings.
Evolution of Vanessa cardui and Vanessa kershawi
The TS-type modifications can be induced not only by temperature conditions but also by certain chemicals, such as sodium tungstate (Otaki, 1998) and dextran sulfate (Serfas and Carroll, 2005). However, it is important to stress that the TS-type does not result from a teratological response (Otaki, 2008a). Indeed, the TS-type modifications are independent of general stress response and ecdysteroid effects (Otaki et al., 2005). A different type of color-pattern modification can be induced by a general stress response (Otaki et al., 2005).
Here, a color-pattern comparison between the cosmopolitan V. cardui and the Australian V. kershawi is rewarding. Molecular phylogenetic analyses confirmed that they are sister species (Wahlberg et al., 2005; Wahlberg and Rubinoff, 2011). They have very similar life histories. However, it is important to note that the latter tends to prefer a “stressful” arid environment. When V. cardui is experimentally exposed to chemical stress, it produces all of the traits that are unique to V. kershawi (Otaki, 2007a). It is probable that the ancestral species of both V. cardui and V. kershawi had similar phenotypic plasticity in response to environmental stress. It was proposed that an extreme expression of this ancestral phenotypic plasticity was assimilated genetically during the process of adaptation to a stressful environment (Otaki, 2007a).
Many similar cases of color-pattern evolution and speciation in butterflies are expected. Here, we indicate one other possible case of stress-induced modifications and speciation: a pair of Japanese Neope butterflies, Neope goschkevitschii, and N. niphonica, found in mainland Japan. Experimental validation is required to clarify the relationships between these two species.
The pale grass blue Zizeeria maha at the northern range margin
These Vanessa cases are remarkable. However, an entire historical reconstruction is difficult, if not impossible, because their speciation process occurred long ago. If these cases can be generalized, favorable opportunities may arise to observe such an evolutionary process in the field in real time. The pale grass blue Z. maha in Fukaura, Japan, may provide a good model of such processes (Buckley et al., 2010; Otaki et al., 2010). This small butterfly has expanded the margin of its range to the north. It is probable that this range expansion is a result of global warming. This process exposed the species to severe cold temperatures. At the northern margin of the range, more than 15% of all individuals had color-patterns similar, if not identical, to the TS-type, although the severity varied among individuals (Otaki et al., 2010; Figure 2). For unknown reasons, three different modification types were observed simultaneously: the reduction type, the inward type, and the outward type. The TS-type outbreak lasted a few years and spanned at least 10 generations. It is probable that the TS-type modifications were genetically assimilated in the population at that time.
Figure 2.
Zizeeria maha individuals with modified color-patterns in Fukaura, Aomori, Japan in 2002. Pictures taken in the field (top) and specimens of the three modification types (bottom) are shown. The normal-type individual was obtained from Hiratsuka, Kanagawa, Japan. Original photographs courtesy of Tadashi Kudo.
After a rearing system was established for this species (Hiyama et al., 2010), this genetic assimilation process was reproduced in a laboratory experiment (Otaki et al., 2010). Although the natural population later became nearly extinct, it is remarkable that the modified individuals strongly resemble other lycaenid species that inhabit relatively cold areas (Otaki and Yamamoto, 2003; Otaki et al., 2010; Figure 3). The modified color-patterns observed in this population were very similar to those seen in Maculinea species that live in high mountainous areas (Otaki and Yamamoto, 2003), and the sexually dimorphic response to cold-shock parallels the sexually dimorphic color-patterns of Lycaena dispar.
Figure 3.

Various color-patterns of lycaenid species that resemble the modified color-patterns of Zizeeria maha. (A) Lycaena heteronea, which resembles the reduction type. (B) Maculinea teleius, which resembles the normal-type. (C) M. arionides, which resembles the outward type. (D,E) L. dispar, male and female. The male resembles the reduction type, whereas the female resembles the outward type.
Side-Effect Model and Epigenetic Inheritance
Side-effects allow non-functional or neutral traits to be assimilated genetically
There are several possible mechanistic explanations for these plasticity-oriented evolutionary cases. The so-called “side-effect model” was proposed to explain the evolution of Vanessa (Otaki, 2008c). This model first makes the reasonable assumption that Vanessa is a group of species that favor a temperate environment and are not highly resistant to temperature shock. In addition, it is assumed that the ancestral species of the group had relatively high vagility, similar to that of V. indica and V. atalanta. Due to its vagility, the ancestral species can expand its range margin to tropical areas, but the temperature of these areas is too high to allow the next generation of the species to be produced. However, even in tropical regions, the species can live in mountainous areas where relatively low temperatures occur. There, large daily temperature fluctuations serve as natural cold-shocks. Natural selection operates to favor cold resistance in the isolated Vanessa populations. Color-pattern changes accompany this increase in cold resistance. However, the changed color traits have no functional relationship to cold resistance. Thus, color-pattern modifications are an opportunistic side-effect of cold resistance, i.e., color-pattern changes or new color-pattern traits are “parasitic” to cold resistance (Otaki, 2008c). Specifically, because the color-pattern changes are induced by a humoral factor in the hemolymph known as cold-shock hormone (CSH; Mahdi et al., 2010, 2011), the ability to efficiently secrete CSH into the hemolymph may be directly related to cold resistance and may be opportunistically linked to color-pattern modifications. As a result, neutral or non-functional color-patterns may be fixed in a population through a process of genetic assimilation.
To support this model, studies of the relationship of temperature to Vanessa’s life history, behavior, and physiology must be conducted. Further clear identification of the CSH is required. Hopefully, the genetic assimilation of the plastic phenotype will be reproduced in the laboratory, although rearing Vanessa for generations in an artificial environment would be relatively difficult from a technical perspective.
A fundamentally similar history may be hypothesized in the case of V. cardui and V. kershawi, although different molecular pathways may be involved in the color-pattern modifications of these species. It is probable that the common ancestor had color-patterns and phenotypic plasticity similar to those of V. cardui, a cosmopolitan species. As this ancestral species invaded a relatively arid environment, stress resistance developed over the generations through natural selection. As a side-effect, color-pattern modifications occurred and were eventually genetically assimilated in the population.
Similarly, in the case of Z. maha, the northward migration selected for cold resistance. As a side-effect, the color-patterns were modified at the previous northern margin of the range even before the population reached the Fukaura area. Although the modified traits were not well assimilated in the population at the previous northern margin of the range, a small number of individuals migrated farther to the north and established a regional population in the Fukaura area. It is probable that this population was genetically unstable and that this instability produced an outbreak of unstable TS-type modifications.
Epigenetic traits could facilitate stress adaptation
The proposed opportunistic link between stress resistance and color-patterns has not yet been demonstrated. If there is no such link, it would be difficult for the modified color-patterns to evolve by natural selection because it is probable that they are functionally neutral at best. It is even more probable that these color-patterns are selectively inferior to the normal patterns. An alternative explanation is that stress-induced traits in a given generation are heritable in the next generation. These two explanations are not mutually exclusive. It is possible that the transgenerational effect of epigenetic traits could involve the three cases discussed above. In Drosophila, epigenetic changes are mediated by the formation of a heritable heterochromatin in response to temperature stress (Seong et al., 2011). Given the prevalence of epigenetic modifications (Jablonka and Raz, 2009), similar molecular mechanisms can be envisioned in butterflies. Other mechanisms of epigenetic changes, such as DNA methylation, histone modifications, and regulation by non-coding RNAs, could also occur.
However, epigenetic changes induced by temperature shock do not persist for many generations in Drosophila (Seong et al., 2011). To establish a new trait in a population, a mechanism should exist to genetically fix such a new trait into the DNA sequences. If the cold-shock resistance itself is epigenetically inherited, this form of inheritance allows the population to develop resistance relatively rapidly and results in a higher probability of survival and of avoidance of extinction for the population. This process furnishes an opportunity for the natural selection of stress-induced phenotypes, because the operation of natural selection requires a relatively high number of generations. This process could eventually cause the assimilation of a new trait in the population and could ultimately promote speciation.
It is important to emphasize that the new trait discussed above need not be a functional trait. It can be neutral or non-functional (Otaki, 2008c). Neutral or non-functional traits will create opportunities for subsequent functional evolution. If all traits were fully functional, subsequent evolution would only damage functional traits, leading to the deterioration of the species. In this sense, neutral or non-functional traits have an evolutionary “function” as a source of opportunities for subsequent evolution and speciation. This concept is similar to the idea that neutral mutations “function” by furnishing a foundation for subsequent evolutionary adaptation at the molecular level (Wagner, 2008).
Concluding Remarks
The three cases of evolution presented in this paper involve coping with environmental stress and the emergence of extreme phenotypes. The coincidence between physiologically induced phenotypes in a species and the normal phenotypes of related species that live in “stressful” environments is striking. These cases demonstrate the involvement of phenotypic plasticity in evolution. Experimental demonstration and field observation are further required to convincingly demonstrate the roles of phenotypic plasticity and environmental stress in the color-pattern evolution and speciation of butterflies. Theoretical frameworks are also expected to emerge from these considerations (Behera and Nanjundiah, 2004; Lande, 2009; Danchin et al., 2011; Espinosa-Soto et al., 2011; Fierst, 2011).
We do not yet accurately know how plastic phenotypes are genetically assimilated in a given butterfly population to produce an independent species, nor do we know the possible roles of epigenetic inheritance in the evolution of butterfly color-patterns. The active role of phenotypic plasticity in evolution is a concept that has gained popularity relatively recently in biology (Pigliucci and Murren, 2003; Pigliucci et al., 2006; Pfennig et al., 2010) despite its original formulation in the 1940s (Waddington, 1942). Epigenetic inheritance is also a relatively new field of biology. Considering that the integration of evolutionary biology and genetics (or the integration of phenotypic biology and genotypic biology) was fruitful in the past, a similar yet higher-level integration of the evolutionary roles of phenotypic plasticity and molecular epigenetics is now opening up a very fruitful and attractive field of biology.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Tadashi Kudo for sharing photographs of Zizeeria maha and members of the BCPH Unit of Molecular Physiology for discussion. This work was partly supported by International Research Hub Project for Climate Change and Coral Reef/Island Dynamics from University of the Ryukyus.
References
- Aubret F., Shine R. (2009). Genetic assimilation and the postcolonization erosion of phenotypic plasticity in island tiger snakes. Curr. Biol. 19, 1932–1936 10.1016/j.cub.2009.09.061 [DOI] [PubMed] [Google Scholar]
- Badyaev A. V. (2005). Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proc. R. Soc. Lond. B Biol. Sci. 272, 877–886 10.1098/rspb.2005.3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera N., Nanjundiah V. (2004). Phenotypic plasticity can potentiate rapid evolutionary change. J. Theor. Biol. 226, 177–184 10.1016/j.jtbi.2003.08.011 [DOI] [PubMed] [Google Scholar]
- Buckley J., Bridle J. R., Pomiankowski A. (2010). Novel variation associated with species range expansion. BMC Evol. Biol. 10, 382. 10.1186/1471-2148-10-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin É., Charmantier A., Champagne F. A., Mesoudi A., Pujol B., Blanchet S. (2011). Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat. Rev. Genet. 12, 475–486 10.1038/nrg3028 [DOI] [PubMed] [Google Scholar]
- Espinosa-Soto C., Martin O. C., Wagner A. (2011). Phenotypic plasticity can facilitate adaptive evolution in gene regulatory circuits. BMC Evol. Biol. 11, 5. 10.1186/1471-2148-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierst J. L. (2011). A history of phenotypic plasticity accelerates adaptation to a new environment. J. Evol. Biol. 24, 1992–2001 10.1111/j.1420-9101.2011.02333.x [DOI] [PubMed] [Google Scholar]
- Fujioka K. (1975). Butterflies of Japan. Tokyo: Kodansha [Google Scholar]
- Hiyama A., Iwata M., Otaki J. M. (2010). Rearing the pale grass blue Zizeeria maha (Lepidoptera, Lycaenidae): toward the establishment of a lycaenid model system for butterfly physiology and genetics. Entomol. Sci. 13, 293–302 10.1111/j.1479-8298.2010.00387.x [DOI] [Google Scholar]
- Jablonka E., Raz G. (2009). Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 84, 131–176 10.1086/598822 [DOI] [PubMed] [Google Scholar]
- Lande R. (2009). Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446 10.1111/j.1420-9101.2009.01754.x [DOI] [PubMed] [Google Scholar]
- Mahdi S. H., Gima S., Tomita Y., Yamasaki H., Otaki J. M. (2010). Physiological characterization of the cold-shock-induced humoral factor for wing color-pattern changes in butterflies. J. Insect Physiol. 56, 1022–1031 10.1016/j.jinsphys.2010.02.013 [DOI] [PubMed] [Google Scholar]
- Mahdi S. H., Yamasaki H., Otaki J. M. (2011). Heat-shock-induced color-pattern changes of the blue pansy butterfly Junonia orithya: physiological and evolutionary implications. J. Therm. Biol. 36, 312–321 [Google Scholar]
- Muschick M., Barluenga M., Salzburger W., Meyer A. (2011). Adaptive phenotypic plasticity in the Midas cichlid fish pharyngeal jaw and its relevance in adaptive radiation. BMC Evol. Biol. 11, 116. 10.1186/1471-2148-11-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout H. F. (1984). Colour pattern modification by cold-shock in Lepidoptera. J. Embryol. Exp. Morphol. 81, 287–305 [PubMed] [Google Scholar]
- Nijhout H. F. (1991). The Development and Evolution of Butterfly Wing Patterns. Washington, DC: Smithsonian Institution Press [Google Scholar]
- Otaki J. M. (1998). Color-pattern modifications of butterfly wings induced by transfusion and oxyanions. J. Insect Physiol. 44, 1181–1190 10.1016/S0022-1910(98)00083-3 [DOI] [PubMed] [Google Scholar]
- Otaki J. M. (2007a). Stress-induced color-pattern modifications and evolution of the painted lady butterflies Vanessa cardui and Vanessa kershawi. Zool. Sci. 24, 811–819 10.2108/zsj.24.811 [DOI] [PubMed] [Google Scholar]
- Otaki J. M. (2007b). Reversed type of color-pattern modifications of butterfly wings: a physiological mechanism of wing-wide color-pattern determination. J. Insect Physiol. 53, 526–537 10.1016/j.jinsphys.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Otaki J. M. (2008a). Physiologically induced color-pattern changes in butterfly wings: mechanistic and evolutionary implications. J. Insect Physiol. 54, 1099–1112 10.1016/j.jinsphys.2008.05.006 [DOI] [PubMed] [Google Scholar]
- Otaki J. M. (2008b). Phenotypic plasticity of wing color patterns revealed by temperature and chemical applications in a nymphalid butterfly Vanessa indica. J. Therm. Biol. 33, 128–139 10.1016/j.jtherbio.2007.11.004 [DOI] [Google Scholar]
- Otaki J. M. (2008c). Physiological side-effect model for diversification of non-functional or neutral traits: a possible evolutionary history of Vanessa butterflies (Lepidoptera, Nymphalidae). Trans. Lepidopterol. Soc. Jpn. 59, 87–102 [Google Scholar]
- Otaki J. M. (2009). Color-pattern analysis of parafocal elements in butterfly wings. Entomol. Sci. 12, 74–83 10.1111/j.1479-8298.2009.00306.x [DOI] [Google Scholar]
- Otaki J. M., Hiyama A., Iwata M., Kudo S. (2010). Phenotypic plasticity in the range-margin population of the lycaenid butterfly Zizeeria maha. BMC Evol. Biol. 10, 252. 10.1186/1471-2148-10-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaki J. M., Kimura Y., Yamamoto H. (2006). Molecular phylogeny and color-pattern evolution of Vanessa butterflies (Lepidoptera, Nymphalidae). Trans. Lepidopterol. Soc. Jpn. 57, 359–370 [Google Scholar]
- Otaki J. M., Ogasawara T., Yamamoto H. (2005). Tungstate-induced color-pattern modifications of butterfly wings are independent of stress response and ecdysteroid effect. Zool. Sci. 22, 635–644 10.2108/zsj.22.635 [DOI] [PubMed] [Google Scholar]
- Otaki J. M., Yamamoto H. (2003). Color-pattern modifications and speciation in lycaenid butterflies. Trans. Lepidopterol. Soc. Jpn. 54, 197–205 [Google Scholar]
- Otaki J. M., Yamamoto H. (2004a). Species-specific color-pattern modifications on butterfly wings. Dev. Growth Differ. 46, 1–14 10.1111/j.1440-169X.2004.00721.x [DOI] [PubMed] [Google Scholar]
- Otaki J. M., Yamamoto H. (2004b). Color-pattern modifications and speciation in butterflies of the genus Vanessa and its related genera Cynthia and Bassaris. Zool. Sci. 21, 967–976 10.2108/zsj.21.967 [DOI] [PubMed] [Google Scholar]
- Pfennig D. W., Wund M. A., Snell-Rood E. C., Cruickshank T., Schlichting C. D., Moczek A. P. (2010). Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol. Evol. (Amst.) 25, 459–467 10.1016/j.tree.2010.05.006 [DOI] [PubMed] [Google Scholar]
- Pigliucci M., Murren C. J. (2003). Genetic assimilation and a possible evolutionary paradox: can macroevolution sometimes be so fast as to pass us by? Evolution 57, 1455–1464 10.1554/02-381 [DOI] [PubMed] [Google Scholar]
- Pigliucci M., Murren C. J., Schlichting C. D. (2006). Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 209, 2362–2367 10.1242/jeb.02070 [DOI] [PubMed] [Google Scholar]
- Russwurm A. D. A. (1978). Aberrations of British Butterflies. Faringdon: Classey [Google Scholar]
- Sakaguti K. (1979). Insects of the World. 1. Southeast Asia I Including Australia. Osaka: Hoikusha [Google Scholar]
- Sakaguti K. (1981a). Insects of the World. 2. Southeast Asia II Including Australia. Osaka: Hoikusha [Google Scholar]
- Sakaguti K. (1981b). Insects of the World. 5. Eurasia. Osaka: Hoikusha [Google Scholar]
- Schlichting C. D., Smith H. (2002). Phenotypic plasticity: linking molecular mechanisms with evolutionary outcomes. Evol. Ecol. 16, 189–211 10.1023/A:1019624425971 [DOI] [Google Scholar]
- Scoville A. G., Pfrender M. E. (2010). Phenotypic plasticity facilitates recurrent rapid adaptation to introduced predators. Proc. Natl. Acad. Sci. U.S.A. 107, 4260–4263 10.1073/pnas.0912748107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong K. H., Li D., Shimizu H., Nakamura R., Ishii S. (2011). Inheritance of stress-induced, ATF-2-dependent epigenetic change. Cell 145, 1049–1061 10.1016/j.cell.2011.05.029 [DOI] [PubMed] [Google Scholar]
- Serfas M. S., Carroll S. B. (2005). Pharmacologic approaches to butterfly wing patterning: sulfated polysaccharides mimic or antagonize cold shock and alter the interpretation of gradients of positional information. Dev. Biol. 287, 416–424 10.1016/j.ydbio.2005.09.014 [DOI] [PubMed] [Google Scholar]
- Shapiro A. M. (1984). “The genetics of seasonal polyphenism and the evolution of ‘general purpose genotypes’ in butterflies,” in Population Biology and Evolution, eds Wöhrmann K., Loeschcke V. (Berlin: Springer-Verlag; ), 16–30 [Google Scholar]
- Sibatani A. (1980). Wing homoeosis in Lepidoptera: a survey. Dev. Biol. 79, 1–18 10.1016/0012-1606(80)90137-2 [DOI] [PubMed] [Google Scholar]
- Waddington C. H. (1942). Canalization of development and the inheritance of acquired characters. Nature 150, 563–565 10.1038/150563a0 [DOI] [PubMed] [Google Scholar]
- Wagner A. (2008). Neutralism and selectionism: a network-based reconciliation. Nat. Rev. Genet. 9, 965–974 10.1038/nrn2456-c1 [DOI] [PubMed] [Google Scholar]
- Wahlberg N., Brower A. V. Z., Nylin S. (2005). Phylogenetic relationships and historical biogeography of tribes and genera in the subfamily Nymphalidae (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. Lond. 86, 227–251 10.1111/j.1095-8312.2005.00531.x [DOI] [Google Scholar]
- Wahlberg N., Rubinoff D. (2011). Vagility across Vanessa (Lepidoptera: Nymphalidae): mobility in butterfly species does not inhibit the formation and persistence of isolated sister taxa. Syst. Entomol. 36, 362–370 10.1111/j.1365-3113.2011.00590.x [DOI] [Google Scholar]
- West-Eberhard M. J. (2003). Developmental Plasticity and Evolution. New York, NY: Oxford University Press [Google Scholar]